NEET Exam > NEET Questions > How many asymmetric carbon atoms are present ...
Start Learning for Free
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?
Verified Answer
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?
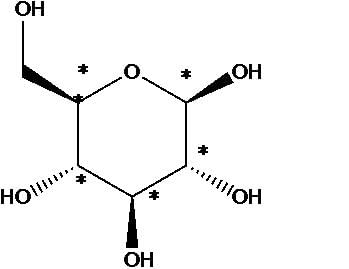
1. A chiral carbon has four different groups attached. α -D-Glucose has no plane of symmetry, so all the carbon atoms are different.
There are five chiral carbons:
2.Three hydroxyl groups are below the plane of the ring.
3. C-5 is attached to an oxygen within the ring and a carbon (C-6) outside the ring.
4.Three of the ring hydrogens (on C-1, C-2, and C-4) are above the plane of the ring, and two (on C-3 and C-5) are below the plane of the ring.
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?
A carbon atom which is bonded to four different groups in an assymetric carbon atom.Glucose with six carbon atom has four assymetric carbon atom.
Community Answer
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?
Asymmetric Carbon Atoms in α-D-Glucose
α-D-glucose is a monosaccharide and a common sugar found in nature. It is a hexose sugar, meaning it has six carbon atoms. To determine the number of asymmetric carbon atoms present in α-D-glucose, we need to understand the concept of chirality and how it relates to carbon atoms.
Chirality and Asymmetric Carbon Atoms
Chirality refers to the property of an object that is not superimposable on its mirror image. In the context of organic chemistry, chiral molecules have a central carbon atom (known as an asymmetric carbon or chiral center) attached to four different substituents. The presence of an asymmetric carbon atom in a molecule leads to the existence of stereoisomers, which are non-superimposable mirror images of each other.
Structure of α-D-Glucose
α-D-glucose has a molecular formula of C6H12O6. It is a cyclic sugar, existing predominantly in the cyclic hemiacetal form. The cyclic structure of glucose consists of a six-membered ring with five carbon atoms and one oxygen atom, known as a pyranose ring.
Number of Asymmetric Carbon Atoms
In α-D-glucose, there are four asymmetric carbon atoms, indicated by the asterisks (*) in the structure below:
H OH
| |
HOCH2----C*----C----OH
| |
H OH
Explanation
1. The carbon atoms numbered 2, 3, 4, and 5 in the structure of α-D-glucose are asymmetric carbon atoms.
2. Each of these carbon atoms is bonded to four different substituents: one hydrogen atom (H) and three hydroxyl groups (OH).
3. The presence of these asymmetric carbon atoms gives rise to different stereoisomers of α-D-glucose.
4. The stereoisomers include enantiomers, which are mirror images of each other, and diastereomers, which are not mirror images.
5. The number of possible stereoisomers for a compound with 'n' asymmetric carbon atoms is 2^n. Therefore, α-D-glucose can exist as 2^4 = 16 stereoisomers.
Conclusion
In α-D-glucose, there are four asymmetric carbon atoms. These carbon atoms are crucial for the formation of different stereoisomers, contributing to the diverse properties and functions of glucose in biological systems. Understanding the presence and arrangement of asymmetric carbon atoms is essential in the study of carbohydrates and their role in various biological processes.
α-D-glucose is a monosaccharide and a common sugar found in nature. It is a hexose sugar, meaning it has six carbon atoms. To determine the number of asymmetric carbon atoms present in α-D-glucose, we need to understand the concept of chirality and how it relates to carbon atoms.
Chirality and Asymmetric Carbon Atoms
Chirality refers to the property of an object that is not superimposable on its mirror image. In the context of organic chemistry, chiral molecules have a central carbon atom (known as an asymmetric carbon or chiral center) attached to four different substituents. The presence of an asymmetric carbon atom in a molecule leads to the existence of stereoisomers, which are non-superimposable mirror images of each other.
Structure of α-D-Glucose
α-D-glucose has a molecular formula of C6H12O6. It is a cyclic sugar, existing predominantly in the cyclic hemiacetal form. The cyclic structure of glucose consists of a six-membered ring with five carbon atoms and one oxygen atom, known as a pyranose ring.
Number of Asymmetric Carbon Atoms
In α-D-glucose, there are four asymmetric carbon atoms, indicated by the asterisks (*) in the structure below:
H OH
| |
HOCH2----C*----C----OH
| |
H OH
Explanation
1. The carbon atoms numbered 2, 3, 4, and 5 in the structure of α-D-glucose are asymmetric carbon atoms.
2. Each of these carbon atoms is bonded to four different substituents: one hydrogen atom (H) and three hydroxyl groups (OH).
3. The presence of these asymmetric carbon atoms gives rise to different stereoisomers of α-D-glucose.
4. The stereoisomers include enantiomers, which are mirror images of each other, and diastereomers, which are not mirror images.
5. The number of possible stereoisomers for a compound with 'n' asymmetric carbon atoms is 2^n. Therefore, α-D-glucose can exist as 2^4 = 16 stereoisomers.
Conclusion
In α-D-glucose, there are four asymmetric carbon atoms. These carbon atoms are crucial for the formation of different stereoisomers, contributing to the diverse properties and functions of glucose in biological systems. Understanding the presence and arrangement of asymmetric carbon atoms is essential in the study of carbohydrates and their role in various biological processes.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?
Question Description
How many asymmetric carbon atoms are present in alpha d ( ) glucose ? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How many asymmetric carbon atoms are present in alpha d ( ) glucose ? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many asymmetric carbon atoms are present in alpha d ( ) glucose ?.
How many asymmetric carbon atoms are present in alpha d ( ) glucose ? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How many asymmetric carbon atoms are present in alpha d ( ) glucose ? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many asymmetric carbon atoms are present in alpha d ( ) glucose ?.
Solutions for How many asymmetric carbon atoms are present in alpha d ( ) glucose ? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of How many asymmetric carbon atoms are present in alpha d ( ) glucose ? defined & explained in the simplest way possible. Besides giving the explanation of
How many asymmetric carbon atoms are present in alpha d ( ) glucose ?, a detailed solution for How many asymmetric carbon atoms are present in alpha d ( ) glucose ? has been provided alongside types of How many asymmetric carbon atoms are present in alpha d ( ) glucose ? theory, EduRev gives you an
ample number of questions to practice How many asymmetric carbon atoms are present in alpha d ( ) glucose ? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























