NEET Exam > NEET Questions > When cell has stalled DNA replication fork, w...
Start Learning for Free
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain?
Verified Answer
When cell has stalled DNA replication fork, which checkpoint should be...
For DNA replication to be accurately completed, the replication fork must frequently overcome a multitude of structurally unrelated obstacles such as DNA lesions, transcribing RNA polymerases, and tightly bound protein–DNA complexes. As a consequence, numerous diverse mechanisms have evolved that either help minimize the frequency or impact of collisions or repair the damage that is left behind. This work will focus solely on the mechanisms that exist in prokaryotes and eukaryotes to facilitate replication on template DNA containing either leading- or lagging-strand polymerization-blocking lesions.
Figure 1 : Intra-S phase checkpoint signaling
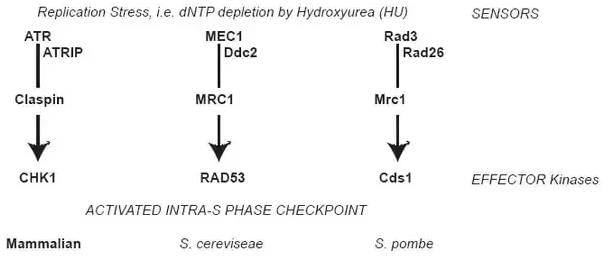
When replication stress is encountered, as during HU exposure, signals are transmitted through a kinase cascade. The paths compared between species are shown, and the given proteins in the pathway have functional similarity between species. At the top, signals are transmitted through the apical kinase: ATR in vertebrates; Mec1 in Saccharomyces cerevisiae (budding yeast); Rad3 in Schizosaccharomyces pombe (fission yeast). These kinases form a complex with adaptor proteins such as Atrip (or Ddc2, or Rad26) and transmit signals through transducers Claspin (or Mrc1 in yeast). For the purpose of this review, the ultimate target is the effector kinase: CHK1 in vertebrates, Rad53 in budding yeast, and Cds1 in fission yeast.
which checkpoint should be predominantly activated?
Answer is: G2-M DNA damage checkpoint
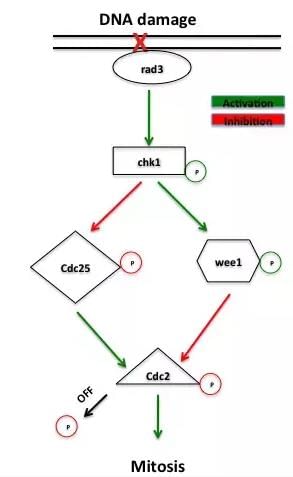
The G2-M DNA damage checkpoint is an important cell cycle checkpoint in eukaryotic organisms ranging from yeast to mammals. This checkpoint ensures that cells don't initiate mitosis before they have a chance to repair damaged DNA after replication. Cells that have a defective G2-M checkpoint enter mitosis before repairing their DNA, leading to death after cell division.
The cell cycle is driven by proteins called cyclin dependent kinases that associate with cyclin regulatory proteins at different points of the cell cycle. Accumulation of cyclin B increases the activity of the cyclin dependent kinase cdc2 as cells prepare to enter mitosis. Cdc2 activity is further regulated by phosphorylation of its tyrosine-15 residue by the kinase wee1. Phosphorylation of tyrosine-15 inhibits cdc2 activity while dephosphorylation by the phosphatase cdc25 activates the mitotic kinase
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
When cell has stalled DNA replication fork, which checkpoint should be...
The answer will be G1 phase..because G1 phase corresponds the initiation of DNA replication..and during this phase the cell continuously grow but doesn't replicate the DNA..when the cell has stalled DNA replication fork it prepares for replication with the help of different enzyme and RNA primer but it doesn't replicate DNA....
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain?
Question Description
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain?.
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain?.
Solutions for When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? defined & explained in the simplest way possible. Besides giving the explanation of
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain?, a detailed solution for When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? has been provided alongside types of When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? theory, EduRev gives you an
ample number of questions to practice When cell has stalled DNA replication fork, which checkpoint should be predominantly activated) A) M B) both G2/M and M C) G1/M D) G2/M Plz explain? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























