Chemistry Exam > Chemistry Questions > Which of the following solutions will show th...
Start Learning for Free
Which of the following solutions will show the minimum value of transference number of Cl–1 ions:
- a)0.1 M NaCl solut ion
- b)0.1 M KCl solut ion
- c)0.1 M HCl solution
- d)0.1 M MgCl2 solution
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following solutions will show the minimum value of transf...
Because 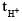 has maximum value of transference no. as co mpared to other cations. Hence
has maximum value of transference no. as co mpared to other cations. Hence 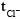 will be minimum in 0.1 M HCl solution
will be minimum in 0.1 M HCl solution
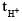 has maximum value of transference no. as co mpared to other cations. Hence
has maximum value of transference no. as co mpared to other cations. Hence 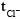 will be minimum in 0.1 M HCl solution
will be minimum in 0.1 M HCl solutionMost Upvoted Answer
Which of the following solutions will show the minimum value of transf...
In above question... From all the options... H+ cation have the highest trasfer no. (t+). And we know that (t+) + (t-) = 1 So species whoes (t+) is highest its (t- ) would be lowest. So in option c) as (t-) = 1 - (t+) will be lowest
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following solutions will show the minimum value of transf...
To determine the solution that will show the minimum value of transference number of Cl, we need to consider the solubility of Cl in different solvents.
1. Water: Chloride ions (Cl-) are highly soluble in water due to its ionic nature. Therefore, water will show a high transference number for Cl-.
2. Nonpolar organic solvents (e.g., hexane, benzene): Chloride ions (Cl-) are not soluble in nonpolar organic solvents. Therefore, these solvents will show a very low or zero transference number for Cl-.
3. Polar organic solvents (e.g., ethanol, acetone): Chloride ions (Cl-) can have some solubility in polar organic solvents, but it is generally lower compared to water. Therefore, polar organic solvents will show a lower transference number for Cl- compared to water.
Based on this information, the solution that will show the minimum value of transference number of Cl is a nonpolar organic solvent, such as hexane or benzene.
1. Water: Chloride ions (Cl-) are highly soluble in water due to its ionic nature. Therefore, water will show a high transference number for Cl-.
2. Nonpolar organic solvents (e.g., hexane, benzene): Chloride ions (Cl-) are not soluble in nonpolar organic solvents. Therefore, these solvents will show a very low or zero transference number for Cl-.
3. Polar organic solvents (e.g., ethanol, acetone): Chloride ions (Cl-) can have some solubility in polar organic solvents, but it is generally lower compared to water. Therefore, polar organic solvents will show a lower transference number for Cl- compared to water.
Based on this information, the solution that will show the minimum value of transference number of Cl is a nonpolar organic solvent, such as hexane or benzene.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer?.
Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following solutions will show the minimum value of transference number of Cl–1 ions:a)0.1 M NaCl solut ionb)0.1 M KCl solut ionc)0.1 M HCl solutiond)0.1 M MgCl2 solutionCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















