How to Master Chemical Bonding and Molecular Structure for NEET? | Chemistry Class 11 PDF Download
Let's explore the Class 11 Chemistry chapter called "Chemical Bonding and Molecular Structure" and understand its importance for the NEET exam. By studying the past years' NEET questions (2016 to 2024), we can see that this chapter holds significant weightage in the exam. If you aim to score well in NEET, it's crucial to grasp the concepts covered in this chapter.
Meeting Your Timetable Goals with EduRev!
The study plan for this chapter offers you a schedule to manage your time effectively for learning and practicing the chapter thoroughly. By following this plan diligently, you'll be well-prepared to tackle even the most challenging questions asked in NEET related to each chapter. EduRev makes your preparation easier and saves you time by providing comprehensive resources for each topic. These resources include chapter notes, videos, and tests for every topic and chapter.
To access these valuable resources, including documents, videos, and tests, simply click here.
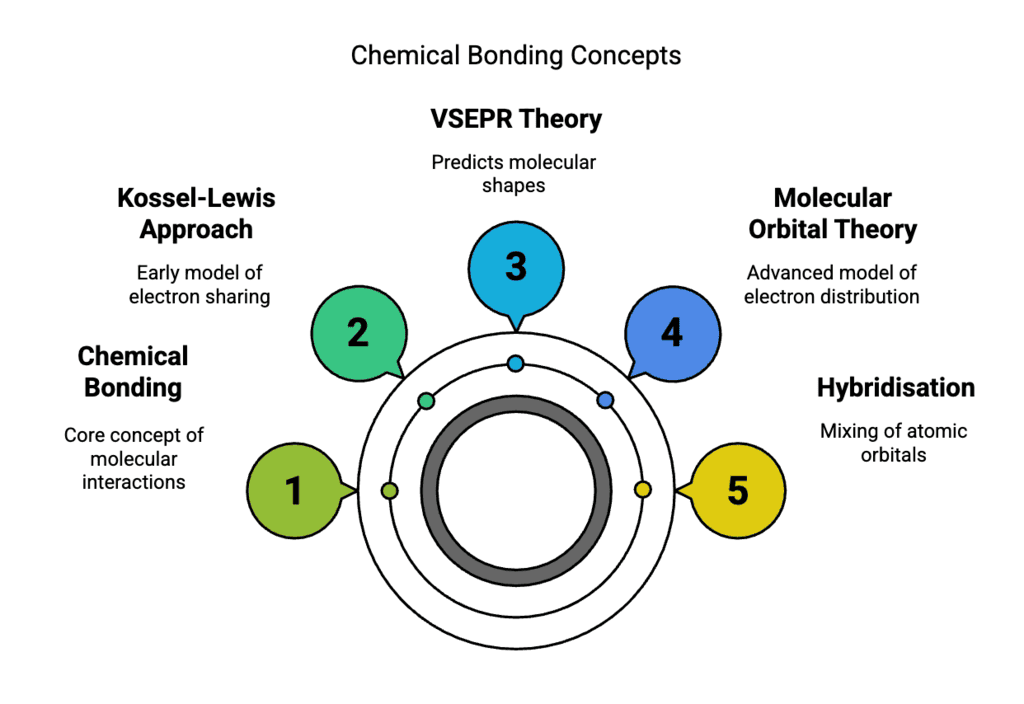
Topics Covered in the Chapter
- Chemical Bonding & Kossel-Lewis Approach
- VSEPR Theory
- Types of Chemical Bonds
- Hydrogen Bonding
- Molecular Orbital Theory
- Valence Bond Theory
- Hybridisation
- Bond Parameters
Day 1: Chemical Bonding & Kossel - Lewis Approach
Start your study plan by understanding the fundamentals of chemical bonding and the Kossel-Lewis approach. Focus on:
- Covalent and ionic bonds
- Octet rule
- Lewis structures
- Formal charges
Refer to the NCERT Chemistry Class 11 textbook for in-depth coverage and attempt questions from it.
Study Tip: Use the mind map to visualize and remember key concepts.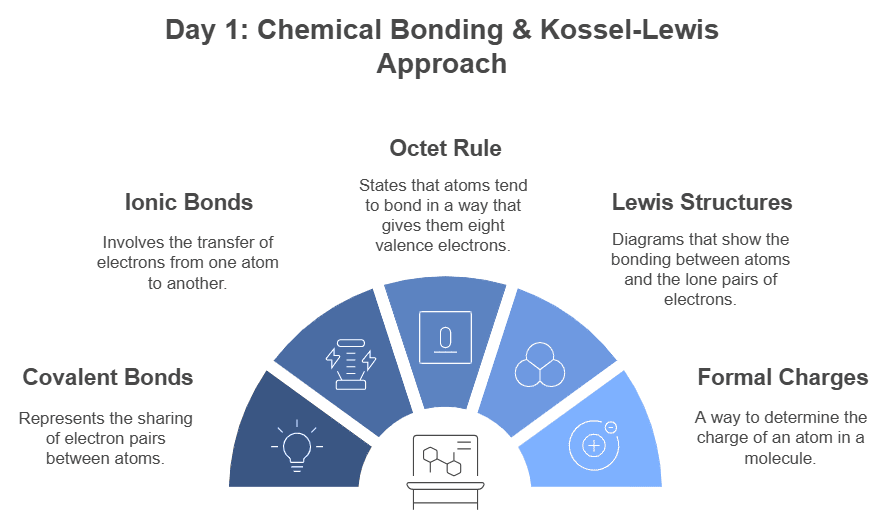
Day 2: VSEPR Theory, Types of Chemical Bonds, and Hydrogen Bonding
On the second day, delve into:
- VSEPR Theory: Understand molecular shapes and bond angles.
- Types of Chemical Bonds: Ionic, covalent, and metallic bonds.
- Hydrogen Bonding: Explore the unique properties and significance of hydrogen bond.
Practice questions from the NCERT textbook and take help from NCERT solutions on EduRev.
Study Tip: Use the PPT on Chemical Bonding for visual aids and memory reinforcement.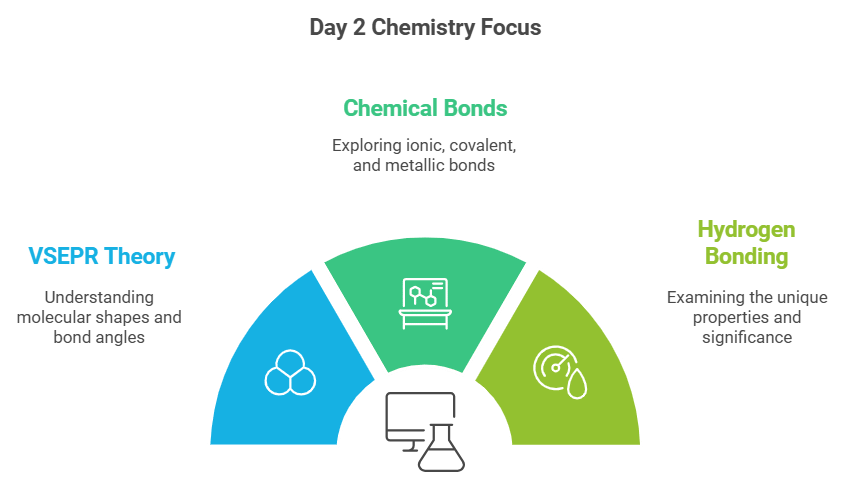
Day 3: Molecular Orbital Theory, Valence Bond Theory, and Hybridisation
Focus on the theories that explain chemical bonding:
- Molecular Orbital Theory: Learn how atomic orbitals combine to form molecular orbitals.
- Valence Bond Theory: Understand the concept of hybridisation.
- Hybridisation: Dive deeper into hybridisation and its applications.
Review important formulas and attempt questions from short and long answer questions on EduRev.
Study Tip: Watch the video on Molecular Orbital Theory to reinforce your understanding.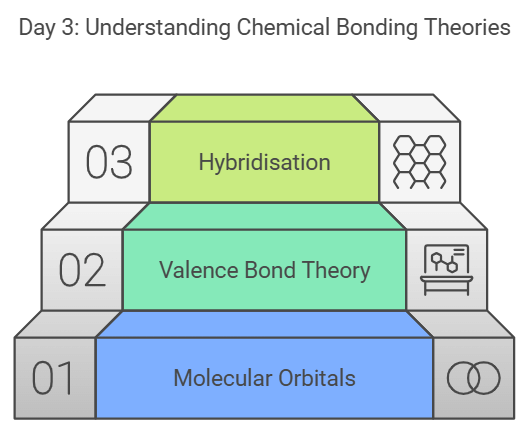
Day 4: Bond Parameters
On the final day of topic-wise study, focus on bond parameters:
- Bond length
- Bond angle
- Bond enthalpy
- Bond polarity
Revise all the topics you've covered during the week to ensure a strong foundation.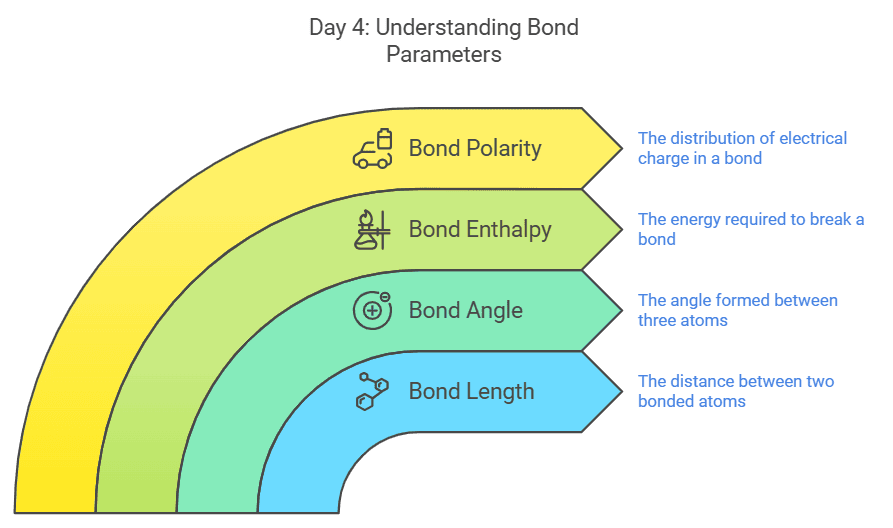
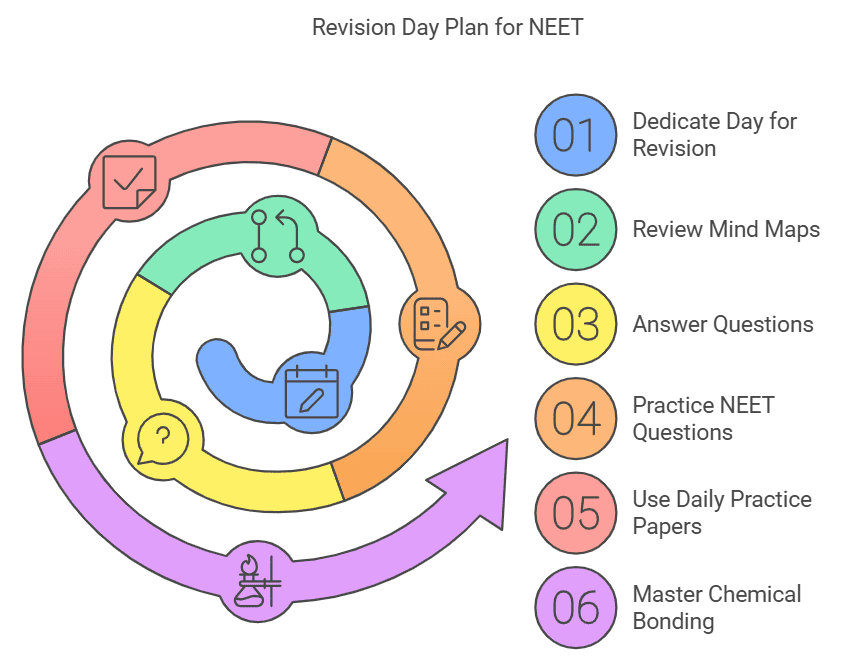
Revision Day
- Dedicate this day solely for revision by reviewing mind map, short and long answer questions, and previous years' NEET questions to solidify your Understanding.
- Remember to practice questions from Daily Practice Papers to test your understanding.
- By following this study plan and taking advantage of the resources available on EduRev, you can master the chapter on "Chemical Bonding and Molecular Structure" and perform exceptionally well in your NEET exam. Good luck!
Here are all the important links and topic-specific links for the chapter "Chemical Bonding and Molecular Structure":
Important Links:
- NEET Exam
- NEET Previous Year Questions (2016-2025)
- Mind Map
- Short & Long Answer Questions: Chemical Bonding & Molecular Structure
- PPT: Chemical Bonding
- NCERT Textbook
- NCERT Solutions
- Video: Molecular Orbital Theory
- Test: Geometry & Shapes of Molecules
- Chapter: Chemical Bonding and Molecular Structure
These links will provide you with valuable resources and topic-specific content to aid in your study of "Chemical Bonding and Molecular Structure."
|
114 videos|263 docs|74 tests
|
FAQs on How to Master Chemical Bonding and Molecular Structure for NEET? - Chemistry Class 11
| 1. What is the Kossel-Lewis approach in chemical bonding? |  |
| 2. How does VSEPR theory help in predicting molecular geometry? |  |
| 3. What are the differences between Valence Bond Theory and Molecular Orbital Theory? |  |
| 4. What are some key bond parameters that can be measured in chemical bonding? |  |
| 5. How does hydrogen bonding influence the properties of substances? |  |





















