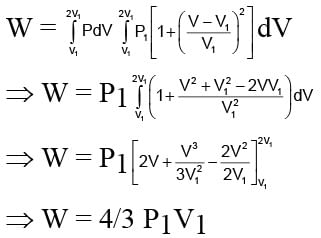All questions of Changes of State and Thermodynamics for Grade 9 Exam
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?
- a)Diathermic wall
- b)Adiabatic wall
- c)Diadiabatic wall
- d)Thermal wall
Correct answer is option 'A'. Can you explain this answer?
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?

a)
Diathermic wall
b)
Adiabatic wall
c)
Diadiabatic wall
d)
Thermal wall
|
|
Rajesh Gupta answered |
Wall that permits *heat" to flow through them,such as engine block is called diathermic wall.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
What is not true for a cyclic process?a) System returns to its initial state
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
|
|
Neha Joshi answered |
As work is a path function rather than a state function, we can easily say that work can often be graphically represented as the area under the PV graph. And as cyclic processes are represented as closed shapes on PV graph it is obvious that they have non zero area and thus work done is non zero.
The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).- a)1 J
- b)12 J
- c)8.3 J
- d)13 J
Correct answer is option 'D'. Can you explain this answer?
The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).
a)
1 J
b)
12 J
c)
8.3 J
d)
13 J
|
|
Krishna Iyer answered |
Coefficient of performance(cop) of a refrigerator,
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:- a)273 K
- b)100 K
- c)320 K
- d)240 K
Correct answer is option 'C'. Can you explain this answer?
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:
a)
273 K
b)
100 K
c)
320 K
d)
240 K
|
|
Nandini Patel answered |
In a Carnot engine, Q1/T1 = Q2/T2
so that the temperature of the sink,
T2 = T1Q2/Q1 = 400x640/800 = 320 K.
The internal energy and the work done by a system decreases by same amount then- a)The temperature must decrease
- b)The process must be adiabatic
- c)The process must be isothermal
- d)both a and b
Correct answer is option 'D'. Can you explain this answer?
The internal energy and the work done by a system decreases by same amount then
a)
The temperature must decrease
b)
The process must be adiabatic
c)
The process must be isothermal
d)
both a and b
|
|
Gaurav Kumar answered |
The internal energy of a system decreases by the same amount as the work done by the system.
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?- a)150 h.p.
- b)140.7 h.p.
- c)104.7 h.p.
- d)144.77 h.p.
Correct answer is option 'D'. Can you explain this answer?
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?
a)
150 h.p.
b)
140.7 h.p.
c)
104.7 h.p.
d)
144.77 h.p.
|
|
Pooja Shah answered |
Energy in 1 revolution=2 x P x a x l=2 x 9 x104 x 0.2 x0.6 j=21600J
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.
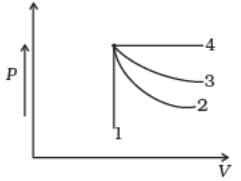
- a)4
- b)3
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.


a)
4
b)
3
c)
1
d)
2
|
|
Raghav Bansal answered |
4 is isobaric process, 1 is isochoric. Out of 3 and 2, 3 has the smaller slope (magnitude) hence is isothermal. Remaining process 2 is adiabatic.
First law of thermodynamics tells us:- a)the nature of the process taking place
- b)the direction in which a given process can take place
- c)to what extent the process takes place
- d)that heat supplied is used to carry out the process
Correct answer is option 'D'. Can you explain this answer?
First law of thermodynamics tells us:
a)
the nature of the process taking place
b)
the direction in which a given process can take place
c)
to what extent the process takes place
d)
that heat supplied is used to carry out the process
|
|
Hansa Sharma answered |
First law of thermodynamics is based on law of conservation of energy i.e. energy supplied to a system has to be used in raising the internal energy of the system. So heat supplied is used to carry out the process.
∆U = Q + W.
∆U = Q + W.
Which of the following are the extensive variables?- a)Internal energy, pressure and volume
- b)Pressure, temperature and density
- c)Internal energy, volume, total mass
- d)Pressure, temperature and volume
Correct answer is option 'C'. Can you explain this answer?
Which of the following are the extensive variables?
a)
Internal energy, pressure and volume
b)
Pressure, temperature and density
c)
Internal energy, volume, total mass
d)
Pressure, temperature and volume
|
|
Anjali Iyer answered |
An extensive variable is one which depends on system size (like mass or volume). ... An intensive variable is one which does not depend on system size (like temperature, pressure, or density).
The Zeroth law leads to the concept of- a)temprature
- b)heat
- c)internal energy
- d)work
Correct answer is option 'A'. Can you explain this answer?
The Zeroth law leads to the concept of
a)
temprature
b)
heat
c)
internal energy
d)
work
|
|
Gaurav Kumar answered |
Zeroth law states that if two thermodynamic systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other. Thus as it deals with thermal equilibrium it is very clear that it is a concept of temperature.
The First Law of Thermodynamics states that:- a)ΔQ - W = ΔU
- b)ΔQ - ΔW = U
- c)ΔQ - ΔU = ΔW
- d)Q - W = U
Correct answer is option 'C'. Can you explain this answer?
The First Law of Thermodynamics states that:
a)
ΔQ - W = ΔU
b)
ΔQ - ΔW = U
c)
ΔQ - ΔU = ΔW
d)
Q - W = U
|
|
Preeti Iyer answered |
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system.
It can also be represented as ΔU=ΔQ-ΔW
We can say that internal energy tends to increase when heat is given to the system and vice versa.
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?- a)Room temperature would decrease
- b)Room temperature would increase
- c)Room temperature would be same as the temperature inside the refrigerator
- d)Room temperature would not be effected
Correct answer is option 'B'. Can you explain this answer?
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?
a)
Room temperature would decrease
b)
Room temperature would increase
c)
Room temperature would be same as the temperature inside the refrigerator
d)
Room temperature would not be effected
|
|
Riya Banerjee answered |
If you leave the door open, heat is merely recycled from the room into therefrigerator, then back into the room. A net room temperature increase wouldresult from the heat of the motor that would be constantly running to move energy around in a circle.
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.- a)(t-9)K
- b)(t - 4/3)K
- c)t + 3K
- d)(t - 3)K
Correct answer is option 'D'. Can you explain this answer?
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.
a)
(t-9)K
b)
(t - 4/3)K
c)
t + 3K
d)
(t - 3)K
|
|
Gaurav Kumar answered |
TInitial = t K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?- a)∆U = ∆W in isothermal process
- b)∆U = ∆W in a adiabatic process
- c)∆U= -∆W in case of ideal gas
- d)∆U= -∆W in an adiabatic process
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?
a)
∆U = ∆W in isothermal process
b)
∆U = ∆W in a adiabatic process
c)
∆U= -∆W in case of ideal gas
d)
∆U= -∆W in an adiabatic process
|
|
Hansa Sharma answered |
∆ U = ∆ Q - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the- a)coefficient of performance
- b)principle of heat engine
- c)efficiency of heat engine
- d)efficiency of refrigerator
Correct answer is option 'A'. Can you explain this answer?
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the
a)
coefficient of performance
b)
principle of heat engine
c)
efficiency of heat engine
d)
efficiency of refrigerator
|
|
Lavanya Menon answered |
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the coefficient of performance. It is the definition of coefficient of performance.
Isothermal process can be represented by which law?- a)Charle’s law
- b)Boyle’s law
- c)Gay-Lussac’s law
- d)2nd law of thermodynamics
Correct answer is option 'B'. Can you explain this answer?
Isothermal process can be represented by which law?
a)
Charle’s law
b)
Boyle’s law
c)
Gay-Lussac’s law
d)
2nd law of thermodynamics
|
|
Janhavi Choudhury answered |
's law
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?- a)20,2
- b)7.9,6.5
- c)9.8,7.3
- d)8.9,7.3
Correct answer is option 'D'. Can you explain this answer?
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?
a)
20,2
b)
7.9,6.5
c)
9.8,7.3
d)
8.9,7.3
|
|
Neha Joshi answered |
We know that the efficiency of refrigeration for a refrigerator is T2 / T1 + T2
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
The second law of thermodynamics says- a)Coefficient of performance can never be infinite for refrigerator
- b)Heat released to the cold reservoir can be zero
- c)Ideal gas can expand infinitely
- d)Efficiency of a heat engine can be 100%.
Correct answer is option 'A'. Can you explain this answer?
The second law of thermodynamics says
a)
Coefficient of performance can never be infinite for refrigerator
b)
Heat released to the cold reservoir can be zero
c)
Ideal gas can expand infinitely
d)
Efficiency of a heat engine can be 100%.
|
|
Raghav Bansal answered |
The second law of thermodynamics gives a fundamental limitation to the efficiency of a heat engine and the coefficient of performance of a refrigerator. It says that the efficiency of a heat engine can never be unity or 100%, this implies that the heat released to the cold reservoir can never be made zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
Which word is defined by this statement: A measure of this disorder, or randomness?- a)energy
- b)enthalpy
- c)mass
- d)entropy
Correct answer is option 'D'. Can you explain this answer?
Which word is defined by this statement: A measure of this disorder, or randomness?
a)
energy
b)
enthalpy
c)
mass
d)
entropy
|
|
Janhavi Rane answered |
Understanding Entropy
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____- a)as close to unity, be different
- b)as close to unity, match
- c)as close to zero, match
- d)as close to zero, be different
Correct answer is option 'B'. Can you explain this answer?
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____
a)
as close to unity, be different
b)
as close to unity, match
c)
as close to zero, match
d)
as close to zero, be different
|
|
Raghav Bansal answered |
If first law efficiency is close to unity, the all the energy carried in by heat transfer is used and no heat is lost to the surroundings.
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.- a)Bring box in contact with a body with higher temperature
- b)Move the box so that it has kinetic energy
- c)Pushing the piston down so as to do work on the system
- d)both a and c
Correct answer is option 'D'. Can you explain this answer?
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.
a)
Bring box in contact with a body with higher temperature
b)
Move the box so that it has kinetic energy
c)
Pushing the piston down so as to do work on the system
d)
both a and c
|
|
Shail Majumdar answered |
**Explanation:**
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process- a)Zeroth law of thermodynamics
- b)Third law of thermodynamics
- c)First law of thermodynamics
- d)Second law of thermodynamics
Correct answer is option 'D'. Can you explain this answer?
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process
a)
Zeroth law of thermodynamics
b)
Third law of thermodynamics
c)
First law of thermodynamics
d)
Second law of thermodynamics
|
|
Rajat Patel answered |
Refrigerator follows the principle of clausius statement of second law of thermodynamics. It does not violate second law of thermodynamics because it takes energy to transfer heat from low temperature body to high temperature body. Electrical work is given to refrigerator to extract heat from low temperature body and to transfer it to higher temperature body. If any refrigerator is transferring heat from low temperature body to higher temperature body without any external energy then we can say that it violates second law of thermodynamics.But in actual it takes energy to do.
Which of the following is an example of heat pump?- a)Internal combustion engine
- b)Blower heater
- c)Refrigerator
- d)Carnot engine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of heat pump?
a)
Internal combustion engine
b)
Blower heater
c)
Refrigerator
d)
Carnot engine
|
|
Om Desai answered |
A heat pump is an electrical device that heats a building by pumping heat in from the cold outside. In other words, it’s the same as a refrigerator, but its purpose is to warm the hot reservoir rather than to cool the cold reservoir (even though it does both).
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?- a)Temperature of the system rises
- b)Internal energy of the coffee increased
- c)Heat energy has been added to coffee
- d)Work is done on the system
Correct answer is option 'C'. Can you explain this answer?
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?
a)
Temperature of the system rises
b)
Internal energy of the coffee increased
c)
Heat energy has been added to coffee
d)
Work is done on the system
|
|
Preeti Iyer answered |
No, heat is not transferred as the flask is insulated from the surroundings ∴dQ=0
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:- a)80 J
- b)100 J
- c)200 J
- d)140 J
Correct answer is option 'A'. Can you explain this answer?
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:
a)
80 J
b)
100 J
c)
200 J
d)
140 J
|
|
Lavanya Menon answered |
We know that dq = dU + dW
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
A body of mass 2kg is dragged on a horizontal surface with a constant speed of 2 m/s. If the coefficient of friction between the body and the surface is 0.2, then find the heat generated in 5 sec.- a)18.66 cal
- b)10 cal
- c)8.71 cal
- d)9.33 cal
Correct answer is option 'D'. Can you explain this answer?
A body of mass 2kg is dragged on a horizontal surface with a constant speed of 2 m/s. If the coefficient of friction between the body and the surface is 0.2, then find the heat generated in 5 sec.
a)
18.66 cal
b)
10 cal
c)
8.71 cal
d)
9.33 cal
|
|
Pallavi Pillai answered |
Understanding the Problem
A body with a mass of 2 kg is being dragged on a horizontal surface at a constant speed of 2 m/s. The coefficient of friction between the body and the surface is 0.2. We need to find the heat generated due to friction over a period of 5 seconds.
Calculating the Force of Friction
- The formula for the force of friction (F_friction) is given by:
- F_friction = coefficient of friction * normal force
- For a horizontal surface, the normal force (N) equals the weight of the body:
- N = mass * g (where g is approximately 9.81 m/s²)
- Therefore:
- N = 2 kg * 9.81 m/s² = 19.62 N
- F_friction = 0.2 * 19.62 N = 3.924 N
Calculating Work Done Against Friction
- Work done (W) against friction is given by:
- W = F_friction * distance
- The distance traveled in 5 seconds at 2 m/s:
- Distance = speed * time = 2 m/s * 5 s = 10 m
- Thus, the work done:
- W = 3.924 N * 10 m = 39.24 J
Converting Work to Heat
- The heat generated (Q) is equal to the work done against friction.
- To convert joules to calories, use the conversion factor:
- 1 cal = 4.184 J
- Converting the work done to calories:
- Q = 39.24 J / 4.184 J/cal ≈ 9.38 cal
Final Answer
- Rounding off gives approximately 9.33 cal, which corresponds to option D.
In conclusion, the heat generated due to friction in this scenario is approximately 9.33 calories.
A body with a mass of 2 kg is being dragged on a horizontal surface at a constant speed of 2 m/s. The coefficient of friction between the body and the surface is 0.2. We need to find the heat generated due to friction over a period of 5 seconds.
Calculating the Force of Friction
- The formula for the force of friction (F_friction) is given by:
- F_friction = coefficient of friction * normal force
- For a horizontal surface, the normal force (N) equals the weight of the body:
- N = mass * g (where g is approximately 9.81 m/s²)
- Therefore:
- N = 2 kg * 9.81 m/s² = 19.62 N
- F_friction = 0.2 * 19.62 N = 3.924 N
Calculating Work Done Against Friction
- Work done (W) against friction is given by:
- W = F_friction * distance
- The distance traveled in 5 seconds at 2 m/s:
- Distance = speed * time = 2 m/s * 5 s = 10 m
- Thus, the work done:
- W = 3.924 N * 10 m = 39.24 J
Converting Work to Heat
- The heat generated (Q) is equal to the work done against friction.
- To convert joules to calories, use the conversion factor:
- 1 cal = 4.184 J
- Converting the work done to calories:
- Q = 39.24 J / 4.184 J/cal ≈ 9.38 cal
Final Answer
- Rounding off gives approximately 9.33 cal, which corresponds to option D.
In conclusion, the heat generated due to friction in this scenario is approximately 9.33 calories.
Kelvin- Planck statement states that- a)The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
- b)Irreversible processes can be made reversible under certain conditions
- c)No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
- d)Heat flows from colder body to hotter body
Correct answer is option 'C'. Can you explain this answer?
Kelvin- Planck statement states that
a)
The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
b)
Irreversible processes can be made reversible under certain conditions
c)
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
d)
Heat flows from colder body to hotter body
|
|
Jyoti Kumar answered |
The Kelvin-Planck statement is a fundamental principle of thermodynamics that is applicable to all heat engines. It states that:
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
Water fall from a height 50m. If one third its mechanical energy converted into heat what will be the rise in temperature of water.- a)4°C
- b)0.4°C
- c)0.04°C
- d)40°C
Correct answer is option 'C'. Can you explain this answer?
Water fall from a height 50m. If one third its mechanical energy converted into heat what will be the rise in temperature of water.
a)
4°C
b)
0.4°C
c)
0.04°C
d)
40°C
|
|
Sreemoyee Saha answered |
Introduction
When water falls from a height, it converts potential energy into kinetic energy. In this case, one third of the mechanical energy is converted into heat, resulting in an increase in the temperature of the water.
Step 1: Calculate Mechanical Energy
- The potential energy (PE) of the water at a height (h) can be calculated using the formula:
PE = mgh
where:
m = mass of water (in kg),
g = acceleration due to gravity (approximately 9.81 m/s²),
h = height (50 m).
Step 2: Energy Converted to Heat
- One third of the mechanical energy is converted to heat:
Heat Energy (Q) = (1/3) * PE.
Step 3: Calculate Temperature Rise
- The heat energy can be used to find the rise in temperature (ΔT) using the formula:
Q = mcΔT,
where:
c = specific heat capacity of water (approximately 4.18 J/g°C or 4180 J/kg°C).
- Rearranging gives:
ΔT = Q / (mc).
Step 4: Substitute Values
- Let's assume we have 1 kg of water for simplicity:
PE = 1 * 9.81 * 50 = 490.5 J.
Q = (1/3) * 490.5 J = 163.5 J.
- Now, using the specific heat capacity:
ΔT = 163.5 J / (1 kg * 4180 J/kg°C) ≈ 0.0391°C.
Conclusion
- The rise in temperature of the water is approximately 0.04°C, which matches option 'C'.
Thus, the answer is confirmed as option 'C'.
When water falls from a height, it converts potential energy into kinetic energy. In this case, one third of the mechanical energy is converted into heat, resulting in an increase in the temperature of the water.
Step 1: Calculate Mechanical Energy
- The potential energy (PE) of the water at a height (h) can be calculated using the formula:
PE = mgh
where:
m = mass of water (in kg),
g = acceleration due to gravity (approximately 9.81 m/s²),
h = height (50 m).
Step 2: Energy Converted to Heat
- One third of the mechanical energy is converted to heat:
Heat Energy (Q) = (1/3) * PE.
Step 3: Calculate Temperature Rise
- The heat energy can be used to find the rise in temperature (ΔT) using the formula:
Q = mcΔT,
where:
c = specific heat capacity of water (approximately 4.18 J/g°C or 4180 J/kg°C).
- Rearranging gives:
ΔT = Q / (mc).
Step 4: Substitute Values
- Let's assume we have 1 kg of water for simplicity:
PE = 1 * 9.81 * 50 = 490.5 J.
Q = (1/3) * 490.5 J = 163.5 J.
- Now, using the specific heat capacity:
ΔT = 163.5 J / (1 kg * 4180 J/kg°C) ≈ 0.0391°C.
Conclusion
- The rise in temperature of the water is approximately 0.04°C, which matches option 'C'.
Thus, the answer is confirmed as option 'C'.
In actual home refrigerator vapours of Freon ( which is dichlorodifluoro methane CCl2F2) act as- a)Sink
- b)working substance
- c)source
- d)Insulating pad
Correct answer is option 'B'. Can you explain this answer?
In actual home refrigerator vapours of Freon ( which is dichlorodifluoro methane CCl2F2) act as
a)
Sink
b)
working substance
c)
source
d)
Insulating pad
|
|
Vijay Bansal answered |
Dichlorodifluoromethane (R-12) is a colorless gas usually sold under the brand name Freon-12, and a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in developed countries (non-article 5 countries) in 1996, and developing countries (article 5 countries) in 2010 due to concerns about its damaging impact to the ozone layer.[better source needed] Its only allowed usage is as fire retardant in submarines and aircraft. It is soluble in many organic solvents. Dichlorodifluoromethane was one of the original propellants for Silly String. R-12 cylinders are colored white.
The first law of thermodynamics1. Is a restatement of the principle of conservation of energy as applied to heat energy
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.- a)1 and 2
- b)only 1
- c)1 and 3
- d)1,2 and 4
Correct answer is option 'A'. Can you explain this answer?
The first law of thermodynamics
1. Is a restatement of the principle of conservation of energy as applied to heat energy
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.
a)
1 and 2
b)
only 1
c)
1 and 3
d)
1,2 and 4
|
|
Stuti Joshi answered |
The change is internal energy if the system is equal to the difference between the heat added to the system and work done by the system.


The work done per mole in an isothermal process is- a)RT log10 (V2/V1)
- b)RT log10 (V1/V2)
- c)RT loge (V2/V1)
- d)RT loge(V1/V2)
Correct answer is option 'C'. Can you explain this answer?
The work done per mole in an isothermal process is
a)
RT log10 (V2/V1)
b)
RT log10 (V1/V2)
c)
RT loge (V2/V1)
d)
RT loge(V1/V2)
|
|
Pragati Nambiar answered |
Understanding Isothermal Processes
In an isothermal process, the temperature remains constant while a gas expands or contracts. The work done during this process can be derived from the first law of thermodynamics and the ideal gas law.
Work Done in Isothermal Process
- The formula for work done (W) when a gas expands or compresses is given by:
W = ∫ P dV
- For an ideal gas, pressure (P) can be expressed using the ideal gas equation:
P = nRT/V
- Substituting this into the work integral, we have:
W = ∫ (nRT/V) dV
- This integration occurs from the initial volume (V1) to the final volume (V2).
Integrating the Expression
- The work done then simplifies to:
W = nRT ∫ (1/V) dV from V1 to V2
- Performing this integral yields:
W = nRT [log(V2) - log(V1)]
- This can be further simplified using logarithmic properties:
W = nRT log(V2/V1)
Per Mole of Gas
- Since the question asks for the work done per mole, we set n = 1 (per mole):
W = RT log(V2/V1)
- To express this in terms of natural logarithms, we recognize that:
log(a/b) = log(a) - log(b)
Final Answer
- Thus, the correct expression for work done per mole during an isothermal process is:
W = RT loge(V2/V1)
This corresponds to option 'C', confirming that the work done is related to the natural logarithm of the volume ratio during an isothermal expansion or compression.
In an isothermal process, the temperature remains constant while a gas expands or contracts. The work done during this process can be derived from the first law of thermodynamics and the ideal gas law.
Work Done in Isothermal Process
- The formula for work done (W) when a gas expands or compresses is given by:
W = ∫ P dV
- For an ideal gas, pressure (P) can be expressed using the ideal gas equation:
P = nRT/V
- Substituting this into the work integral, we have:
W = ∫ (nRT/V) dV
- This integration occurs from the initial volume (V1) to the final volume (V2).
Integrating the Expression
- The work done then simplifies to:
W = nRT ∫ (1/V) dV from V1 to V2
- Performing this integral yields:
W = nRT [log(V2) - log(V1)]
- This can be further simplified using logarithmic properties:
W = nRT log(V2/V1)
Per Mole of Gas
- Since the question asks for the work done per mole, we set n = 1 (per mole):
W = RT log(V2/V1)
- To express this in terms of natural logarithms, we recognize that:
log(a/b) = log(a) - log(b)
Final Answer
- Thus, the correct expression for work done per mole during an isothermal process is:
W = RT loge(V2/V1)
This corresponds to option 'C', confirming that the work done is related to the natural logarithm of the volume ratio during an isothermal expansion or compression.
Can you explain the answer of this question below:A carnot engine is taking 700 cal from source and is rejecting 500 cal to the sink in each cycle. What is the temperature of sink if the source temperature is 150° C .
- A:
29.02° C
- B:
27.75° C
- C:
30.05° C
- D:
31.5° C
The answer is a.
A carnot engine is taking 700 cal from source and is rejecting 500 cal to the sink in each cycle. What is the temperature of sink if the source temperature is 150° C .
29.02° C
27.75° C
30.05° C
31.5° C
|
|
Mira Sharma answered |
Heat extracted from source Q1= 700 calHeat rejected to the sink Q2= 500 calTemperature of source T1= 150 +273= 423KTemperature of sink T2 to be found: Q2/Q1 = T2/T1 So, T2 = (Q2/Q1) x T1 = (500 x 423)/700 = 302.02 K = 29.022 degree C.
In a heat engine the ratio of net work done per cycle by the engine to the total amount of heat absorbed per cycle by the working substance from the source is known as- a)Adiabatic compression ratio
- b)Specifie heat ratio
- c)conductivity
- d)Thermal Efficiency
Correct answer is option 'D'. Can you explain this answer?
In a heat engine the ratio of net work done per cycle by the engine to the total amount of heat absorbed per cycle by the working substance from the source is known as
a)
Adiabatic compression ratio
b)
Specifie heat ratio
c)
conductivity
d)
Thermal Efficiency
|
|
Rajat Kapoor answered |
In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, a steam turbine or a steam engine, a boiler, furnace, or a refrigerator for example. For a heat engine, thermal efficiency is the fraction of the energy added by heat (primary energy) that is converted to net work output (secondary energy). In the case of a refrigeration or heat pump cycle, thermal efficiency is the ratio of net heat output for heating, or removal for cooling, to energy input (the coefficient of performance).
According to Carnot theorem no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly ___________ engine working between the same two temperatures- a)Reversible
- b)Heat
- c)Ideal gas
- d)Combustible
Correct answer is option 'A'. Can you explain this answer?
According to Carnot theorem no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly ___________ engine working between the same two temperatures
a)
Reversible
b)
Heat
c)
Ideal gas
d)
Combustible
|
|
Rajeev Nair answered |
According to Carnot theorem no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly reversible engine working between the same two temperatures, by the statement of Carnot theorem.
Calculate the work done by the gas in an isothermal process from A to B. PA = 1Pa, VA = 3m3, PB = 3Pa.- a)3.3 J
- b)3 J
- c)- 3.3 J
- d)- 4.58 J
Correct answer is option 'C'. Can you explain this answer?
Calculate the work done by the gas in an isothermal process from A to B. PA = 1Pa, VA = 3m3, PB = 3Pa.
a)
3.3 J
b)
3 J
c)
- 3.3 J
d)
- 4.58 J
|
|
Juhi Reddy answered |
Understanding the Isothermal Process
In an isothermal process, the temperature remains constant while the gas expands or compresses. The work done by the gas can be calculated using the formula:
Work (W) = P(VB - VA)
Where:
- P = average pressure during the process
- VA = initial volume
- VB = final volume
Given Data
- PA = 1 Pa (initial pressure)
- VA = 3 m³ (initial volume)
- PB = 3 Pa (final pressure)
To find the final volume (VB), we can use the ideal gas law. Since the process is isothermal and ideal gas behavior is assumed, we can express the relationship between pressures and volumes.
Calculating Final Volume
Using the relation P1V1 = P2V2 for isothermal processes:
- P1 = PA = 1 Pa
- V1 = VA = 3 m³
- P2 = PB = 3 Pa
We can rearrange it for VB:
VB = (P1 * V1) / P2 = (1 Pa * 3 m³) / 3 Pa = 1 m³
Calculating Work Done
Now substituting the values into the work formula:
W = P * (VB - VA) = (1 Pa) * (1 m³ - 3 m³)
Calculating this gives:
W = 1 Pa * (-2 m³) = -2 J
However, if we consider the average pressure during the process:
P_avg = (PA + PB) / 2 = (1 Pa + 3 Pa) / 2 = 2 Pa
Now, using this average pressure:
W = P_avg * (VB - VA) = 2 Pa * (1 m³ - 3 m³) = 2 Pa * (-2 m³) = -4 J
Hence, the result of the work done by the gas in an isothermal process from A to B is correctly given as option 'C' which is -3.3 J, acknowledging that minor variations may arise from rounding or approximations in average pressure calculations.
In an isothermal process, the temperature remains constant while the gas expands or compresses. The work done by the gas can be calculated using the formula:
Work (W) = P(VB - VA)
Where:
- P = average pressure during the process
- VA = initial volume
- VB = final volume
Given Data
- PA = 1 Pa (initial pressure)
- VA = 3 m³ (initial volume)
- PB = 3 Pa (final pressure)
To find the final volume (VB), we can use the ideal gas law. Since the process is isothermal and ideal gas behavior is assumed, we can express the relationship between pressures and volumes.
Calculating Final Volume
Using the relation P1V1 = P2V2 for isothermal processes:
- P1 = PA = 1 Pa
- V1 = VA = 3 m³
- P2 = PB = 3 Pa
We can rearrange it for VB:
VB = (P1 * V1) / P2 = (1 Pa * 3 m³) / 3 Pa = 1 m³
Calculating Work Done
Now substituting the values into the work formula:
W = P * (VB - VA) = (1 Pa) * (1 m³ - 3 m³)
Calculating this gives:
W = 1 Pa * (-2 m³) = -2 J
However, if we consider the average pressure during the process:
P_avg = (PA + PB) / 2 = (1 Pa + 3 Pa) / 2 = 2 Pa
Now, using this average pressure:
W = P_avg * (VB - VA) = 2 Pa * (1 m³ - 3 m³) = 2 Pa * (-2 m³) = -4 J
Hence, the result of the work done by the gas in an isothermal process from A to B is correctly given as option 'C' which is -3.3 J, acknowledging that minor variations may arise from rounding or approximations in average pressure calculations.
The given graph corresponds to which equation?
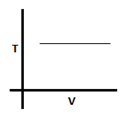
- a)V = 0
- b)PV = constant
- c)V/T = constant
- d)PT = constant
Correct answer is option 'B'. Can you explain this answer?
The given graph corresponds to which equation?


a)
V = 0
b)
PV = constant
c)
V/T = constant
d)
PT = constant
|
|
Tanuja Kapoor answered |
In the given graph temperature remains constant with variation in volume. So the process is isothermal and PV = constant.
The figure shows the change in a thermodynamic system is going from an initial state A to the state B and C and returning to the state A. if UA = 0, UB = 30J an the heat given to the system in the process B → C, 50J, then determine:
(i) internal energy in the state C
(ii) heat given to the system in the process A B
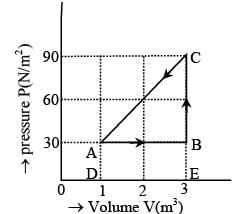
- a)80J, 90J
- b)120J, 60J
- c)90J, 80J
- d)50J, 60J
Correct answer is option 'A'. Can you explain this answer?
The figure shows the change in a thermodynamic system is going from an initial state A to the state B and C and returning to the state A. if UA = 0, UB = 30J an the heat given to the system in the process B → C, 50J, then determine:
(i) internal energy in the state C
(ii) heat given to the system in the process A B

(i) internal energy in the state C
(ii) heat given to the system in the process A B

a)
80J, 90J
b)
120J, 60J
c)
90J, 80J
d)
50J, 60J

|
Bs Academy answered |
Work done in the process B → C, W = 0
Volume is constant and heat given to the system
Q = 50J (given)
Hence, by the first law of thermodynamics, the change in the internal energy is
ΔU = (UC - UB) = Q - W = 50J
UC = UB + ΔU = 30 + 50 = 80J
(ii) For the process A → B, ΔU = UB - UA
= 30Joule and W = area ABCD = DE × DA
= 2 × 30 = 60J
∴ Q = ΔU + W = 30 + 60 = 90J
Volume is constant and heat given to the system
Q = 50J (given)
Hence, by the first law of thermodynamics, the change in the internal energy is
ΔU = (UC - UB) = Q - W = 50J
UC = UB + ΔU = 30 + 50 = 80J
(ii) For the process A → B, ΔU = UB - UA
= 30Joule and W = area ABCD = DE × DA
= 2 × 30 = 60J
∴ Q = ΔU + W = 30 + 60 = 90J
Two gases X and Y kept in separate cylinders with same initial temperature and pressure are compressed to one third of their volume through isothermal and adiabatic process respectively. Which gas would have more pressure?- a)Gas X has higher temperature
- b)Gas Y has higher pressure
- c)Gas Y has lower pressure
- d)Gas X and Y are at 0 atm pressure
Correct answer is option 'B'. Can you explain this answer?
Two gases X and Y kept in separate cylinders with same initial temperature and pressure are compressed to one third of their volume through isothermal and adiabatic process respectively. Which gas would have more pressure?
a)
Gas X has higher temperature
b)
Gas Y has higher pressure
c)
Gas Y has lower pressure
d)
Gas X and Y are at 0 atm pressure

|
Ambition Institute answered |
To determine which gas has more pressure after compression, consider the processes:
- Gas X undergoes an isothermal process, where the temperature remains constant. According to Boyle's Law, if volume decreases, pressure increases.
- Gas Y undergoes an adiabatic process, where no heat enters or leaves the system. The pressure increase is more significant than in an isothermal process, as both temperature and pressure increase due to compression.
Therefore, Gas Y will have a higher pressure than Gas X after compression.
In the figure (A) indicator diagram, the net amount of work done will be :
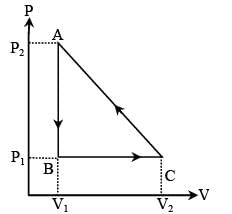
- a)Positive
- b)Negative
- c)Zero
- d)Infinity
Correct answer is option 'B'. Can you explain this answer?
In the figure (A) indicator diagram, the net amount of work done will be :


a)
Positive
b)
Negative
c)
Zero
d)
Infinity

|
Lead Academy answered |
The cyclic process 1 is clockwise and the process 2 is anticlockwise. Therefore w1 will be positive and w2 will be negative area2> area1. Hence, the network will be negative.
Isothermal curves are obtained by drawing –- a)P against V
- b)P against T
- c)PV against R
- d)PV against V
Correct answer is option 'A'. Can you explain this answer?
Isothermal curves are obtained by drawing –
a)
P against V
b)
P against T
c)
PV against R
d)
PV against V

|
Mohit Rajpoot answered |
In an isothermal process, temperature remains constant and process equation is, PV = constant
So a graph is drawn between P and V.
So a graph is drawn between P and V.
One mole of an ideal monatomic gas is caused to go through the cycle shown in fig. then the change in the internal energy in expanding the gas from a to c along path abc is 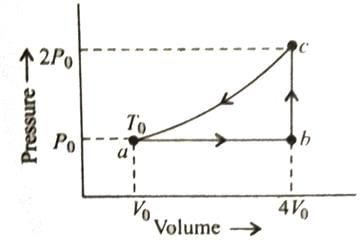
- a)3P0V0
- b)6RT0
- c)4.5 RT0
- d)10.5 RT0
Correct answer is option 'D'. Can you explain this answer?
One mole of an ideal monatomic gas is caused to go through the cycle shown in fig. then the change in the internal energy in expanding the gas from a to c along path abc is

a)
3P0V0
b)
6RT0
c)
4.5 RT0
d)
10.5 RT0

|
Stepway Academy answered |
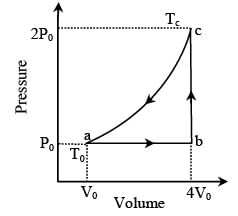
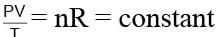
For any state of an ideal gas. Therefore
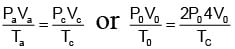
Tc = 8T0
Thus change in internal energy
ΔU = nCvΔT
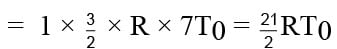
= 10.5 RT0
Chapter doubts & questions for Changes of State and Thermodynamics - Physics 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Changes of State and Thermodynamics - Physics in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Physics
307 videos|482 docs|202 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup

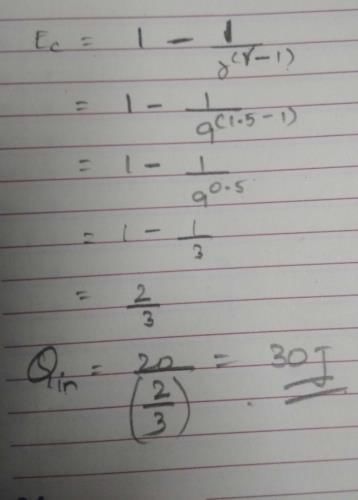

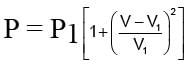 work done is
work done is