NEET Exam > NEET Questions > n-Butylbenzene on oxidation will givea)benzo...
Start Learning for Free
n-Butylbenzene on oxidation will give
- a)benzoic acid
- b)butanoic acid
- c)benzyl alcohol
- d)benzaldehyde
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)b...
In this reaction, the alkyl chain is oxidized to carboxylic acid in presence of oxidants like KMnO4 or chromic acid irrespective of the number of carbon atoms in the chain.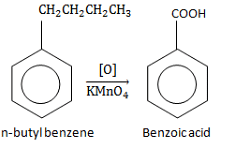
So the n-butyl benzene is converted into benzoic acid.

Free Test
FREE
| Start Free Test |
Community Answer
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)b...
Introduction:
n-Butylbenzene is a compound with a butyl group attached to the benzene ring. On oxidation, it undergoes a reaction called side-chain oxidation, where the alkyl group is oxidized to form a carboxylic acid. The specific product formed depends on the conditions of the reaction.
Explanation:
The oxidation of n-butylbenzene can proceed through different pathways, resulting in different products. However, in this case, the correct answer is option 'A', which states that the product is benzoic acid. Let's explore why this is the case.
Side-chain oxidation:
Side-chain oxidation involves the oxidation of the alkyl group attached to the benzene ring. In the case of n-butylbenzene, the butyl group (C4H9) is oxidized.
Reagents:
The reagents used for the oxidation of n-butylbenzene can vary. Common oxidizing agents include potassium permanganate (KMnO4) and chromic acid (H2CrO4).
Reaction conditions:
The reaction conditions also play a crucial role in determining the product. In this case, we assume that the reaction is carried out under controlled conditions to favor the formation of benzoic acid.
Product formation:
Under controlled conditions, n-butylbenzene undergoes oxidation to form benzoic acid. The butyl group (C4H9) is oxidized to a carboxylic acid group (-COOH) attached to the benzene ring, resulting in the formation of benzoic acid (C6H5COOH).
Other possible products:
It is worth noting that under different reaction conditions, other products can also be formed. For example, if the reaction is carried out under harsher conditions or with a different oxidizing agent, the butyl group can be further oxidized to form butanoic acid (C3H7COOH) instead.
Conclusion:
In summary, the oxidation of n-butylbenzene can result in different products depending on the reaction conditions. However, under controlled conditions, it primarily forms benzoic acid.
n-Butylbenzene is a compound with a butyl group attached to the benzene ring. On oxidation, it undergoes a reaction called side-chain oxidation, where the alkyl group is oxidized to form a carboxylic acid. The specific product formed depends on the conditions of the reaction.
Explanation:
The oxidation of n-butylbenzene can proceed through different pathways, resulting in different products. However, in this case, the correct answer is option 'A', which states that the product is benzoic acid. Let's explore why this is the case.
Side-chain oxidation:
Side-chain oxidation involves the oxidation of the alkyl group attached to the benzene ring. In the case of n-butylbenzene, the butyl group (C4H9) is oxidized.
Reagents:
The reagents used for the oxidation of n-butylbenzene can vary. Common oxidizing agents include potassium permanganate (KMnO4) and chromic acid (H2CrO4).
Reaction conditions:
The reaction conditions also play a crucial role in determining the product. In this case, we assume that the reaction is carried out under controlled conditions to favor the formation of benzoic acid.
Product formation:
Under controlled conditions, n-butylbenzene undergoes oxidation to form benzoic acid. The butyl group (C4H9) is oxidized to a carboxylic acid group (-COOH) attached to the benzene ring, resulting in the formation of benzoic acid (C6H5COOH).
Other possible products:
It is worth noting that under different reaction conditions, other products can also be formed. For example, if the reaction is carried out under harsher conditions or with a different oxidizing agent, the butyl group can be further oxidized to form butanoic acid (C3H7COOH) instead.
Conclusion:
In summary, the oxidation of n-butylbenzene can result in different products depending on the reaction conditions. However, under controlled conditions, it primarily forms benzoic acid.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer?
Question Description
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer?.
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer?.
Solutions for n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice n-Butylbenzene on oxidation will givea)benzoic acidb)butanoic acidc)benzyl alcohold)benzaldehydeCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















