NEET Exam > NEET Questions > Why ethyl acetate and methyl propionate are n...
Start Learning for Free
Why ethyl acetate and methyl propionate are not position isomers?
Verified Answer
Why ethyl acetate and methyl propionate are not position isomers?
Positional isomers are defined as constitutional isomers that have the same carbon skeleton and the same functional group but which differ from each other in the position of the functional group in or on the carbon chain. The term is then most simply applied to isomers where an atom or group is attached to a unique carbon skeleton. Thus 1-butanol and 2-butanol are positional isomers with the same unbranched carbon skeleton and differing only in the position of the functional group.

Other positional isomers would arise in the same way if the hydroxyl group were replaced by, for example, a halogen or an amino group. However, esters are formed by the condensation of a carboxylic acid and an alcohol.
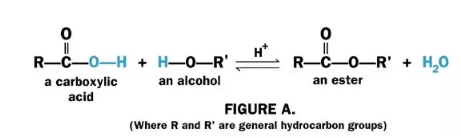
An ester linkage is then not a group which can be attached to different positions on a carbon skeleton but is incorporated within the molecule. However, esters can show constitutional isomerism and C4H8O2 represents a number of compounds, including the four esters: propyl formate HCOOCH2CH2CH3, ethyl acetate CH3COOCH2CH3, methyl propionate CH3CH2COOCH3 and isopropyl formate HCOOCH(CH3)2
The first three compounds have the same unbranched carbon skeleton and, insofar as the definition of positional isomerism refers to the position of a functional moiety on or within the chain, they can be regarded as positional isomers.
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Why ethyl acetate and methyl propionate are not position isomers?
Question Description
Why ethyl acetate and methyl propionate are not position isomers? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Why ethyl acetate and methyl propionate are not position isomers? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Why ethyl acetate and methyl propionate are not position isomers?.
Why ethyl acetate and methyl propionate are not position isomers? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Why ethyl acetate and methyl propionate are not position isomers? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Why ethyl acetate and methyl propionate are not position isomers?.
Solutions for Why ethyl acetate and methyl propionate are not position isomers? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Why ethyl acetate and methyl propionate are not position isomers? defined & explained in the simplest way possible. Besides giving the explanation of
Why ethyl acetate and methyl propionate are not position isomers?, a detailed solution for Why ethyl acetate and methyl propionate are not position isomers? has been provided alongside types of Why ethyl acetate and methyl propionate are not position isomers? theory, EduRev gives you an
ample number of questions to practice Why ethyl acetate and methyl propionate are not position isomers? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























