All Exams >
MCAT >
MCAT Biological and Biochemical Foundations >
All Questions
All questions of Control of Enzyme Activity (BIO, BC) for MCAT Exam
Select the right option regarding the given graph.
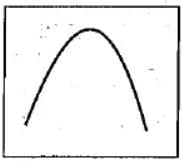
- a)X - axis = Rate of reaction
Y - axis = Enzymatic activity
- b)X - axis = Enzymatic activity
Y - axis = Rate of reaction
- c)X - axis = pH/Temperature
Y - axis = Enzymatic activity
- d)X - axis = Enzymatic activity
Y - axis = pH/Temperature
Correct answer is option 'C'. Can you explain this answer?
Select the right option regarding the given graph.

a)
X - axis = Rate of reaction
Y - axis = Enzymatic activity
Y - axis = Enzymatic activity
b)
X - axis = Enzymatic activity
Y - axis = Rate of reaction
Y - axis = Rate of reaction
c)
X - axis = pH/Temperature
Y - axis = Enzymatic activity
Y - axis = Enzymatic activity
d)
X - axis = Enzymatic activity
Y - axis = pH/Temperature
Y - axis = pH/Temperature
|
|
Jyoti Sengupta answered |
The variable with which we have to find the relation should always have to be on the y axis and the variable which does not show variation and will always increase will be on the x-axis.
Which of the following is an example of isozyme?- a)α-amylase
- b)Glucokinase
- c)Lactate dehydrogenases
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an example of isozyme?
a)
α-amylase
b)
Glucokinase
c)
Lactate dehydrogenases
d)
All of these
|
|
Dev Patel answered |
The multiple molecular forms of an enzyme occuring in the same organism and having a similar substrate activity are called isoenymes or isozymes. They have similar properties but different molecular weights and locations. Over 100 enzymes are known to have isoenzymes. α-amylase of wheat endosperm has 16 isozymes, lactate dehydrogenase has 5 isozymes. Glucokinase is an isozyme of hexokinase.
Dihydroxyacetone-3-phosphate and glyceraldehyde-3- phosphate are interconvertible. The enzyme responsible for this interconversion belongs to the cateogry of- a)isomerases
- b)ligases
- c)lyases
- d)hydrolases
Correct answer is option 'A'. Can you explain this answer?
Dihydroxyacetone-3-phosphate and glyceraldehyde-3- phosphate are interconvertible. The enzyme responsible for this interconversion belongs to the cateogry of
a)
isomerases
b)
ligases
c)
lyases
d)
hydrolases
|
|
Dev Patel answered |
Isomerases catalyse the change of a substrate into a related isomeric form by rearrangement of molecules.
What is meant by feedback inhibition?- a)Inhibition of enzyme activity at an active site by an inhibitor, which is structurally similar to a substrate
- b)Inhibition of first step of biosynthesis by the end-product of reaction
- c)Inhibition by a structurally different inhibitor at a place other than active site
- d)Denaturation of enzyme
Correct answer is option 'B'. Can you explain this answer?
What is meant by feedback inhibition?
a)
Inhibition of enzyme activity at an active site by an inhibitor, which is structurally similar to a substrate
b)
Inhibition of first step of biosynthesis by the end-product of reaction
c)
Inhibition by a structurally different inhibitor at a place other than active site
d)
Denaturation of enzyme

|
Lead Academy answered |
Inhibition of an enzyme controlling an early stage of a series of biochemical reactions by the end product when it reaches a critical concentration.
An enzyme extract, when subjected to electric field, separates into two fractions, each catalysing the same reaction. These fractions are:- a)Allosteric enzymes
- b)Inducible enzymes
- c)Isoenzymes
- d)Coenzymes
Correct answer is option 'C'. Can you explain this answer?
An enzyme extract, when subjected to electric field, separates into two fractions, each catalysing the same reaction. These fractions are:
a)
Allosteric enzymes
b)
Inducible enzymes
c)
Isoenzymes
d)
Coenzymes

|
EduRev NEET answered |
- Isoenzymes (also called isozymes) are alternative forms of the same enzyme activity that exist in different proportions in different tissues.
- Isoenzymes differ in amino acid composition and sequence and multimeric quaternary structure; mostly, but not always, they have similar (conserved) structures.
How would the addition of a catalyst to the reaction S⇋P affect the difference between the free energies of S and P in their ground states (∆G’°)?- a)∆G’° would become less negative
- b)The change in ∆G’° cannot be predicted without more information
- c)∆G’° would not change
- d)∆G’° would become more negative
Correct answer is option 'C'. Can you explain this answer?
How would the addition of a catalyst to the reaction S⇋P affect the difference between the free energies of S and P in their ground states (∆G’°)?
a)
∆G’° would become less negative
b)
The change in ∆G’° cannot be predicted without more information
c)
∆G’° would not change
d)
∆G’° would become more negative
|
|
Oliver Williams answered |
Explanation:
Effect of Catalyst on G’°:
- The addition of a catalyst to a reaction does not change the free energy of the reactants or products in their ground states (G’°).
- A catalyst speeds up the rate of reaction by providing an alternative pathway with lower activation energy, but it does not affect the overall energy difference between the reactants and products.
- Therefore, the addition of a catalyst would not change the free energy difference between S and P in their ground states (G’°).
Conclusion:
- In this case, the free energy of the reaction (G’°) would remain unchanged with the addition of a catalyst.
- The catalyst would only affect the kinetics of the reaction by lowering the activation energy, leading to a faster conversion of reactants to products without altering the thermodynamics of the reaction.
Effect of Catalyst on G’°:
- The addition of a catalyst to a reaction does not change the free energy of the reactants or products in their ground states (G’°).
- A catalyst speeds up the rate of reaction by providing an alternative pathway with lower activation energy, but it does not affect the overall energy difference between the reactants and products.
- Therefore, the addition of a catalyst would not change the free energy difference between S and P in their ground states (G’°).
Conclusion:
- In this case, the free energy of the reaction (G’°) would remain unchanged with the addition of a catalyst.
- The catalyst would only affect the kinetics of the reaction by lowering the activation energy, leading to a faster conversion of reactants to products without altering the thermodynamics of the reaction.
Which of the following statements about enzymes are correct?
(i) Enzymes do not alter the overall change in free energy for a reaction.
(ii) Enzymes are proteins whose three dimensional shape is key to their functions.
(iii) Enzymes speed up reactions by lowering activation energy.
(iv) Enzymes are highly specific for reactions.
(v) The energy input needed to start a chemical reaction is called activation energy.- a)(i) and (v)
- b)(ii) and (iv)
- c)(i), (ii) and (iv)
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements about enzymes are correct?
(i) Enzymes do not alter the overall change in free energy for a reaction.
(ii) Enzymes are proteins whose three dimensional shape is key to their functions.
(iii) Enzymes speed up reactions by lowering activation energy.
(iv) Enzymes are highly specific for reactions.
(v) The energy input needed to start a chemical reaction is called activation energy.
(i) Enzymes do not alter the overall change in free energy for a reaction.
(ii) Enzymes are proteins whose three dimensional shape is key to their functions.
(iii) Enzymes speed up reactions by lowering activation energy.
(iv) Enzymes are highly specific for reactions.
(v) The energy input needed to start a chemical reaction is called activation energy.
a)
(i) and (v)
b)
(ii) and (iv)
c)
(i), (ii) and (iv)
d)
All of these
|
|
Maitri Deshpande answered |
**Enzymes are biological catalysts that play a crucial role in speeding up chemical reactions in living organisms. Let's analyze each statement to determine which ones are correct:**
(i) Enzymes do not alter the overall change in free energy for a reaction.
- This statement is correct. Enzymes do not affect the overall change in free energy for a reaction. They only lower the activation energy required for the reaction to occur, but they do not change the energy difference between the reactants and products.
(ii) Enzymes are proteins whose three-dimensional shape is key to their functions.
- This statement is correct. Enzymes are proteins, and their three-dimensional shape is critical for their proper functioning. The active site of an enzyme, where the substrate binds and the reaction takes place, is precisely shaped to accommodate specific substrates.
(iii) Enzymes speed up reactions by lowering activation energy.
- This statement is correct. Enzymes work by lowering the activation energy required for a chemical reaction to occur. Activation energy is the energy input needed to start a reaction, and enzymes facilitate this process by providing an alternative pathway with a lower energy barrier.
(iv) Enzymes are highly specific for reactions.
- This statement is correct. Enzymes are highly specific for particular reactions. Each enzyme is designed to catalyze a specific chemical reaction or a group of closely related reactions. This specificity is determined by the enzyme's active site and its ability to recognize and bind to specific substrates.
(v) The energy input needed to start a chemical reaction is called activation energy.
- This statement is correct. Activation energy refers to the energy input needed to initiate a chemical reaction. It is the energy required to break the bonds of the reactant molecules and reach the transition state, from which the reaction proceeds to form the products.
**In conclusion, all of the given statements are correct. Enzymes do not alter the overall change in free energy, their three-dimensional shape is crucial for their function, they lower activation energy, they are highly specific for reactions, and activation energy is the energy input needed to start a chemical reaction. Therefore, the correct answer is option 'D'.
(i) Enzymes do not alter the overall change in free energy for a reaction.
- This statement is correct. Enzymes do not affect the overall change in free energy for a reaction. They only lower the activation energy required for the reaction to occur, but they do not change the energy difference between the reactants and products.
(ii) Enzymes are proteins whose three-dimensional shape is key to their functions.
- This statement is correct. Enzymes are proteins, and their three-dimensional shape is critical for their proper functioning. The active site of an enzyme, where the substrate binds and the reaction takes place, is precisely shaped to accommodate specific substrates.
(iii) Enzymes speed up reactions by lowering activation energy.
- This statement is correct. Enzymes work by lowering the activation energy required for a chemical reaction to occur. Activation energy is the energy input needed to start a reaction, and enzymes facilitate this process by providing an alternative pathway with a lower energy barrier.
(iv) Enzymes are highly specific for reactions.
- This statement is correct. Enzymes are highly specific for particular reactions. Each enzyme is designed to catalyze a specific chemical reaction or a group of closely related reactions. This specificity is determined by the enzyme's active site and its ability to recognize and bind to specific substrates.
(v) The energy input needed to start a chemical reaction is called activation energy.
- This statement is correct. Activation energy refers to the energy input needed to initiate a chemical reaction. It is the energy required to break the bonds of the reactant molecules and reach the transition state, from which the reaction proceeds to form the products.
**In conclusion, all of the given statements are correct. Enzymes do not alter the overall change in free energy, their three-dimensional shape is crucial for their function, they lower activation energy, they are highly specific for reactions, and activation energy is the energy input needed to start a chemical reaction. Therefore, the correct answer is option 'D'.
Feedback inhibition of enzyme is influenced by- a)Enzyme
- b)External factors
- c)End product
- d)Substrate
Correct answer is option 'C'. Can you explain this answer?
Feedback inhibition of enzyme is influenced by
a)
Enzyme
b)
External factors
c)
End product
d)
Substrate
|
|
Suresh Iyer answered |
In feedback inhibition, product of a reaction inhibits the enzyme catalysing that reaction. It is a type of control mechanism at the enzyme level. If the product is produced in sufficient amount, in inhibits the enzyme to stop the further production.
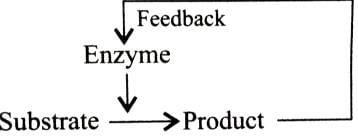
Holoenzyme is the complete enzyme consisting of an apoenzyme and a co-factor. Select the option that correctly identifies the nature of apoenzyme and co-factor.- a)Apoenzyme - Protein
Co - factor - Non-Protein - b)Apoenzyme - Non - Protein
Co - factor - Protein - c)Apoenzyme - Protein
Co - factor - Protein - d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Holoenzyme is the complete enzyme consisting of an apoenzyme and a co-factor. Select the option that correctly identifies the nature of apoenzyme and co-factor.
a)
Apoenzyme - Protein
Co - factor - Non-Protein
Co - factor - Non-Protein
b)
Apoenzyme - Non - Protein
Co - factor - Protein
Co - factor - Protein
c)
Apoenzyme - Protein
Co - factor - Protein
Co - factor - Protein
d)
None of the above
|
|
Mira Joshi answered |
Enzyme may be broadly classified into two types depending on their chemical composition-simple enzymes and conjugated enzymes are wholly made up of proteins and any additional substance or group is absent, e.g., pepsin, trypsin, etc. Conjugated enzymes (or holoenzymes) are formed of two parts -a protein part called apoenzyme and a non-protein part named co-factor. The complete conjugated enzyme consisting of an apoenzyme and a co-factor is called holoenzyme. Holoenzyme is the functional unit of enzyme.
Co-factor may be inorgainc or roganic in nature. Catalytic activity is lost when co-factor is removed from the enzyme which indicates that it plays a crucial role in catalytic activity of enzymes.
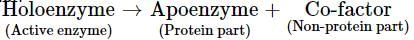
Co-factor may be inorgainc or roganic in nature. Catalytic activity is lost when co-factor is removed from the enzyme which indicates that it plays a crucial role in catalytic activity of enzymes.
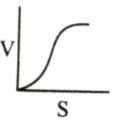
Which of the following graphs shows the relationship between the rate of an enzymatic activity and substrate concentration (S)?- a)
- b)
- c)
- d)
Correct answer is option 'D'. Can you explain this answer?
Which of the following graphs shows the relationship between the rate of an enzymatic activity and substrate concentration (S)?
a)

b)

c)

d)

|
|
Ajay Yadav answered |
Increase in substrate concentration increases the rate of reaction due to two factors: (i) occupation of more and more active sites by the substrate molecules, (ii) higher number of collisions between substrate molecules. The rise in velocity is quite high in the beginning but it decreases progressively with the increase in substate concentration. If a graph is plotted for substrate concentration versus reaction velocity, it appears as a hyperbolic curve. A stage is reached where velocity is maximum. It does not increase further by increasing the substrate concentration. At this stage the anzyme molecule becomes fully saturated and no active site is left free to bind additional substrate molecules.
Michaelis Menten Constant (Km) is equal to- a)The rate of reaction
- b)The rate of enzymatic activity
- c)Substrate concentration at which the reaction attains half of its maximum velocity
- d)Substrate concentration at which the rate of reaction is maximum
Correct answer is option 'C'. Can you explain this answer?
Michaelis Menten Constant (Km) is equal to
a)
The rate of reaction
b)
The rate of enzymatic activity
c)
Substrate concentration at which the reaction attains half of its maximum velocity
d)
Substrate concentration at which the rate of reaction is maximum
|
|
Ajay Yadav answered |
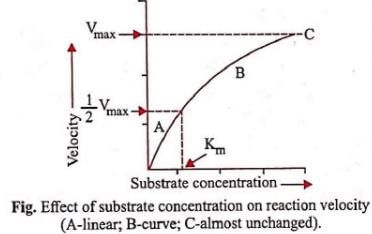
Km or the Michaelis-Menten constant is defined as the substrate concentration (expressed in moles/l) at which half-maximum velocity in an enzyme catalysed reaction is achieved. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value. It was given by Leonor Michaelis and Maud Menten (1913). Km value is a characteristic feature of a given enzyme. It is a representative for measuring the strength of ES complex. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them. For majority of enzymes, the Km values are in the range of 10−5 to 10−2 moles.

A.
Release of products of the reaction
B.
Binding of substrate to the active site of the enzyme
C.
Formation of enzyme-substrate complex
D.
Alteration in the shape of the enzyme
E.
Enzyme free to bind another molecule of substrate
Given above are the steps involved in the catalytic cycle of enzyme action. Choose the correct answer from the options given below: - a)B > C > D > A > E
- b)B > D > C > A > E
- c)B > D > A > E > C
- d)B > E > D > C > A
Correct answer is option 'B'. Can you explain this answer?
A.
Release of products of the reaction
B.
Binding of substrate to the active site of the enzyme
C.
Formation of enzyme-substrate complex
D.
Alteration in the shape of the enzyme
E.
Enzyme free to bind another molecule of substrate
Given above are the steps involved in the catalytic cycle of enzyme action. Choose the correct answer from the options given below:
Release of products of the reaction
B.
Binding of substrate to the active site of the enzyme
C.
Formation of enzyme-substrate complex
D.
Alteration in the shape of the enzyme
E.
Enzyme free to bind another molecule of substrate
Given above are the steps involved in the catalytic cycle of enzyme action. Choose the correct answer from the options given below:
a)
B > C > D > A > E
b)
B > D > C > A > E
c)
B > D > A > E > C
d)
B > E > D > C > A

|
EduRev NEET answered |
Ans: 2
Sol: During the state where substrate is bound to the enzyme active site, a new structure of the substrate called transition state structure is formed. Very soon, after the expected bond breaking/making is completed, the product is released from the active site
Sol: During the state where substrate is bound to the enzyme active site, a new structure of the substrate called transition state structure is formed. Very soon, after the expected bond breaking/making is completed, the product is released from the active site
Read the given statements and select the correct options.
Statement 1: Low temperature destroys enzymes by causing their denaturation.
Statement 2: High temperature preserves the enzymes in their inactive stages.- a)Both Statements 1 and 2 are correct
- b)Statement 1 is correct but statement 2 is incorrect
- c)Statement 1 is incorrect but statement 2 is correct
- d)Both Statements 1 and 2 are incorrect
Correct answer is option 'D'. Can you explain this answer?
Read the given statements and select the correct options.
Statement 1: Low temperature destroys enzymes by causing their denaturation.
Statement 2: High temperature preserves the enzymes in their inactive stages.
Statement 1: Low temperature destroys enzymes by causing their denaturation.
Statement 2: High temperature preserves the enzymes in their inactive stages.
a)
Both Statements 1 and 2 are correct
b)
Statement 1 is correct but statement 2 is incorrect
c)
Statement 1 is incorrect but statement 2 is correct
d)
Both Statements 1 and 2 are incorrect
|
|
Suresh Iyer answered |
Low temperature preserves the enzymes in their inactive state. Therefore it is used in preservation of foods inside cold storage. Low temperature present inside cold storage prevents spoilage of food.
High temperature destroys enzymes by causing their denaturation, This occurs at 50oC or so. In between the minimum and maximum temperature the reaction velocity doubles for every 10oC in temperature (general rule of thumb).
The inhibitor which does not resemble the substrate in structure and binds to the enzyme at site other than the active site is called- a)Competitive inhibitor
- b)Non-competitive inhibitor
- c)Activator
- d)Substrate analogue
Correct answer is option 'B'. Can you explain this answer?
The inhibitor which does not resemble the substrate in structure and binds to the enzyme at site other than the active site is called
a)
Competitive inhibitor
b)
Non-competitive inhibitor
c)
Activator
d)
Substrate analogue
|
|
Juhi Bose answered |
Non-competitive inhibitor:
Non-competitive inhibitors are molecules that bind to an enzyme at a site other than the active site. They do not resemble the substrate in structure. These inhibitors inhibit enzyme activity by changing the shape of the enzyme, making it less effective in catalyzing the reaction.
Mechanism of action:
- Non-competitive inhibitors bind to the enzyme at an allosteric site, causing a conformational change in the enzyme.
- This conformational change may prevent the substrate from binding to the active site or inhibit the catalytic activity of the enzyme.
- Since non-competitive inhibitors do not compete with the substrate for the active site, increasing substrate concentration will not overcome their inhibitory effect.
Characteristics:
- Non-competitive inhibitors do not compete with the substrate for binding to the enzyme.
- They can bind to the enzyme-substrate complex as well as the free enzyme.
- The inhibition by non-competitive inhibitors is not easily reversible.
- These inhibitors are not affected by changes in substrate concentration.
Examples:
- Heavy metal ions like mercury and lead are examples of non-competitive inhibitors.
- Drugs like aspirin and certain antibiotics also act as non-competitive inhibitors of specific enzymes.
In conclusion, non-competitive inhibitors are molecules that bind to an enzyme at a site other than the active site, causing a conformational change that inhibits enzyme activity.
Non-competitive inhibitors are molecules that bind to an enzyme at a site other than the active site. They do not resemble the substrate in structure. These inhibitors inhibit enzyme activity by changing the shape of the enzyme, making it less effective in catalyzing the reaction.
Mechanism of action:
- Non-competitive inhibitors bind to the enzyme at an allosteric site, causing a conformational change in the enzyme.
- This conformational change may prevent the substrate from binding to the active site or inhibit the catalytic activity of the enzyme.
- Since non-competitive inhibitors do not compete with the substrate for the active site, increasing substrate concentration will not overcome their inhibitory effect.
Characteristics:
- Non-competitive inhibitors do not compete with the substrate for binding to the enzyme.
- They can bind to the enzyme-substrate complex as well as the free enzyme.
- The inhibition by non-competitive inhibitors is not easily reversible.
- These inhibitors are not affected by changes in substrate concentration.
Examples:
- Heavy metal ions like mercury and lead are examples of non-competitive inhibitors.
- Drugs like aspirin and certain antibiotics also act as non-competitive inhibitors of specific enzymes.
In conclusion, non-competitive inhibitors are molecules that bind to an enzyme at a site other than the active site, causing a conformational change that inhibits enzyme activity.
Hormones are found in the body in very low concentrations, but tend to have a strong effect. What type of receptor are hormones most likely to act on?
I. Ligand-gated ion channels
II. Enzyme-linked receptors
III. G protein-coupled receptors- a)I only
- b)III only
- c)II and III only
- d)I, II, and III
Correct answer is option 'C'. Can you explain this answer?
Hormones are found in the body in very low concentrations, but tend to have a strong effect. What type of receptor are hormones most likely to act on?
I. Ligand-gated ion channels
II. Enzyme-linked receptors
III. G protein-coupled receptors
I. Ligand-gated ion channels
II. Enzyme-linked receptors
III. G protein-coupled receptors
a)
I only
b)
III only
c)
II and III only
d)
I, II, and III

|
Orion Classes answered |
For a ligand present in low quantities to have a strong action, we expect it to initiate a second messenger cascade system. Second messenger systems amplify signals because enzymes can catalyze a reaction more than once while they are active, and often activate other enzymes. Both enzyme-linked receptors and G protein-coupled receptors use second messenger systems, while ion channels do not.
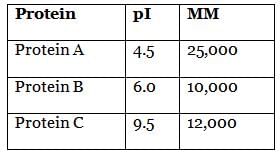
At what pH can protein A best be obtained through electrophoresis? (Note: MM = molar mass)
- a)2.5
- b)3.5
- c)4.5
- d)5.5
Correct answer is option 'D'. Can you explain this answer?
At what pH can protein A best be obtained through electrophoresis? (Note: MM = molar mass)


a)
2.5
b)
3.5
c)
4.5
d)
5.5

|
Orion Classes answered |
In most electrophoresis experiments, we attempt to separate out one component from the others. Because we are attempting to isolate protein A only, a pH that causes protein A to be negative while proteins B and C are neutral or positive will be best. pH 5.5 accomplishes this goal; proteins B and C will be positively charged. A pH of 4.5, choice (C), would make protein A neutral, and it would thus not migrate across the gel. Any neutral impurities would also remain in the well with protein A, making this pH not the best choice.
In competitive inhibition, how does the presence of an inhibitor affect the apparent Km and Vmax values in enzyme kinetics?- a)Apparent Km increases, and Vmax decreases.
- b)Apparent Km decreases, and Vmax increases.
- c)Apparent Km increases, and Vmax remains unchanged.
- d)Apparent Km decreases, and Vmax remains unchanged.
Correct answer is option 'A'. Can you explain this answer?
In competitive inhibition, how does the presence of an inhibitor affect the apparent Km and Vmax values in enzyme kinetics?
a)
Apparent Km increases, and Vmax decreases.
b)
Apparent Km decreases, and Vmax increases.
c)
Apparent Km increases, and Vmax remains unchanged.
d)
Apparent Km decreases, and Vmax remains unchanged.

|
Orion Classes answered |
In competitive inhibition, the inhibitor competes with the substrate for binding to the active site of the enzyme. This results in an increase in the apparent Km value since a higher substrate concentration is required to reach half of the Vmax. However, the inhibitor does not affect the maximum velocity (Vmax) of the reaction. Therefore, option A is the correct answer.
What is denoted by X and Y in the given graph?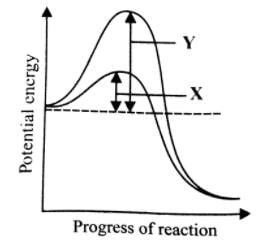
- a)X-Activation energy without enzyme; Y-Activation energy with enzyme
- b)X-Activation energy with enzyme; Y-Activation energy without enzyme
- c)X-Substrate concentration with enzyme; Y- Substrate concentration without enzyme
- d)X-Substrate concentration without enzyme; Y-Substrate concentration with enzyme
Correct answer is option 'B'. Can you explain this answer?
What is denoted by X and Y in the given graph?

a)
X-Activation energy without enzyme; Y-Activation energy with enzyme
b)
X-Activation energy with enzyme; Y-Activation energy without enzyme
c)
X-Substrate concentration with enzyme; Y- Substrate concentration without enzyme
d)
X-Substrate concentration without enzyme; Y-Substrate concentration with enzyme
|
|
Raghav Bansal answered |
Most reactions do not start automatically because the reactions have an energy barrier which may be due to hydrogen bonding, absence of precise collisions due to small reactive site, mutual repulsion, etc. External supply of energy needed to overcome this barrier is known as activation energy. The enzyme lowers the activation energy of the reaction and allows a large number of molecules to react at a time.
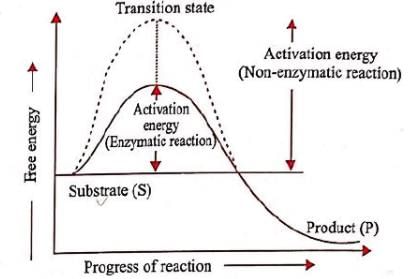
Fig. Lowering of activation energy by enzyme in the energy relations of a chemical reaction
Which of the following characteristics is NOT attributed to antibodies?- a)Antibodies bind to more than one distinct antigen.
- b)Antibodies label antigens for targeting by other immune cells.
- c)Antibodies can cause agglutination by interaction with antigen.
- d)Antibodies have two heavy chains and two light chains.
Correct answer is option 'A'. Can you explain this answer?
Which of the following characteristics is NOT attributed to antibodies?
a)
Antibodies bind to more than one distinct antigen.
b)
Antibodies label antigens for targeting by other immune cells.
c)
Antibodies can cause agglutination by interaction with antigen.
d)
Antibodies have two heavy chains and two light chains.

|
Orion Classes answered |
Antibodies are specific to a single antigen. Each B-cell produces a single type of antibody with a constant region that is specific to the host and a variable region that is specific to an antigen.
Which of the following is NOT involved in cell migration?- a)Dynein
- b)Flagella
- c)Actin
- d)Centrioles
Correct answer is option 'D'. Can you explain this answer?
Which of the following is NOT involved in cell migration?
a)
Dynein
b)
Flagella
c)
Actin
d)
Centrioles

|
Orion Classes answered |
From the given choices, all of them are involved in cell movement with the exception of choice (D). Centrioles are composed of microtubules, but are involved in mitosis, not cell motility.
A drug molecule binds to an enzyme and reduces its activity. This effect is observed regardless of whether the substrate is present or not. What type of inhibition is exhibited by the drug molecule?- a)Competitive inhibition
- b)Noncompetitive inhibition
- c)Uncompetitive inhibition
- d)Mixed inhibition
Correct answer is option 'B'. Can you explain this answer?
A drug molecule binds to an enzyme and reduces its activity. This effect is observed regardless of whether the substrate is present or not. What type of inhibition is exhibited by the drug molecule?
a)
Competitive inhibition
b)
Noncompetitive inhibition
c)
Uncompetitive inhibition
d)
Mixed inhibition

|
Orion Classes answered |
Noncompetitive inhibition. Noncompetitive inhibitors bind to an allosteric site on the enzyme, causing a conformational change that reduces the enzyme's activity. Unlike competitive inhibitors, noncompetitive inhibitors do not compete with the substrate for binding to the active site and their inhibitory effect is not affected by substrate concentration. Therefore, the drug molecule described in the question is an example of noncompetitive inhibition.
What property of protein-digesting enzymes allows for a sequence to be determined without fully degrading the protein?- a)Selectivity
- b)Sensitivity
- c)Turnover
- d)Inhibition
Correct answer is option 'A'. Can you explain this answer?
What property of protein-digesting enzymes allows for a sequence to be determined without fully degrading the protein?
a)
Selectivity
b)
Sensitivity
c)
Turnover
d)
Inhibition

|
Orion Classes answered |
The selective cleavage of proteins by digestive enzymes allows fragments of different lengths with known amino acid endpoints to be created. By cleaving the protein with several different enzymes, a basic outline of the amino acid sequence can be created.
Which of the following proteins is most likely to be found extracellularly?- a)Tubulin
- b)Myosin
- c)Collagen
- d)Actin
Correct answer is option 'C'. Can you explain this answer?
Which of the following proteins is most likely to be found extracellularly?
a)
Tubulin
b)
Myosin
c)
Collagen
d)
Actin

|
Orion Classes answered |
The most prevalent extracellular proteins are keratin, elastin, and collagen. Tubulin and actin are the primary cytoskeletal proteins, while myosin is a motor protein.
If the enzyme-catalyzed reaction E + S ⇋ ES ⇋ E + P is proceeding at or near the Vmax of E, what can be deduced about the relative concentrations of S and ES?- a)S is abundant, [ES] is at its highest point
- b)[S] is vanishingly low, [ES] is at its highest point
- c)[S] is vanishingly low, [ES] is vanishingly low
- d)S is abundant, [ES] is vanishingly low
Correct answer is option 'A'. Can you explain this answer?
If the enzyme-catalyzed reaction E + S ⇋ ES ⇋ E + P is proceeding at or near the Vmax of E, what can be deduced about the relative concentrations of S and ES?
a)
S is abundant, [ES] is at its highest point
b)
[S] is vanishingly low, [ES] is at its highest point
c)
[S] is vanishingly low, [ES] is vanishingly low
d)
S is abundant, [ES] is vanishingly low

|
Orion Classes answered |
At or near Vmax, the enzyme is saturated with substrate (S), meaning that there is an excess of substrate available for binding to the enzyme. The concentration of free substrate (S) is high because it is not being rapidly converted to product (P). At the same time, the concentration of the enzyme-substrate complex (ES) is at its highest point because the enzyme is bound to substrate molecules. This is the point where the enzyme is working at its maximum capacity, and the rate of formation of the ES complex is balanced by the rate of its conversion to product. Therefore, [S] is abundant, and [ES] is at its highest point.
What is the function of sodium dodecyl sulfate (SDS) in SDS-PAGE?- a)SDS stabilizes the gel matrix, improving resolution during electrophoresis.
- b)SDS solubilizes proteins to give them uniformly negative charges, so the separation is based purely on size.
- c)SDS raises the pH of the gel, separating multiunit proteins into individual subunits.
- d)SDS solubilizes proteins to give them uniformly positive charges, so separation is based purely on pH.
Correct answer is option 'B'. Can you explain this answer?
What is the function of sodium dodecyl sulfate (SDS) in SDS-PAGE?
a)
SDS stabilizes the gel matrix, improving resolution during electrophoresis.
b)
SDS solubilizes proteins to give them uniformly negative charges, so the separation is based purely on size.
c)
SDS raises the pH of the gel, separating multiunit proteins into individual subunits.
d)
SDS solubilizes proteins to give them uniformly positive charges, so separation is based purely on pH.

|
Orion Classes answered |
Sodium dodecyl sulfate is a detergent and will digest proteins to form micelles with uniform negative charges. Because the protein is sequestered within the micelle, other factors such as charge of the protein and shape have minimal roles during separation. In essence, the protein micelles can be modeled as being spheres, dependent only on size.
Enzymes are regarded as the:- a)Messengers
- b)Activators
- c)Biocatalysts
- d)Anti bodies
Correct answer is option 'C'. Can you explain this answer?
Enzymes are regarded as the:
a)
Messengers
b)
Activators
c)
Biocatalysts
d)
Anti bodies

|
Stepway Academy answered |
- Enzymes are regarded as the biocatalysts.
- Enzymes are proteins made from amino acids.
- It is made up of hundreds and thousands of amino acids stringed together in a very specific and unique order.
- Any chemical reaction inside a cell or any work that goes on inside a cell is the handiwork of enzymes inside the cell.
Which amino acids contribute most significantly to the pI of a protein?
I. Lysine
II. Glycine
III. Arginine- a)I only
- b)I and II only
- c)I and III only
- d)II and III only
Correct answer is option 'C'. Can you explain this answer?
Which amino acids contribute most significantly to the pI of a protein?
I. Lysine
II. Glycine
III. Arginine
I. Lysine
II. Glycine
III. Arginine
a)
I only
b)
I and II only
c)
I and III only
d)
II and III only

|
Orion Classes answered |
The overall pI of a protein is determined by the relative number of acidic and basic amino acids. The basic amino acids arginine, lysine, and histidine, and the acidic amino acids aspartic acid and glutamic acid will therefore contribute most significantly. Glycine’s side chain is a hydrogen atom, so it will have the least contribution of all the amino acids.
Assuming all other reaction conditions remain constant and that the reaction is allowed to proceed to equilibrium, how would the reaction S⇋P be affected by the addition of an enzyme catalyst?- a)Both increased reaction rate and increased formation of product
- b)Neither increased reaction rate or increased formation of product
- c)Increased total product formed
- d)Increased reaction rate
Correct answer is option 'D'. Can you explain this answer?
Assuming all other reaction conditions remain constant and that the reaction is allowed to proceed to equilibrium, how would the reaction S⇋P be affected by the addition of an enzyme catalyst?
a)
Both increased reaction rate and increased formation of product
b)
Neither increased reaction rate or increased formation of product
c)
Increased total product formed
d)
Increased reaction rate

|
Orion Classes answered |
The addition of an enzyme catalyst to the reaction S⇋P would increase the rate at which the reaction proceeds. Enzymes are biological catalysts that facilitate chemical reactions by lowering the activation energy required for the reaction to occur. They do this by providing an alternative reaction pathway with a lower energy barrier.
When an enzyme is added to a reaction, it can bind to the substrate (S) and form an enzyme-substrate complex (ES). This binding event lowers the activation energy required for the conversion of substrate to product (P). By lowering the activation energy, the enzyme accelerates the rate at which the reaction reaches equilibrium.
Importantly, the addition of an enzyme catalyst does not affect the equilibrium position of the reaction. The equilibrium is determined by the relative concentrations of the reactants and products and the free energy difference (∆G) between them. The presence of an enzyme catalyst does not change the overall thermodynamics or equilibrium constant of the reaction.
Therefore, the addition of an enzyme catalyst primarily affects the reaction rate, allowing the reaction to reach equilibrium more quickly. It does not directly affect the total amount of product formed at equilibrium (option C), as that is determined by the equilibrium constant and the initial concentrations of reactants.
Given that ∆G’° for the reaction S⇋P is negative in the direction of S→P, reaction equilibrium favors the formation of which substance?- a)P
- b)The reaction does not proceed in either direction
- c)S
- d)The reaction proceeds equally in both directions
Correct answer is option 'A'. Can you explain this answer?
Given that ∆G’° for the reaction S⇋P is negative in the direction of S→P, reaction equilibrium favors the formation of which substance?
a)
P
b)
The reaction does not proceed in either direction
c)
S
d)
The reaction proceeds equally in both directions

|
Orion Classes answered |
The sign of ΔG'° indicates the direction in which the reaction is thermodynamically favorable. In this case, since ΔG'° is negative in the direction of S → P, it means that the formation of product P is thermodynamically favored over the formation of substrate S. Therefore, the reaction equilibrium favors the formation of substance P.
Which of the following is NOT a component of all trimeric G proteins?- a)Gα
- b)Gβ
- c)Gγ
- d)Gi
Correct answer is option 'D'. Can you explain this answer?
Which of the following is NOT a component of all trimeric G proteins?
a)
Gα
b)
Gβ
c)
Gγ
d)
Gi

|
Orion Classes answered |
All trimeric G proteins have α, β, and γ subunits—choices (A), (B), and (C), respectively. Gs, Gi, and Gq are subtypes of the Gα subunit of the trimeric G protein and differ depending on the G protein-coupled receptor’s function.
Which of the following best describes the Michaelis-Menten constant (Km) in enzyme kinetics?- a)The substrate concentration at which the reaction rate is half of Vmax
- b)The enzyme concentration at which the reaction rate is half of Vmax
- c)The rate of the enzyme-substrate complex formation
- d)The rate of enzyme catalysis
Correct answer is option 'A'. Can you explain this answer?
Which of the following best describes the Michaelis-Menten constant (Km) in enzyme kinetics?
a)
The substrate concentration at which the reaction rate is half of Vmax
b)
The enzyme concentration at which the reaction rate is half of Vmax
c)
The rate of the enzyme-substrate complex formation
d)
The rate of enzyme catalysis

|
Orion Classes answered |
The Michaelis-Menten constant (Km) is a measure of the affinity between an enzyme and its substrate. It represents the substrate concentration at which the reaction rate is half of the maximum velocity (Vmax) of the reaction. At Km, half of the enzyme active sites are occupied by substrate molecules, leading to an optimal rate of catalysis. Therefore, option A is the correct answer.
Molecule ‘X’ is an enzyme inhibitor that reversibly binds to an enzyme at a site that is distinct from its active site. Molecule ‘X’ must NOT be what type of inhibitor?- a)Mixed inhibitor
- b)Competitive inhibitor
- c)Uncompetitive inhibitor
- d)Noncompetitive inhibitor
Correct answer is option 'B'. Can you explain this answer?
Molecule ‘X’ is an enzyme inhibitor that reversibly binds to an enzyme at a site that is distinct from its active site. Molecule ‘X’ must NOT be what type of inhibitor?
a)
Mixed inhibitor
b)
Competitive inhibitor
c)
Uncompetitive inhibitor
d)
Noncompetitive inhibitor

|
Orion Classes answered |
A competitive inhibitor is a type of enzyme inhibitor that competes with the substrate for binding to the active site of the enzyme. It binds reversibly to the active site, preventing the substrate from binding and reducing the enzyme's activity. In competitive inhibition, the inhibitor and substrate cannot bind to the enzyme simultaneously.
In the given scenario, molecule 'X' binds to a site on the enzyme that is distinct from the active site. This type of inhibition is characteristic of noncompetitive inhibitors or uncompetitive inhibitors.
A noncompetitive inhibitor binds to an allosteric site on the enzyme, which is different from the active site. The binding of the inhibitor to this site induces a conformational change in the enzyme that affects its activity, regardless of whether the substrate is bound or not.
An uncompetitive inhibitor, on the other hand, binds to the enzyme-substrate complex, forming an enzyme-inhibitor-substrate ternary complex. This type of inhibition only occurs when the substrate is bound to the enzyme.
Therefore, since molecule 'X' binds to a site distinct from the active site, it cannot be a competitive inhibitor (option B). It is more likely to be a noncompetitive inhibitor (option D) or an uncompetitive inhibitor (option C). The exact classification would depend on whether molecule 'X' binds to the enzyme alone or to the enzyme-substrate complex.
Which protein properties allow UV spectroscopy to be used as a method of determining concentration?- a)Proteins have partially planar characteristics in peptide bonds.
- b)Globular proteins cause scattering of light.
- c)Proteins contain aromatic groups in certain amino acids.
- d)All organic macromolecules can be assessed with UV spectroscopy.
Correct answer is option 'C'. Can you explain this answer?
Which protein properties allow UV spectroscopy to be used as a method of determining concentration?
a)
Proteins have partially planar characteristics in peptide bonds.
b)
Globular proteins cause scattering of light.
c)
Proteins contain aromatic groups in certain amino acids.
d)
All organic macromolecules can be assessed with UV spectroscopy.

|
Orion Classes answered |
UV spectroscopy is best used with conjugated systems of double bonds. While the double bond in the peptide bond does display resonance, this is not adequate for UV absorption. However, aromatic systems are conjugated, and phenylalanine, tyrosine, and tryptophan all contain aromatic ring structures.
A certain enzyme has an optimum pH of 7.5. If the pH of the reaction environment is increased to 9.0, what effect would this have on the enzyme's activity?- a)No effect on enzyme activity
- b)Increased enzyme activity
- c)Decreased enzyme activity
- d) Enzyme activity would fluctuate
Correct answer is option 'C'. Can you explain this answer?
A certain enzyme has an optimum pH of 7.5. If the pH of the reaction environment is increased to 9.0, what effect would this have on the enzyme's activity?
a)
No effect on enzyme activity
b)
Increased enzyme activity
c)
Decreased enzyme activity
d)
Enzyme activity would fluctuate

|
Orion Classes answered |
Decreased enzyme activity. Enzymes have an optimum pH at which they exhibit maximum activity. Deviations from this pH can disrupt the enzyme's structure and alter its catalytic efficiency. In this case, the higher pH of 9.0 would likely lead to decreased enzyme activity.
With respect to the binding of regulatory compounds, what properties define an enzyme as being allosteric?- a)Irreversible, covalent binding of regulatory compounds
- b)Reversible, noncovalent binding of regulatory compounds
- c)Reversible, covalent binding of regulatory compounds
- d)Irreversible, noncovalent binding of regulatory compounds
Correct answer is option 'B'. Can you explain this answer?
With respect to the binding of regulatory compounds, what properties define an enzyme as being allosteric?
a)
Irreversible, covalent binding of regulatory compounds
b)
Reversible, noncovalent binding of regulatory compounds
c)
Reversible, covalent binding of regulatory compounds
d)
Irreversible, noncovalent binding of regulatory compounds

|
Orion Classes answered |
Allosteric enzymes are enzymes that can be regulated by the binding of specific molecules, known as allosteric regulators, to sites on the enzyme other than the active site. The binding of these regulatory compounds can either enhance or inhibit the enzyme's activity. Importantly, the binding of allosteric regulators is reversible, meaning that the compounds can bind and unbind from the enzyme.
The binding of allosteric regulators typically occurs through noncovalent interactions, such as hydrogen bonds, electrostatic interactions, or hydrophobic interactions. These interactions allow the regulatory compounds to bind to specific allosteric sites on the enzyme, causing a conformational change in the enzyme's structure. This conformational change can alter the enzyme's activity and its affinity for the substrate.
In contrast, options A, C, and D do not accurately describe the properties of allosteric enzymes. Irreversible binding implies a permanent and covalent modification of the enzyme, which is not characteristic of allosteric regulation. Covalent binding, in general, refers to the formation of a covalent bond between the enzyme and a molecule, which is not a defining feature of allosteric regulation.
Chapter doubts & questions for Control of Enzyme Activity (BIO, BC) - MCAT Biological and Biochemical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Control of Enzyme Activity (BIO, BC) - MCAT Biological and Biochemical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Biological and Biochemical Foundations
367 videos|157 docs|88 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









