NEET Exam > NEET Questions > If on adding FeCl3 solution to acidified Lass...
Start Learning for Free
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence of
- a)S
- b)N
- c)N and S
- d)S and Cl
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If on adding FeCl3 solution to acidified Lassaigne solution, a blood r...
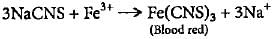
Most Upvoted Answer
If on adding FeCl3 solution to acidified Lassaigne solution, a blood r...
Presence of Nitrogen in Acidified Lassaigne Solution
To understand why the blood red coloration indicates the presence of nitrogen in acidified Lassaigne solution, we need to examine the chemical reactions involved.
1. Formation of Sodium Fusion Extract:
Lassaigne's test is used to detect the presence of nitrogen and sulfur in an organic compound. The compound is first heated with sodium metal, which forms a fusion extract containing sodium derivatives of the elements present in the compound.
2. Acidification of Sodium Fusion Extract:
The fusion extract is dissolved in water and then acidified with dilute HNO3. This step helps to convert the sodium derivatives into their respective acids.
3. Reaction with FeCl3:
After acidification, FeCl3 solution is added to the acidified Lassaigne solution. The addition of FeCl3 is crucial in detecting the presence of nitrogen.
Explanation of the Blood Red Coloration:
When FeCl3 is added to the acidified Lassaigne solution, a blood red coloration is observed. This coloration is indicative of the presence of nitrogen compounds, specifically nitro groups (NO2-).
The blood red coloration is due to the formation of a complex between FeCl3 and the nitro group. The FeCl3 oxidizes the nitro group to form a stable complex called ferric nitroso-ortho-sulfonate. This complex has a characteristic blood red color.
FeCl3 + NO2- → [Fe(III)-NO2]2- (blood red complex)
Since the nitro group is derived from nitrogen, the blood red coloration confirms the presence of nitrogen in the acidified Lassaigne solution.
Significance of Acidification:
Acidification of the Lassaigne solution is important in this test because it converts the sodium derivatives of nitrogen compounds into their corresponding acids. This step allows for the reaction between FeCl3 and the nitro group to occur, resulting in the blood red coloration.
Conclusion:
The blood red coloration produced upon adding FeCl3 to acidified Lassaigne solution indicates the presence of nitrogen compounds, specifically nitro groups. Acidification of the solution is necessary to convert the sodium derivatives into their respective acids, enabling the reaction with FeCl3 to occur. This test is widely used in organic chemistry to detect the presence of nitrogen in organic compounds.
To understand why the blood red coloration indicates the presence of nitrogen in acidified Lassaigne solution, we need to examine the chemical reactions involved.
1. Formation of Sodium Fusion Extract:
Lassaigne's test is used to detect the presence of nitrogen and sulfur in an organic compound. The compound is first heated with sodium metal, which forms a fusion extract containing sodium derivatives of the elements present in the compound.
2. Acidification of Sodium Fusion Extract:
The fusion extract is dissolved in water and then acidified with dilute HNO3. This step helps to convert the sodium derivatives into their respective acids.
3. Reaction with FeCl3:
After acidification, FeCl3 solution is added to the acidified Lassaigne solution. The addition of FeCl3 is crucial in detecting the presence of nitrogen.
Explanation of the Blood Red Coloration:
When FeCl3 is added to the acidified Lassaigne solution, a blood red coloration is observed. This coloration is indicative of the presence of nitrogen compounds, specifically nitro groups (NO2-).
The blood red coloration is due to the formation of a complex between FeCl3 and the nitro group. The FeCl3 oxidizes the nitro group to form a stable complex called ferric nitroso-ortho-sulfonate. This complex has a characteristic blood red color.
FeCl3 + NO2- → [Fe(III)-NO2]2- (blood red complex)
Since the nitro group is derived from nitrogen, the blood red coloration confirms the presence of nitrogen in the acidified Lassaigne solution.
Significance of Acidification:
Acidification of the Lassaigne solution is important in this test because it converts the sodium derivatives of nitrogen compounds into their corresponding acids. This step allows for the reaction between FeCl3 and the nitro group to occur, resulting in the blood red coloration.
Conclusion:
The blood red coloration produced upon adding FeCl3 to acidified Lassaigne solution indicates the presence of nitrogen compounds, specifically nitro groups. Acidification of the solution is necessary to convert the sodium derivatives into their respective acids, enabling the reaction with FeCl3 to occur. This test is widely used in organic chemistry to detect the presence of nitrogen in organic compounds.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer?
Question Description
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer?.
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer?.
Solutions for If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence ofa)Sb)Nc)N and Sd)S and ClCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























