NEET Exam > NEET Questions > In Williamson's synthesis, tertiary alkyl ha...
Start Learning for Free
In Williamson's synthesis, tertiary alkyl halides cannot be used because
- a)they readily decompose to give olefin along with ethers
- b)they are not reactive
- c)the reaction becomes reversible
- d)it is difficult to remove halogen atom
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
In Williamson's synthesis, tertiary alkyl halides cannot be used beca...
Williamson Ether Synthesis usually takes place as an SN2 reaction of a primary or secondary alkyl halide with an alkoxide ion. Tertiary alkyl halides undergo elimination reactions readily rather than SN2 under Williamson Ether Synthesis conditions. Tertiary alkyl halides produces alkene via elimination reaction.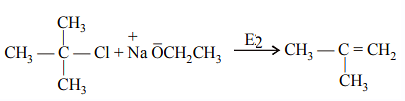
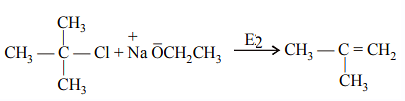
Free Test
FREE
| Start Free Test |
Community Answer
In Williamson's synthesis, tertiary alkyl halides cannot be used beca...
Answer:
In Williamson's synthesis, tertiary alkyl halides cannot be used because they readily decompose to give olefin along with ethers. This is due to the stability of tertiary carbocations and the elimination reaction that occurs under the reaction conditions.
Explanation:
Williamson's synthesis: Williamson's synthesis is a method used to prepare ethers by the reaction of alkyl halides with alkoxides. The reaction involves the nucleophilic substitution of the halide by the alkoxide.
Tertiary alkyl halides: Tertiary alkyl halides are compounds in which the halogen atom is attached to a carbon atom that is bonded to three other carbon atoms. These compounds have a highly branched structure and are sterically hindered.
Decomposition to give olefin: Tertiary alkyl halides readily undergo elimination reactions, where the halide is eliminated along with the formation of a double bond (olefin). This is due to the stability of tertiary carbocations, which can be formed during the reaction.
Reactivity: Tertiary alkyl halides are less reactive compared to primary and secondary alkyl halides due to the steric hindrance caused by the three alkyl groups attached to the carbon atom. This steric hindrance makes it difficult for the nucleophile (alkoxide) to attack the carbon atom and undergo substitution.
Reversibility of the reaction: The reaction between tertiary alkyl halides and alkoxides is reversible. The formation of the olefin is favored under the reaction conditions, leading to an equilibrium between the reactants and products. This makes it difficult to obtain a high yield of the desired ether product.
Difficulty in removing the halogen atom: Tertiary alkyl halides are more stable compared to primary and secondary alkyl halides, making it difficult to remove the halogen atom. The C-X bond in tertiary alkyl halides is stronger and less reactive, requiring harsher reaction conditions to cleave the bond.
Overall, the combination of the decomposition of tertiary alkyl halides to give olefin along with ethers, their low reactivity, and the reversibility of the reaction make them unsuitable for use in Williamson's synthesis.
In Williamson's synthesis, tertiary alkyl halides cannot be used because they readily decompose to give olefin along with ethers. This is due to the stability of tertiary carbocations and the elimination reaction that occurs under the reaction conditions.
Explanation:
Williamson's synthesis: Williamson's synthesis is a method used to prepare ethers by the reaction of alkyl halides with alkoxides. The reaction involves the nucleophilic substitution of the halide by the alkoxide.
Tertiary alkyl halides: Tertiary alkyl halides are compounds in which the halogen atom is attached to a carbon atom that is bonded to three other carbon atoms. These compounds have a highly branched structure and are sterically hindered.
Decomposition to give olefin: Tertiary alkyl halides readily undergo elimination reactions, where the halide is eliminated along with the formation of a double bond (olefin). This is due to the stability of tertiary carbocations, which can be formed during the reaction.
Reactivity: Tertiary alkyl halides are less reactive compared to primary and secondary alkyl halides due to the steric hindrance caused by the three alkyl groups attached to the carbon atom. This steric hindrance makes it difficult for the nucleophile (alkoxide) to attack the carbon atom and undergo substitution.
Reversibility of the reaction: The reaction between tertiary alkyl halides and alkoxides is reversible. The formation of the olefin is favored under the reaction conditions, leading to an equilibrium between the reactants and products. This makes it difficult to obtain a high yield of the desired ether product.
Difficulty in removing the halogen atom: Tertiary alkyl halides are more stable compared to primary and secondary alkyl halides, making it difficult to remove the halogen atom. The C-X bond in tertiary alkyl halides is stronger and less reactive, requiring harsher reaction conditions to cleave the bond.
Overall, the combination of the decomposition of tertiary alkyl halides to give olefin along with ethers, their low reactivity, and the reversibility of the reaction make them unsuitable for use in Williamson's synthesis.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer?
Question Description
In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer?.
In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer?.
Solutions for In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In Williamson's synthesis, tertiary alkyl halides cannot be used becausea)they readily decompose to give olefin along with ethersb)they are not reactivec)the reaction becomes reversibled)it is difficult to remove halogen atomCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























