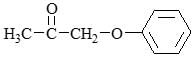All Exams >
Chemistry >
Mock Test Series for IIT JAM Chemistry >
All Questions
All questions of Mock Tests for Chemistry Exam
Which of the following form stable for CN—CH2—CH2—CN? - a)Anti-form
- b)Partially-eclipsed
- c)Gauche-form
- d)Eclipsed-form
Correct answer is option 'C'. Can you explain this answer?
Which of the following form stable for CN—CH2—CH2—CN?
a)
Anti-form
b)
Partially-eclipsed
c)
Gauche-form
d)
Eclipsed-form

|
Veda Institute answered |
Correct answer is Option (C).
Explanation:
In the given molecule CN—CH2—CH2—CN, we can analyze the stable conformations based on the dihedral angle between the CN groups. The conformations are:
A: Gauche-form
- In this conformation, the dihedral angle between the CN groups is around 60°.
- The molecule will have a slight steric hindrance between the CN groups as they are close to each other.
- This form is not the most stable conformation.
B: Partially-eclipsed
- In this conformation, the dihedral angle between the CN groups is around 120°.
- The molecule will have a moderate steric hindrance between the CN groups.
- This form is less stable than the anti-form.
C: Anti-form
- In this conformation, the dihedral angle between the CN groups is 180°.
- This conformation has the least steric hindrance between the CN groups as they are farthest apart.
- This form is the most stable conformation.
D: Eclipsed-form
- In this conformation, the dihedral angle between the CN groups is 0°.
- The molecule will have maximum steric hindrance between the CN groups as they are directly overlapping.
- This form is the least stable conformation.
Hence, the most stable conformation for the given molecule CN—CH2—CH2—CN is the anti-form (Option C), where the dihedral angle between the CN groups is 180° and there is minimum steric hindrance.
Explanation:
In the given molecule CN—CH2—CH2—CN, we can analyze the stable conformations based on the dihedral angle between the CN groups. The conformations are:
A: Gauche-form
- In this conformation, the dihedral angle between the CN groups is around 60°.
- The molecule will have a slight steric hindrance between the CN groups as they are close to each other.
- This form is not the most stable conformation.
B: Partially-eclipsed
- In this conformation, the dihedral angle between the CN groups is around 120°.
- The molecule will have a moderate steric hindrance between the CN groups.
- This form is less stable than the anti-form.
C: Anti-form
- In this conformation, the dihedral angle between the CN groups is 180°.
- This conformation has the least steric hindrance between the CN groups as they are farthest apart.
- This form is the most stable conformation.
D: Eclipsed-form
- In this conformation, the dihedral angle between the CN groups is 0°.
- The molecule will have maximum steric hindrance between the CN groups as they are directly overlapping.
- This form is the least stable conformation.
Hence, the most stable conformation for the given molecule CN—CH2—CH2—CN is the anti-form (Option C), where the dihedral angle between the CN groups is 180° and there is minimum steric hindrance.
If the pressure P(system) is greater than the P(surroundings), then: - a)Work is done on the system by the surroundings.
- b)Work is done on the surroundings by the system.
- c)Work done on the system by the surroundings is equal to the work done on the surroundings by the system.
- d)Internal energy of the system increases.
Correct answer is option 'B'. Can you explain this answer?
If the pressure P(system) is greater than the P(surroundings), then:
a)
Work is done on the system by the surroundings.
b)
Work is done on the surroundings by the system.
c)
Work done on the system by the surroundings is equal to the work done on the surroundings by the system.
d)
Internal energy of the system increases.
|
|
Tanisha Senghal answered |
If pressure of system is greater than the surrounding, it will undergo expansion and hence option b
For the following equilibrium,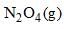 →
→ 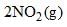 Kp is found to be equal to Kc. This is attained when:
Kp is found to be equal to Kc. This is attained when:- a)0°C
- b)273 K
- c)1 K
- d)12.18 K
Correct answer is option 'D'. Can you explain this answer?
For the following equilibrium,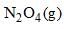 →
→ 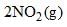 Kp is found to be equal to Kc. This is attained when:
Kp is found to be equal to Kc. This is attained when:
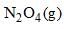 →
→ 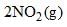 Kp is found to be equal to Kc. This is attained when:
Kp is found to be equal to Kc. This is attained when:a)
0°C
b)
273 K
c)
1 K
d)
12.18 K

|
Asf Institute answered |
Correct Answer: D (12.18 K)
Explanation:
The relationship between Kp and Kc is given by the equation:
Kp = Kc(RT)^(Δn)
where Δn is the change in the number of moles of gas in the reaction, R is the ideal gas constant, and T is the temperature in Kelvin.
For the given equilibrium, N2O4(g) → 2NO2(g), the change in the number of moles of gas, Δn, is:
Δn = (moles of products) - (moles of reactants)
Δn = 2 - 1
Δn = 1
We know that Kp = Kc, so:
Kc(RT)^(Δn) = Kc
This equation simplifies to:
RT = 1
Let's consider the possibility that the constant R used in the question is given in a different unit, such as atm·L/(mol·K). In this case, R = 0.0821 atm·L/(mol·K). We can solve for T again:
T = 1/R
T = 1/0.0821
T ≈ 12.18 K
This value matches option D, so the correct answer is 12.18 K.
Explanation:
The relationship between Kp and Kc is given by the equation:
Kp = Kc(RT)^(Δn)
where Δn is the change in the number of moles of gas in the reaction, R is the ideal gas constant, and T is the temperature in Kelvin.
For the given equilibrium, N2O4(g) → 2NO2(g), the change in the number of moles of gas, Δn, is:
Δn = (moles of products) - (moles of reactants)
Δn = 2 - 1
Δn = 1
We know that Kp = Kc, so:
Kc(RT)^(Δn) = Kc
This equation simplifies to:
RT = 1
Let's consider the possibility that the constant R used in the question is given in a different unit, such as atm·L/(mol·K). In this case, R = 0.0821 atm·L/(mol·K). We can solve for T again:
T = 1/R
T = 1/0.0821
T ≈ 12.18 K
This value matches option D, so the correct answer is 12.18 K.
Consider the following statements:- a)For a one component system, the maximum number of phases that can exits in equilibrium is three.
- b)A system as have negative degrees of freedom
- c)The number of phases in a system does not depend not he amounts of various substances present at equilibrium.
- d)The number of triple point in phase diagram of sulfur is 3
Correct answer is option 'A,C'. Can you explain this answer?
Consider the following statements:
a)
For a one component system, the maximum number of phases that can exits in equilibrium is three.
b)
A system as have negative degrees of freedom
c)
The number of phases in a system does not depend not he amounts of various substances present at equilibrium.
d)
The number of triple point in phase diagram of sulfur is 3

|
Veda Institute answered |
a) If there is only one component, there are no degrees of freedom (F = 0) when there are three phases.
d) The phase diagram of sulfur contains a new feature: there are two solid phases, rhombic and monoclinic. The names refer to the crystal structures in which the S8 molecules arrange themselves. This gives rise to three triple points, indicated by the numbers on the diagram.
2 moles of ideal gas is expanded isothermally & reversibly from 1 liter to 10 liter. Find the enthalpy change in (kJ mol-1,rounded up to first decimal place ).
Correct answer is '0'. Can you explain this answer?
2 moles of ideal gas is expanded isothermally & reversibly from 1 liter to 10 liter. Find the enthalpy change in (kJ mol-1,rounded up to first decimal place ).

|
Anshu Kumar answered |
Given conditions,
No. of moles(n) = 2 moles.
Temperature(T) = 300 K.
V₂ = 10 L, and V₁ = 1 L.
Universal Gas Constant (R) = 8.31 J/moleK.
Using the formula,
Work done = -2.303nRTlog(V₂/V₁) [For Chemistry.]
∴ Work done = -2.303 x 2 x 8.31 x 300 x log(10/1)
∴ Work done = -11482.758 J.
∴ Work done = -11.483 kJ.
Now, Process is Isothermal, therefore, Internal Energy will also be zero.
Thus, Using first Law of thermodynamics (of Chemistry and not of physics)
∴ ΔU = ΔQ + w
∴ ΔQ = - w
∴ ΔQ = - (-11.483)
∴ ΔQ = 11.483 kJ ≈ 11.4 kJ.
Now, ΔQ is the enthaly because heat constant at constant pressure is enthalpy.
Number of 2c -2e- bonds in Al2(CH3)6 :
Correct answer is '22'. Can you explain this answer?
Number of 2c -2e- bonds in Al2(CH3)6 :

|
Anirban Kapoor answered |
Calculation of number of 2c-2e bonds in Al2(CH3)6:
- The molecule Al2(CH3)6 can be divided into two Al(CH3)3 groups which are connected by a covalent bond between the two Al atoms.
- Each Al(CH3)3 group contains one Al atom, three CH3 groups, and three covalent bonds between the Al atom and the CH3 groups.
- Each covalent bond between the Al atom and a CH3 group is a 2-center-2-electron (2c-2e) bond, because it involves two atoms sharing two electrons.
- Therefore, each Al(CH3)3 group contains 3x2 = 6 2c-2e bonds.
- The two Al(CH3)3 groups are connected by a covalent bond between the two Al atoms, which is also a 2c-2e bond.
- Therefore, the molecule Al2(CH3)6 contains a total of 2x6 + 1 = 13 2c-2e bonds.
- However, each CH3 group also contains a covalent bond between the C atom and one of the H atoms, which is also a 2c-2e bond.
- Each Al(CH3)3 group contains 3 CH3 groups, so there are a total of 3x3 = 9 CH3 groups in the molecule.
- Therefore, there are 9 additional 2c-2e bonds in the molecule.
- Thus, the total number of 2c-2e bonds in Al2(CH3)6 is 13 + 9 = 22.
- The molecule Al2(CH3)6 can be divided into two Al(CH3)3 groups which are connected by a covalent bond between the two Al atoms.
- Each Al(CH3)3 group contains one Al atom, three CH3 groups, and three covalent bonds between the Al atom and the CH3 groups.
- Each covalent bond between the Al atom and a CH3 group is a 2-center-2-electron (2c-2e) bond, because it involves two atoms sharing two electrons.
- Therefore, each Al(CH3)3 group contains 3x2 = 6 2c-2e bonds.
- The two Al(CH3)3 groups are connected by a covalent bond between the two Al atoms, which is also a 2c-2e bond.
- Therefore, the molecule Al2(CH3)6 contains a total of 2x6 + 1 = 13 2c-2e bonds.
- However, each CH3 group also contains a covalent bond between the C atom and one of the H atoms, which is also a 2c-2e bond.
- Each Al(CH3)3 group contains 3 CH3 groups, so there are a total of 3x3 = 9 CH3 groups in the molecule.
- Therefore, there are 9 additional 2c-2e bonds in the molecule.
- Thus, the total number of 2c-2e bonds in Al2(CH3)6 is 13 + 9 = 22.
Number of complexes in which color is produced due to LMCT transition:
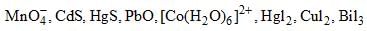
Correct answer is '6'. Can you explain this answer?
Number of complexes in which color is produced due to LMCT transition:
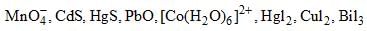
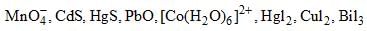

|
Sourav Dutta answered |
1,2,3,4,6,8 are take place by LMCT
Find the value of lmax in (nm) in following compound.
Given: Base value of Heteroannular component = 214 nm
Homoannular component = 253 nm
Increment for each extra double bond = 30 nm
Exocyclic bond = 5 nm; Alkyl/Ring residues = 5 nm
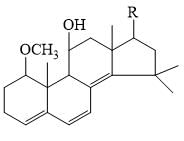
Correct answer is '284'. Can you explain this answer?
Find the value of lmax in (nm) in following compound.
Given: Base value of Heteroannular component = 214 nm
Homoannular component = 253 nm
Increment for each extra double bond = 30 nm
Exocyclic bond = 5 nm; Alkyl/Ring residues = 5 nm

Given: Base value of Heteroannular component = 214 nm
Homoannular component = 253 nm
Increment for each extra double bond = 30 nm
Exocyclic bond = 5 nm; Alkyl/Ring residues = 5 nm


|
Sourav Dutta answered |
The ans should be 279. Because here 2 exocyclic bonds are present.
Arrange the following in decreasing order of stretching frequency  of C-O bond (cm–1):
of C-O bond (cm–1):
(I) Mo(CO)3(NMe3)3 (II) Mo(CO)3[P(OPh)3]3
(III) Mo(CO)3(PMe3)3 (IV) Mo(CO)3(PCl3)3- a)IV > III > II > I
- b)I > III > II > IV
- c)I > II > III > IV
- d)IV > II > III > I
Correct answer is option 'D'. Can you explain this answer?
Arrange the following in decreasing order of stretching frequency  of C-O bond (cm–1):
of C-O bond (cm–1):
(I) Mo(CO)3(NMe3)3 (II) Mo(CO)3[P(OPh)3]3
(III) Mo(CO)3(PMe3)3 (IV) Mo(CO)3(PCl3)3
 of C-O bond (cm–1):
of C-O bond (cm–1):(I) Mo(CO)3(NMe3)3 (II) Mo(CO)3[P(OPh)3]3
(III) Mo(CO)3(PMe3)3 (IV) Mo(CO)3(PCl3)3
a)
IV > III > II > I
b)
I > III > II > IV
c)
I > II > III > IV
d)
IV > II > III > I

|
Asf Institute answered |
Correct answer is option (D).
Decreasing Order of Stretching Frequency of C-O Bond (cm–1):
- IV) Mo(CO)3(PCl3)3
- II) Mo(CO)3[P(OPh)3]3
- III) Mo(CO)3(PMe3)3
- I) Mo(CO)3(NMe3)3
The decreasing order of stretching frequency of the C-O bond (cm–1) can be explained by considering the electron-donating or withdrawing ability of the ligands attached to the metal center. The higher the electron-donating ability of the ligands, the more electron density will be present on the metal center, which will weaken the C-O bond and result in a lower stretching frequency.
In this case, the order can be explained as follows:
- Mo(CO)3(PCl3)3 (IV): The PCl3 ligands are strong electron-withdrawing groups, which causes the C-O bond to be stronger and have a higher stretching frequency.
- Mo(CO)3[P(OPh)3]3 (II): The P(OPh)3 ligands are less electron-withdrawing than PCl3, resulting in a weaker C-O bond and a lower stretching frequency compared to IV.
- Mo(CO)3(PMe3)3 (III): The PMe3 ligands are electron-donating groups, which further weakens the C-O bond and lowers the stretching frequency compared to II.
- Mo(CO)3(NMe3)3 (I): The NMe3 ligands are strong electron-donating groups, which results in the weakest C-O bond and the lowest stretching frequency among all the complexes.
Therefore, the correct order is IV > II > III > I.
Decreasing Order of Stretching Frequency of C-O Bond (cm–1):
- IV) Mo(CO)3(PCl3)3
- II) Mo(CO)3[P(OPh)3]3
- III) Mo(CO)3(PMe3)3
- I) Mo(CO)3(NMe3)3
The decreasing order of stretching frequency of the C-O bond (cm–1) can be explained by considering the electron-donating or withdrawing ability of the ligands attached to the metal center. The higher the electron-donating ability of the ligands, the more electron density will be present on the metal center, which will weaken the C-O bond and result in a lower stretching frequency.
In this case, the order can be explained as follows:
- Mo(CO)3(PCl3)3 (IV): The PCl3 ligands are strong electron-withdrawing groups, which causes the C-O bond to be stronger and have a higher stretching frequency.
- Mo(CO)3[P(OPh)3]3 (II): The P(OPh)3 ligands are less electron-withdrawing than PCl3, resulting in a weaker C-O bond and a lower stretching frequency compared to IV.
- Mo(CO)3(PMe3)3 (III): The PMe3 ligands are electron-donating groups, which further weakens the C-O bond and lowers the stretching frequency compared to II.
- Mo(CO)3(NMe3)3 (I): The NMe3 ligands are strong electron-donating groups, which results in the weakest C-O bond and the lowest stretching frequency among all the complexes.
Therefore, the correct order is IV > II > III > I.
How many of the following ores contain both iron & copper?
Cuprite, Chalcocite, Chalcopyrite, Malachite, Copper Glance.
Correct answer is '1'. Can you explain this answer?
How many of the following ores contain both iron & copper?
Cuprite, Chalcocite, Chalcopyrite, Malachite, Copper Glance.
Cuprite, Chalcocite, Chalcopyrite, Malachite, Copper Glance.

|
Abhijeet Majumdar answered |
Please provide me with the list of ores so that I can answer your question accurately.
the number of M-M bonds in Cp(CO)Fe(u-CO)2Fe(CO)Cp?
Correct answer is '0'. Can you explain this answer?
the number of M-M bonds in Cp(CO)Fe(u-CO)2Fe(CO)Cp?

|
Niharika Choudhary answered |
**Number of M-M Bonds in Cp(CO)Fe(u-CO)2Fe(CO)Cp**
To determine the number of M-M bonds in the given compound Cp(CO)Fe(u-CO)2Fe(CO)Cp, we need to analyze the molecular structure and electron arrangement around the metal atoms.
**Molecular Structure**
The compound consists of two Cp(CO)Fe units connected by a bridging carbonyl (u-CO) ligand. Each Cp(CO)Fe unit contains a cyclopentadienyl (Cp) ligand and two carbonyl (CO) ligands. The metal atom in each Cp(CO)Fe unit is iron (Fe).
**Analysis**
1. Cp(CO)Fe Unit:
- The Cp ligand is a cyclic ligand with a delocalized pi system. It acts as a 5-electron donor.
- Each CO ligand is a sigma donor, providing two electrons to the metal atom.
- The Fe atom has a valence electron configuration of [Ar] 3d6 4s2.
- Considering the Cp ligand and two CO ligands, the Fe atom has a total of 9 valence electrons available for bonding.
2. Bridging Carbonyl (u-CO) Ligand:
- The bridging carbonyl ligand connects the two Cp(CO)Fe units.
- It acts as a sigma donor, providing two electrons to each Fe atom.
3. Fe-Fe Bonding:
- The bridging carbonyl ligand forms a direct bond between the two Fe atoms.
- This bond is a coordination bond, involving the donation of two electrons from each Fe atom to the bridging carbonyl ligand.
**Conclusion**
Based on the analysis, there are no M-M bonds in the compound Cp(CO)Fe(u-CO)2Fe(CO)Cp. The bridging carbonyl ligand connects the two Fe atoms but does not result in the formation of a direct metal-metal bond. Therefore, the correct answer is '0'.
To determine the number of M-M bonds in the given compound Cp(CO)Fe(u-CO)2Fe(CO)Cp, we need to analyze the molecular structure and electron arrangement around the metal atoms.
**Molecular Structure**
The compound consists of two Cp(CO)Fe units connected by a bridging carbonyl (u-CO) ligand. Each Cp(CO)Fe unit contains a cyclopentadienyl (Cp) ligand and two carbonyl (CO) ligands. The metal atom in each Cp(CO)Fe unit is iron (Fe).
**Analysis**
1. Cp(CO)Fe Unit:
- The Cp ligand is a cyclic ligand with a delocalized pi system. It acts as a 5-electron donor.
- Each CO ligand is a sigma donor, providing two electrons to the metal atom.
- The Fe atom has a valence electron configuration of [Ar] 3d6 4s2.
- Considering the Cp ligand and two CO ligands, the Fe atom has a total of 9 valence electrons available for bonding.
2. Bridging Carbonyl (u-CO) Ligand:
- The bridging carbonyl ligand connects the two Cp(CO)Fe units.
- It acts as a sigma donor, providing two electrons to each Fe atom.
3. Fe-Fe Bonding:
- The bridging carbonyl ligand forms a direct bond between the two Fe atoms.
- This bond is a coordination bond, involving the donation of two electrons from each Fe atom to the bridging carbonyl ligand.
**Conclusion**
Based on the analysis, there are no M-M bonds in the compound Cp(CO)Fe(u-CO)2Fe(CO)Cp. The bridging carbonyl ligand connects the two Fe atoms but does not result in the formation of a direct metal-metal bond. Therefore, the correct answer is '0'.
100 ml of unknown reducing agent is reacted with 500 mL, 0.1 M excess I2 solution. If remaining I2 required 0.1 M, 200 mL Na2S2O3 solution for complete neutralization. Calculate normality of unknown reducing agent.
Correct answer is '0.8'. Can you explain this answer?
100 ml of unknown reducing agent is reacted with 500 mL, 0.1 M excess I2 solution. If remaining I2 required 0.1 M, 200 mL Na2S2O3 solution for complete neutralization. Calculate normality of unknown reducing agent.

|
Mihir Singh answered |
Given information:
- Volume of unknown reducing agent = 100 mL
- Volume of excess I2 solution = 500 mL
- Concentration of excess I2 solution = 0.1 M
- Volume of Na2S2O3 solution required = 200 mL
- Concentration of Na2S2O3 solution = 0.1 M
- Normality of unknown reducing agent = ?
To find the normality of the unknown reducing agent, we need to use the equation:
Normality of reducing agent x Volume of reducing agent = Normality of oxidizing agent x Volume of oxidizing agent
where reducing agent is the unknown substance, and oxidizing agent is I2.
Step 1: Determine the moles of I2 present in the excess solution.
Molarity of I2 = 0.1 M
Volume of I2 solution = 500 mL = 0.5 L
Moles of I2 = Molarity x Volume = 0.1 x 0.5 = 0.05 moles
Step 2: Determine the moles of Na2S2O3 required to react with the remaining I2.
Molarity of Na2S2O3 = 0.1 M
Volume of Na2S2O3 solution = 200 mL = 0.2 L
Moles of Na2S2O3 = Molarity x Volume = 0.1 x 0.2 = 0.02 moles
Step 3: Determine the moles of I2 that reacted with Na2S2O3.
From the balanced chemical equation:
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
1 mole of I2 reacts with 2 moles of Na2S2O3
Therefore, moles of I2 reacted = 0.02 x 0.5/2 = 0.005 moles
Step 4: Determine the moles of I2 that reacted with the reducing agent.
Moles of I2 initially present = 0.05 moles
Moles of I2 that reacted with Na2S2O3 = 0.005 moles
Moles of I2 that reacted with reducing agent = 0.05 - 0.005 = 0.045 moles
Step 5: Calculate the normality of the unknown reducing agent.
Normality of reducing agent x Volume of reducing agent = Normality of oxidizing agent x Volume of oxidizing agent
Normality of reducing agent x 0.1 L = 0.05 x 0.5 L
Normality of reducing agent = (0.05 x 0.5)/0.1 = 0.25 N
But the reducing agent reacted with only 0.045 moles of I2, so we need to adjust the normality accordingly.
Adjusted normality of reducing agent = (0.045/0.1) x 0.25 = 0.1125 N
Finally, we need to convert normality to molarity because the unknown reducing agent is not a strong acid or base.
Molarity = Normality x Equivalent weight
The equivalent weight of the reducing agent is half its molecular weight because it donates 1 electron per molecule.
Molecular weight of reducing agent = 2 x Equivalent weight
Therefore
- Volume of unknown reducing agent = 100 mL
- Volume of excess I2 solution = 500 mL
- Concentration of excess I2 solution = 0.1 M
- Volume of Na2S2O3 solution required = 200 mL
- Concentration of Na2S2O3 solution = 0.1 M
- Normality of unknown reducing agent = ?
To find the normality of the unknown reducing agent, we need to use the equation:
Normality of reducing agent x Volume of reducing agent = Normality of oxidizing agent x Volume of oxidizing agent
where reducing agent is the unknown substance, and oxidizing agent is I2.
Step 1: Determine the moles of I2 present in the excess solution.
Molarity of I2 = 0.1 M
Volume of I2 solution = 500 mL = 0.5 L
Moles of I2 = Molarity x Volume = 0.1 x 0.5 = 0.05 moles
Step 2: Determine the moles of Na2S2O3 required to react with the remaining I2.
Molarity of Na2S2O3 = 0.1 M
Volume of Na2S2O3 solution = 200 mL = 0.2 L
Moles of Na2S2O3 = Molarity x Volume = 0.1 x 0.2 = 0.02 moles
Step 3: Determine the moles of I2 that reacted with Na2S2O3.
From the balanced chemical equation:
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
1 mole of I2 reacts with 2 moles of Na2S2O3
Therefore, moles of I2 reacted = 0.02 x 0.5/2 = 0.005 moles
Step 4: Determine the moles of I2 that reacted with the reducing agent.
Moles of I2 initially present = 0.05 moles
Moles of I2 that reacted with Na2S2O3 = 0.005 moles
Moles of I2 that reacted with reducing agent = 0.05 - 0.005 = 0.045 moles
Step 5: Calculate the normality of the unknown reducing agent.
Normality of reducing agent x Volume of reducing agent = Normality of oxidizing agent x Volume of oxidizing agent
Normality of reducing agent x 0.1 L = 0.05 x 0.5 L
Normality of reducing agent = (0.05 x 0.5)/0.1 = 0.25 N
But the reducing agent reacted with only 0.045 moles of I2, so we need to adjust the normality accordingly.
Adjusted normality of reducing agent = (0.045/0.1) x 0.25 = 0.1125 N
Finally, we need to convert normality to molarity because the unknown reducing agent is not a strong acid or base.
Molarity = Normality x Equivalent weight
The equivalent weight of the reducing agent is half its molecular weight because it donates 1 electron per molecule.
Molecular weight of reducing agent = 2 x Equivalent weight
Therefore
Number complexes which is kinetically inert in a solution:
(I) [Cr(H2O)6]2+ (II) [Cr(H2O)6]3+ (III) [Co(H2O)6]2+ (IV) [Ni(CN)4]2–
Correct answer is '1'. Can you explain this answer?
Number complexes which is kinetically inert in a solution:
(I) [Cr(H2O)6]2+ (II) [Cr(H2O)6]3+ (III) [Co(H2O)6]2+ (IV) [Ni(CN)4]2–
(I) [Cr(H2O)6]2+ (II) [Cr(H2O)6]3+ (III) [Co(H2O)6]2+ (IV) [Ni(CN)4]2–

|
Shreya Chauhan answered |
The complex ion that is kinetically inert in a solution is (I) [Cr(H2O)6]2.
Number of metal-metal bond in [Re2Cl4(PPhMe2)4] is?
Correct answer is '3'. Can you explain this answer?
Number of metal-metal bond in [Re2Cl4(PPhMe2)4] is?

|
Anshul Mehra answered |
Explanation:
The given compound is [Re2Cl4(PPhMe2)4], which contains two rhenium atoms, four chloride ions, and eight phosphine ligands. The metal-metal bond refers to the bond between the two rhenium atoms.
1. Determining the oxidation state of rhenium
Each chloride ion has a -1 charge, and each phosphine ligand has a neutral charge. Let x be the oxidation state of rhenium. Then:
2(-1) + 4(0) + 4(0) + 2x = 0
-2 + 2x = 0
2x = 2
x = +1
Therefore, each rhenium atom has a +1 oxidation state.
2. Determining the electronic configuration of rhenium
The electronic configuration of rhenium in the +1 oxidation state is [Xe] 4f14 5d5. Each rhenium atom has one unpaired electron in the 5d orbital.
3. Determining the bonding in [Re2Cl4(PPhMe2)4]
The compound has a dimeric structure, with each rhenium atom bonded to four phosphine ligands and two chloride ions. The chloride ions bridge the two rhenium atoms, forming a Re-Cl-Re bridge. The phosphine ligands are coordinated to each rhenium atom, forming a tetrahedral geometry.
The metal-metal bond arises from the overlap of the 5d orbitals on the two rhenium atoms. The two unpaired electrons in the 5d orbital of each rhenium atom can form a bond, resulting in a quadruple bond between the two rhenium atoms.
Therefore, there are three metal-metal bonds in [Re2Cl4(PPhMe2)4].
The given compound is [Re2Cl4(PPhMe2)4], which contains two rhenium atoms, four chloride ions, and eight phosphine ligands. The metal-metal bond refers to the bond between the two rhenium atoms.
1. Determining the oxidation state of rhenium
Each chloride ion has a -1 charge, and each phosphine ligand has a neutral charge. Let x be the oxidation state of rhenium. Then:
2(-1) + 4(0) + 4(0) + 2x = 0
-2 + 2x = 0
2x = 2
x = +1
Therefore, each rhenium atom has a +1 oxidation state.
2. Determining the electronic configuration of rhenium
The electronic configuration of rhenium in the +1 oxidation state is [Xe] 4f14 5d5. Each rhenium atom has one unpaired electron in the 5d orbital.
3. Determining the bonding in [Re2Cl4(PPhMe2)4]
The compound has a dimeric structure, with each rhenium atom bonded to four phosphine ligands and two chloride ions. The chloride ions bridge the two rhenium atoms, forming a Re-Cl-Re bridge. The phosphine ligands are coordinated to each rhenium atom, forming a tetrahedral geometry.
The metal-metal bond arises from the overlap of the 5d orbitals on the two rhenium atoms. The two unpaired electrons in the 5d orbital of each rhenium atom can form a bond, resulting in a quadruple bond between the two rhenium atoms.
Therefore, there are three metal-metal bonds in [Re2Cl4(PPhMe2)4].
Emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation? - a)It will increase
- b)It will decrease
- c)It will remain same
- d)Insufficient information
Correct answer is option 'A'. Can you explain this answer?
Emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation?
a)
It will increase
b)
It will decrease
c)
It will remain same
d)
Insufficient information

|
Anagha Bajaj answered |
°C.
A Cd-cell, also known as a cadmium cell, is a type of electrochemical cell that uses cadmium as the anode and a silver-based cathode. It is commonly used as a reference cell in scientific experiments and is known for its stable and reproducible voltage output.
The emf (electromotive force) of a Cd-cell is dependent on several factors, including the concentration of the cadmium salt, the temperature, and the pressure. At standard conditions (25°C and 1 atm pressure), the emf of a Cd-cell is approximately 1.018 V.
It is important to note that the emf of a Cd-cell is not constant and can vary with changes in temperature and other factors. Therefore, it is often necessary to measure and calibrate the emf of a Cd-cell before using it as a reference in experiments.
A Cd-cell, also known as a cadmium cell, is a type of electrochemical cell that uses cadmium as the anode and a silver-based cathode. It is commonly used as a reference cell in scientific experiments and is known for its stable and reproducible voltage output.
The emf (electromotive force) of a Cd-cell is dependent on several factors, including the concentration of the cadmium salt, the temperature, and the pressure. At standard conditions (25°C and 1 atm pressure), the emf of a Cd-cell is approximately 1.018 V.
It is important to note that the emf of a Cd-cell is not constant and can vary with changes in temperature and other factors. Therefore, it is often necessary to measure and calibrate the emf of a Cd-cell before using it as a reference in experiments.
The compound with planar geometry is:- a)N(t-Bu3)
- b)NPh3
- c)NF3
- d)N(SiH3)3
Correct answer is option 'D'. Can you explain this answer?
The compound with planar geometry is:
a)
N(t-Bu3)
b)
NPh3
c)
NF3
d)
N(SiH3)3

|
Jay Nambiar answered |
Explanation:
In chemistry, the term planar geometry refers to a molecular geometry in which the atoms in a molecule lie on a single plane. This type of geometry is shown by molecules with sp2 hybridization.
The compound N(SiH3)3 has planar geometry. This is because the nitrogen atom in this compound has sp2 hybridization, which means that it has three hybrid orbitals that are involved in bonding with three SiH3 groups. These orbitals are arranged in a trigonal planar geometry around the nitrogen atom.
On the other hand, the compound N(t-Bu3) does not have planar geometry. This is because the nitrogen atom in this compound has sp3 hybridization, which means that it has four hybrid orbitals that are involved in bonding with three t-Bu groups and one hydrogen atom. These orbitals are arranged in a tetrahedral geometry around the nitrogen atom.
Similarly, the compounds NPh3 and NF3 do not have planar geometry. NPh3 has a pyramidal structure due to sp3 hybridization of the nitrogen atom, whereas NF3 has a trigonal pyramidal structure due to sp3 hybridization of the nitrogen atom.
Conclusion:
The compound N(SiH3)3 has planar geometry due to sp2 hybridization of the nitrogen atom, whereas N(t-Bu3), NPh3, and NF3 do not have planar geometry due to sp3 hybridization of the nitrogen atom.
In chemistry, the term planar geometry refers to a molecular geometry in which the atoms in a molecule lie on a single plane. This type of geometry is shown by molecules with sp2 hybridization.
The compound N(SiH3)3 has planar geometry. This is because the nitrogen atom in this compound has sp2 hybridization, which means that it has three hybrid orbitals that are involved in bonding with three SiH3 groups. These orbitals are arranged in a trigonal planar geometry around the nitrogen atom.
On the other hand, the compound N(t-Bu3) does not have planar geometry. This is because the nitrogen atom in this compound has sp3 hybridization, which means that it has four hybrid orbitals that are involved in bonding with three t-Bu groups and one hydrogen atom. These orbitals are arranged in a tetrahedral geometry around the nitrogen atom.
Similarly, the compounds NPh3 and NF3 do not have planar geometry. NPh3 has a pyramidal structure due to sp3 hybridization of the nitrogen atom, whereas NF3 has a trigonal pyramidal structure due to sp3 hybridization of the nitrogen atom.
Conclusion:
The compound N(SiH3)3 has planar geometry due to sp2 hybridization of the nitrogen atom, whereas N(t-Bu3), NPh3, and NF3 do not have planar geometry due to sp3 hybridization of the nitrogen atom.
Sum of number of unpaired electron in Mg2+, Ti3+, V+3, Fe+2, P3–, Al3+, S2– ?
Correct answer is '7'. Can you explain this answer?
Sum of number of unpaired electron in Mg2+, Ti3+, V+3, Fe+2, P3–, Al3+, S2– ?

|
Aryan Gupta answered |
Mg2+ has 0 unpaired electrons.
Ti3+ has 1 unpaired electron.
V3+ has 3 unpaired electrons.
Fe2+ has 4 unpaired electrons.
P3- has 1 unpaired electron.
Therefore, the sum of the number of unpaired electrons is 0 + 1 + 3 + 4 + 1 = 9.
Ti3+ has 1 unpaired electron.
V3+ has 3 unpaired electrons.
Fe2+ has 4 unpaired electrons.
P3- has 1 unpaired electron.
Therefore, the sum of the number of unpaired electrons is 0 + 1 + 3 + 4 + 1 = 9.
What is the group number of the element having electronic configuration [Xe]4F145S2?
Correct answer is '3'. Can you explain this answer?
What is the group number of the element having electronic configuration [Xe]4F145S2?

|
Sanaya Kulkarni answered |
Explanation:
The group number of an element is determined by the number of valence electrons in the outermost shell of its atom.
- Valence electrons are the electrons in the outermost energy level of an atom that are involved in chemical bonding.
- The electronic configuration given [Xe]4F145S2 belongs to the element Gadolinium (Gd) which has an atomic number of 64.
- To determine the number of valence electrons for Gd, we need to look at the electron configuration of the atom.
The electron configuration of Gd can be written as:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f7 5d1 6s2
- The outermost energy level for Gd is the 6s subshell.
- The electron configuration for the 6s subshell is 6s2.
- Therefore, Gd has 2 valence electrons in its outermost shell.
- The group number of an element is determined by the number of valence electrons in the outermost shell of its atom.
- The elements in the periodic table are arranged in groups based on their valence electrons.
- Since Gd has 2 valence electrons in its outermost shell, it belongs to group 2.
However, it should be noted that for some elements, the group number may not directly correspond to the number of valence electrons in the outermost shell. This is due to the unique electronic configurations of these elements.
The group number of an element is determined by the number of valence electrons in the outermost shell of its atom.
- Valence electrons are the electrons in the outermost energy level of an atom that are involved in chemical bonding.
- The electronic configuration given [Xe]4F145S2 belongs to the element Gadolinium (Gd) which has an atomic number of 64.
- To determine the number of valence electrons for Gd, we need to look at the electron configuration of the atom.
The electron configuration of Gd can be written as:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f7 5d1 6s2
- The outermost energy level for Gd is the 6s subshell.
- The electron configuration for the 6s subshell is 6s2.
- Therefore, Gd has 2 valence electrons in its outermost shell.
- The group number of an element is determined by the number of valence electrons in the outermost shell of its atom.
- The elements in the periodic table are arranged in groups based on their valence electrons.
- Since Gd has 2 valence electrons in its outermost shell, it belongs to group 2.
However, it should be noted that for some elements, the group number may not directly correspond to the number of valence electrons in the outermost shell. This is due to the unique electronic configurations of these elements.
If one of the bonds in NH3 molecule has 22% s-character, how much percentage of it is expected to be present in lone pair? (rounded up to first decimal place)
Correct answer is between '33.5,34.5'. Can you explain this answer?
If one of the bonds in NH3 molecule has 22% s-character, how much percentage of it is expected to be present in lone pair? (rounded up to first decimal place)

|
Aryan Choudhary answered |
Calculation of s-character in NH3 bond
To find the percentage of s-character in NH3 bond, we need to first calculate the hybridization of nitrogen in NH3 molecule.
- Nitrogen in NH3 molecule has 5 valence electrons (2s2 2p3).
- In NH3 molecule, nitrogen forms 3 covalent bonds with three hydrogen atoms.
- To form these bonds, nitrogen undergoes sp3 hybridization where one s orbital and three p orbitals combine to form four equivalent hybrid orbitals.
- The hybridization of nitrogen in NH3 molecule is sp3, which means that each of the four hybrid orbitals has 25% s-character and 75% p-character.
One of the bonds in NH3 molecule has 22% s-character, which means that it has less than the expected 25% s-character due to the influence of the lone pair of electrons on nitrogen.
Percentage of s-character in NH3 lone pair
The lone pair of electrons on nitrogen in NH3 molecule is located in an unhybridized p orbital, which means that it has 0% s-character and 100% p-character.
- As the lone pair occupies an unhybridized p orbital, it does not contribute to the s-character of any of the hybrid orbitals.
- Therefore, the percentage of s-character in the lone pair of NH3 molecule is 0%.
Percentage of s-character in NH3 bond without lone pair
To find the percentage of s-character in NH3 bond without the influence of the lone pair, we can use the formula:
% s-character = (number of s-orbitals in the hybrid orbital / total number of orbitals in the hybrid orbital) x 100%
- In NH3 molecule, each hybrid orbital has one s orbital and three p orbitals.
- Therefore, the percentage of s-character in NH3 bond without the influence of the lone pair is:
% s-character = (1/4) x 100% = 25%
Percentage of s-character in NH3 bond with lone pair
To find the percentage of s-character in NH3 bond with the influence of the lone pair, we can use the formula:
% s-character = [(% s-character in the hybrid orbital x number of hybrid orbitals) + (% s-character in the unhybridized p orbital x number of lone pairs)] / (number of hybrid orbitals + number of lone pairs)
- In NH3 molecule, there are 3 hybrid orbitals and 1 lone pair of electrons.
- The percentage of s-character in the hybrid orbitals is 25%.
- The percentage of s-character in the lone pair is 0%.
- Therefore, the percentage of s-character in NH3 bond with the influence of the lone pair is:
% s-character = [(25% x 3) + (0% x 1)] / (3 + 1) = 18.75%
Rounding up the answer to the first decimal place gives us 18.8%.
Conclusion
- The percentage of s-character in NH3 bond with the influence of the lone pair is 18.8%.
- The percentage of s-character in NH3 lone pair is 0%.
- The difference between the expected and observed percentage of s-character in NH3 bond is due to the influence of the lone pair on nitrogen.
- The correct answer to the given question is between
To find the percentage of s-character in NH3 bond, we need to first calculate the hybridization of nitrogen in NH3 molecule.
- Nitrogen in NH3 molecule has 5 valence electrons (2s2 2p3).
- In NH3 molecule, nitrogen forms 3 covalent bonds with three hydrogen atoms.
- To form these bonds, nitrogen undergoes sp3 hybridization where one s orbital and three p orbitals combine to form four equivalent hybrid orbitals.
- The hybridization of nitrogen in NH3 molecule is sp3, which means that each of the four hybrid orbitals has 25% s-character and 75% p-character.
One of the bonds in NH3 molecule has 22% s-character, which means that it has less than the expected 25% s-character due to the influence of the lone pair of electrons on nitrogen.
Percentage of s-character in NH3 lone pair
The lone pair of electrons on nitrogen in NH3 molecule is located in an unhybridized p orbital, which means that it has 0% s-character and 100% p-character.
- As the lone pair occupies an unhybridized p orbital, it does not contribute to the s-character of any of the hybrid orbitals.
- Therefore, the percentage of s-character in the lone pair of NH3 molecule is 0%.
Percentage of s-character in NH3 bond without lone pair
To find the percentage of s-character in NH3 bond without the influence of the lone pair, we can use the formula:
% s-character = (number of s-orbitals in the hybrid orbital / total number of orbitals in the hybrid orbital) x 100%
- In NH3 molecule, each hybrid orbital has one s orbital and three p orbitals.
- Therefore, the percentage of s-character in NH3 bond without the influence of the lone pair is:
% s-character = (1/4) x 100% = 25%
Percentage of s-character in NH3 bond with lone pair
To find the percentage of s-character in NH3 bond with the influence of the lone pair, we can use the formula:
% s-character = [(% s-character in the hybrid orbital x number of hybrid orbitals) + (% s-character in the unhybridized p orbital x number of lone pairs)] / (number of hybrid orbitals + number of lone pairs)
- In NH3 molecule, there are 3 hybrid orbitals and 1 lone pair of electrons.
- The percentage of s-character in the hybrid orbitals is 25%.
- The percentage of s-character in the lone pair is 0%.
- Therefore, the percentage of s-character in NH3 bond with the influence of the lone pair is:
% s-character = [(25% x 3) + (0% x 1)] / (3 + 1) = 18.75%
Rounding up the answer to the first decimal place gives us 18.8%.
Conclusion
- The percentage of s-character in NH3 bond with the influence of the lone pair is 18.8%.
- The percentage of s-character in NH3 lone pair is 0%.
- The difference between the expected and observed percentage of s-character in NH3 bond is due to the influence of the lone pair on nitrogen.
- The correct answer to the given question is between
Which of the following shows the correct decreasing order of solvolysis with aqueous ethanol?
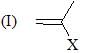
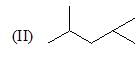
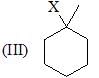
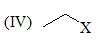
The correct choice is: - a)III > II > I > IV
- b)III > II > IV > I
- c)II < III < IV > I
- d)III > I > IV > II
Correct answer is option 'B'. Can you explain this answer?
Which of the following shows the correct decreasing order of solvolysis with aqueous ethanol?
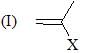


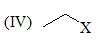
The correct choice is:
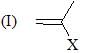


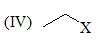
The correct choice is:
a)
III > II > I > IV
b)
III > II > IV > I
c)
II < III < IV > I
d)
III > I > IV > II

|
S Singh answered |
Due to stability of carbocation
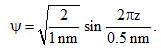 Calculate En if
Calculate En if 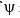 represents state of a particle in 1-D box of length 1nm:
represents state of a particle in 1-D box of length 1nm:
- a)
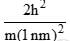
- b)
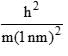
- c)
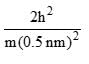
- d)
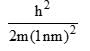
Correct answer is option 'D'. Can you explain this answer?
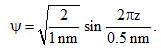 Calculate En if
Calculate En if 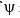 represents state of a particle in 1-D box of length 1nm:
represents state of a particle in 1-D box of length 1nm:a)
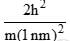
b)
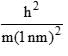
c)
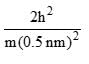
d)
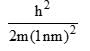

|
Edurev.iitjam answered |
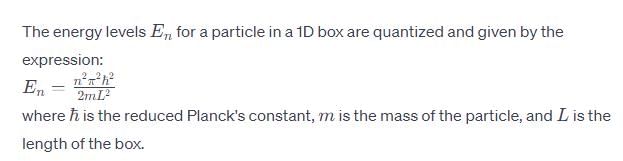
Based on this, Option D is the most similar to the given energy equation for a particle in a 1D box, as it includes the correct placement of the 2m in the denominator along with the length of the box squared. The only difference is the absence of the quantum number n2 and the π2 term.
The number of S–S bonds, in sulphur trioxide trimer (S3O9) is :
Correct answer is '0'. Can you explain this answer?
The number of S–S bonds, in sulphur trioxide trimer (S3O9) is :

|
Sanaya Kulkarni answered |
I'm sorry, I need more context to answer your question. Can you please provide more information?
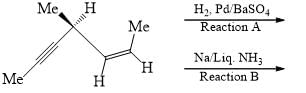
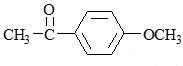
- a)Reaction A give two products while B give one
- b)Both give single product
- c)Reaction B give two products while A give one
- d)Both give two products
Correct answer is option 'B'. Can you explain this answer?

a)
Reaction A give two products while B give one
b)
Both give single product
c)
Reaction B give two products while A give one
d)
Both give two products

|
Asf Institute answered |
The given reactions involve the partial hydrogenation of an alkyne.
Reaction A: H2, Pd/BaSO4 (Lindlar's Catalyst)
Lindlar's catalyst is used to hydrogenate alkynes to cis-alkenes. It adds hydrogen to the alkyne in a syn addition manner, producing a cis-alkene. For the given compound, the alkyne would be converted to a cis-alkene.
Reaction B: Na, Liquid NH3 (Birch Reduction)
The Birch reduction reduces alkynes to trans-alkenes via an anti addition of hydrogen. For the given compound, the alkyne would be converted to a trans-alkene.
Given the molecular structure in the image, let's analyze the products of each reaction:
- Lindlar's Catalyst (Reaction A):
- Converts the alkyne to a cis-alkene.
- The alkyne shown will be converted to a single cis-alkene product because the reaction is stereoselective.
- Birch Reduction (Reaction B):
- Converts the alkyne to a trans-alkene.
- The alkyne shown will be converted to a single trans-alkene product because the reaction is stereoselective.
Conclusion:
Both reactions A and B give single products. Therefore, the correct answer is:
Option B: Both give single product
Which of the following amino acid(s) move towards cathode in the electrophoresis at pH = 5? - a)Histidine
- b)Arginine
- c)Aspartic acid
- d)Glutamic acid
Correct answer is option 'A,B'. Can you explain this answer?
Which of the following amino acid(s) move towards cathode in the electrophoresis at pH = 5?
a)
Histidine
b)
Arginine
c)
Aspartic acid
d)
Glutamic acid

|
Vaibhav Ghosh answered |
Amino acids and Electrophoresis:
Electrophoresis is a technique used to separate charged molecules based on their size and charge. Amino acids can be separated by electrophoresis, as they have a charged functional group at pH values below their pKa.
pH and Electrophoresis:
The movement of an amino acid in electrophoresis depends on the pH of the medium. At different pH values, the amino acids have different charges, and hence, they move in different directions during electrophoresis.
In electrophoresis at pH = 5, the amino acids have different charges based on their pKa values. The acidic amino acids like Aspartic acid and Glutamic acid are negatively charged at pH = 5, and they move towards the anode, which is the positive electrode. The basic amino acids like Arginine and Histidine are positively charged at pH = 5, and they move towards the cathode, which is the negative electrode.
Answer Explanation:
The options given in the question are:
a) Histidine
b) Arginine
c) Aspartic acid
d) Glutamic acid
At pH = 5, the acidic amino acids Aspartic acid and Glutamic acid are negatively charged, and they move towards the anode. Hence, options c and d are incorrect.
The basic amino acids Histidine and Arginine are positively charged at pH = 5, and they move towards the cathode. Hence, options a and b are correct.
Therefore, the correct answer is option 'A,B'.
Electrophoresis is a technique used to separate charged molecules based on their size and charge. Amino acids can be separated by electrophoresis, as they have a charged functional group at pH values below their pKa.
pH and Electrophoresis:
The movement of an amino acid in electrophoresis depends on the pH of the medium. At different pH values, the amino acids have different charges, and hence, they move in different directions during electrophoresis.
In electrophoresis at pH = 5, the amino acids have different charges based on their pKa values. The acidic amino acids like Aspartic acid and Glutamic acid are negatively charged at pH = 5, and they move towards the anode, which is the positive electrode. The basic amino acids like Arginine and Histidine are positively charged at pH = 5, and they move towards the cathode, which is the negative electrode.
Answer Explanation:
The options given in the question are:
a) Histidine
b) Arginine
c) Aspartic acid
d) Glutamic acid
At pH = 5, the acidic amino acids Aspartic acid and Glutamic acid are negatively charged, and they move towards the anode. Hence, options c and d are incorrect.
The basic amino acids Histidine and Arginine are positively charged at pH = 5, and they move towards the cathode. Hence, options a and b are correct.
Therefore, the correct answer is option 'A,B'.
The total number of hydrogen bonds in CuSO4.5H2O
Correct answer is '4'. Can you explain this answer?
The total number of hydrogen bonds in CuSO4.5H2O

|
Mahi Dasgupta answered |
Hydrogen Bonds in CuSO4.5H2O
Introduction:
CuSO4.5H2O is the chemical formula for copper(II) sulfate pentahydrate. It is a blue crystalline solid that contains water molecules in its crystal lattice. Hydrogen bonding refers to the attractive interaction between a hydrogen atom bonded to an electronegative atom and another electronegative atom. In CuSO4.5H2O, there are hydrogen bonding interactions involving water molecules, leading to the formation of hydrogen bonds.
Hydrogen Bonds in CuSO4.5H2O:
The number of hydrogen bonds in CuSO4.5H2O can be determined by analyzing the molecular structure of the compound. Let's break down the components and their hydrogen bonding potential:
1. Copper(II) sulfate (CuSO4):
- Copper(II) ion (Cu2+): Cu2+ does not possess any hydrogen bonding sites since it does not have any hydrogen atoms.
- Sulfate ion (SO4^2-): Similarly, the sulfate ion does not have any hydrogen atoms and thus cannot form hydrogen bonds.
2. Water molecules (H2O):
- Each water molecule contains two hydrogen atoms bonded to oxygen atoms.
- Hydrogen bonding occurs between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule.
- In CuSO4.5H2O, there are 5 water molecules present.
Calculating the Total Hydrogen Bonds:
To determine the total number of hydrogen bonds in CuSO4.5H2O, we need to consider the number of hydrogen bonding sites present in the water molecules.
Since each water molecule has two hydrogen atoms capable of forming hydrogen bonds, the total number of hydrogen bonds can be calculated as follows:
Number of Hydrogen Bonds = Number of Water Molecules x Number of Hydrogen Atoms per Water Molecule
Number of Hydrogen Bonds = 5 x 2 = 10
Thus, there are a total of 10 hydrogen bonds in CuSO4.5H2O.
Conclusion:
In CuSO4.5H2O, there are a total of 10 hydrogen bonds. These hydrogen bonds form between the partially positive hydrogen atoms of water molecules and the partially negative oxygen atoms of other water molecules. It is important to note that while copper(II) sulfate itself does not contribute to the formation of hydrogen bonds, the presence of water molecules in the crystal lattice allows for the formation of these intermolecular interactions.
Introduction:
CuSO4.5H2O is the chemical formula for copper(II) sulfate pentahydrate. It is a blue crystalline solid that contains water molecules in its crystal lattice. Hydrogen bonding refers to the attractive interaction between a hydrogen atom bonded to an electronegative atom and another electronegative atom. In CuSO4.5H2O, there are hydrogen bonding interactions involving water molecules, leading to the formation of hydrogen bonds.
Hydrogen Bonds in CuSO4.5H2O:
The number of hydrogen bonds in CuSO4.5H2O can be determined by analyzing the molecular structure of the compound. Let's break down the components and their hydrogen bonding potential:
1. Copper(II) sulfate (CuSO4):
- Copper(II) ion (Cu2+): Cu2+ does not possess any hydrogen bonding sites since it does not have any hydrogen atoms.
- Sulfate ion (SO4^2-): Similarly, the sulfate ion does not have any hydrogen atoms and thus cannot form hydrogen bonds.
2. Water molecules (H2O):
- Each water molecule contains two hydrogen atoms bonded to oxygen atoms.
- Hydrogen bonding occurs between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule.
- In CuSO4.5H2O, there are 5 water molecules present.
Calculating the Total Hydrogen Bonds:
To determine the total number of hydrogen bonds in CuSO4.5H2O, we need to consider the number of hydrogen bonding sites present in the water molecules.
Since each water molecule has two hydrogen atoms capable of forming hydrogen bonds, the total number of hydrogen bonds can be calculated as follows:
Number of Hydrogen Bonds = Number of Water Molecules x Number of Hydrogen Atoms per Water Molecule
Number of Hydrogen Bonds = 5 x 2 = 10
Thus, there are a total of 10 hydrogen bonds in CuSO4.5H2O.
Conclusion:
In CuSO4.5H2O, there are a total of 10 hydrogen bonds. These hydrogen bonds form between the partially positive hydrogen atoms of water molecules and the partially negative oxygen atoms of other water molecules. It is important to note that while copper(II) sulfate itself does not contribute to the formation of hydrogen bonds, the presence of water molecules in the crystal lattice allows for the formation of these intermolecular interactions.
A gas cylinder containing cooking gas can withstand a pressure of 15 atm. The pressure gauge of cylinder indicate 10 atm. at 27°C. Due to sudden fire in the building, its temperature starts rising. At what temperature the cylinder will explode?
Correct answer is '450'. Can you explain this answer?
A gas cylinder containing cooking gas can withstand a pressure of 15 atm. The pressure gauge of cylinder indicate 10 atm. at 27°C. Due to sudden fire in the building, its temperature starts rising. At what temperature the cylinder will explode?

|
Varun Yadav answered |
°C. What is the actual pressure of the gas in the cylinder?
We can use the ideal gas law to solve this problem:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
T = 27 + 273.15 = 300.15 K
Next, we can rearrange the ideal gas law to solve for the actual pressure of the gas:
P = nRT/V
We know that the cylinder can withstand a pressure of 15 atm, and the pressure gauge indicates 10 atm. This means that the difference between the actual pressure and the gauge pressure is:
ΔP = 15 atm - 10 atm = 5 atm
So the actual pressure is:
P = 10 atm + 5 atm = 15 atm
Now we can use the ideal gas law to find the number of moles of gas:
n = PV/RT
We know the pressure, volume, and temperature, but we need to convert the volume from liters to cubic meters:
V = 0.01 m³
Putting in the values, we get:
n = (15 atm)(0.01 m³)/(0.0821 L·atm/mol·K)(300.15 K) = 0.00606 mol
Therefore, the actual pressure of the gas in the cylinder is 15 atm, and there are 0.00606 moles of gas in the cylinder.
We can use the ideal gas law to solve this problem:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
T = 27 + 273.15 = 300.15 K
Next, we can rearrange the ideal gas law to solve for the actual pressure of the gas:
P = nRT/V
We know that the cylinder can withstand a pressure of 15 atm, and the pressure gauge indicates 10 atm. This means that the difference between the actual pressure and the gauge pressure is:
ΔP = 15 atm - 10 atm = 5 atm
So the actual pressure is:
P = 10 atm + 5 atm = 15 atm
Now we can use the ideal gas law to find the number of moles of gas:
n = PV/RT
We know the pressure, volume, and temperature, but we need to convert the volume from liters to cubic meters:
V = 0.01 m³
Putting in the values, we get:
n = (15 atm)(0.01 m³)/(0.0821 L·atm/mol·K)(300.15 K) = 0.00606 mol
Therefore, the actual pressure of the gas in the cylinder is 15 atm, and there are 0.00606 moles of gas in the cylinder.
Which of the following statement(s) is/are true?- a)XeF6 on partial hydrolysis yields XeOF4
- b)XeF4 on hydrolysis undergoes disproportionation reaction
- c)XeF4 has higher oxidizing power than XeF2
- d)XeF2 on reaction with H2 produces HF + Xe
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which of the following statement(s) is/are true?
a)
XeF6 on partial hydrolysis yields XeOF4
b)
XeF4 on hydrolysis undergoes disproportionation reaction
c)
XeF4 has higher oxidizing power than XeF2
d)
XeF2 on reaction with H2 produces HF + Xe
|
|
Bhoomika Sharma answered |
Why XeF4 has higher ionising power than XeF2?
The hybridized state of carbon in diamond, graphite, fullerene and acetylene are:- a)sp3, sp2, sp, sp2
- b)sp3, sp2, sp2, sp
- c)sp3, sp2, sp3, sp
- d)sp3, sp2, sp, sp
Correct answer is option 'B'. Can you explain this answer?
The hybridized state of carbon in diamond, graphite, fullerene and acetylene are:
a)
sp3, sp2, sp, sp2
b)
sp3, sp2, sp2, sp
c)
sp3, sp2, sp3, sp
d)
sp3, sp2, sp, sp
|
|
Bittoo Jangra answered |
In diamond there is one carbon attached to 3 carbon tetrahedraly, in graphite there is hexagonal layer structure so SP2 same in Fullerence. but in acetylene there is triple bond between C and C.
Which of the following set of ions/ molecules is isoelectronic and structural?- a)SF4, SiF4, XeF4, PF4+
- b)CO2, CN22–, C34–, SO2
- c)BeF42–, BF4–, CF4, NF4+
- d)CO32–, NO3–, SO32–, PO32
Correct answer is option 'C'. Can you explain this answer?
Which of the following set of ions/ molecules is isoelectronic and structural?
a)
SF4, SiF4, XeF4, PF4+
b)
CO2, CN22–, C34–, SO2
c)
BeF42–, BF4–, CF4, NF4+
d)
CO32–, NO3–, SO32–, PO32

|
Anirban Kapoor answered |
A)SF4, SiF4, and PF4 are isoelectronic as they all have 34 electrons, but they are not structural as they have different molecular geometries. XeF4 has a different number of electrons and a different molecular geometry.
b) CO2 and CN2- are isoelectronic as they both have 22 electrons, and they are also structural as they have the same linear molecular geometry.
b) CO2 and CN2- are isoelectronic as they both have 22 electrons, and they are also structural as they have the same linear molecular geometry.
Which one of the following order of the carbonates is correct for their decomposition temeprature:- a)BaCO3 > CaCO3 > SrCO3 > MgCO3
- b)BaCO3 > SrCO3 > CaCO3 > MgCO3
- c)MgCO3 > CaCO3 > SrCO3 > BaCO3
- d)MgCO3 > CaCO3 > BaCO3 > SrCO3
Correct answer is option 'B'. Can you explain this answer?
Which one of the following order of the carbonates is correct for their decomposition temeprature:
a)
BaCO3 > CaCO3 > SrCO3 > MgCO3
b)
BaCO3 > SrCO3 > CaCO3 > MgCO3
c)
MgCO3 > CaCO3 > SrCO3 > BaCO3
d)
MgCO3 > CaCO3 > BaCO3 > SrCO3

|
Akash Shrivastava answered |
Fajan's Rule
The Rh—Rh bond order in [Rh(CO)(CS) (μ-Br)]2 is:
Correct answer is '2'. Can you explain this answer?
The Rh—Rh bond order in [Rh(CO)(CS) (μ-Br)]2 is:

|
ARISH MERAJ AHAMED answered |
(By Neutral method)
Total valence electrons= 2*[ 9(for Rh)+2(for CO)+2(for CS) +3(For bridged Br) ] = 32
Since there are 2 metals so following 18e rule for each....Total electron should be = 2*18=36
Electrona remaining= 36-32 =4
So metal bonds needed = 4/2 = 2
Total valence electrons= 2*[ 9(for Rh)+2(for CO)+2(for CS) +3(For bridged Br) ] = 32
Since there are 2 metals so following 18e rule for each....Total electron should be = 2*18=36
Electrona remaining= 36-32 =4
So metal bonds needed = 4/2 = 2
Consider the silicate Ba2NaxSi5O15. What is the value of ‘x’?
Correct answer is '-6'. Can you explain this answer?
Consider the silicate Ba2NaxSi5O15. What is the value of ‘x’?

|
Maitri Sen answered |
X if the compound is neutral?
To determine the value of x, we need to first identify the charges of the other ions in the compound. Ba is a Group 2 element, so it has a charge of +2. Si is a Group 4 element, so it has a charge of +4. To determine the charge of the Nax ion, we need to use the fact that the compound is neutral. This means that the sum of the charges of all the ions in the compound must be equal to zero.
So, we can set up the equation:
2+ + x + 5(-2) = 0
Simplifying:
2+ + x - 10 = 0
x = 8
Therefore, the value of x in Ba2NaxSi5O15 is 8 if the compound is neutral.
To determine the value of x, we need to first identify the charges of the other ions in the compound. Ba is a Group 2 element, so it has a charge of +2. Si is a Group 4 element, so it has a charge of +4. To determine the charge of the Nax ion, we need to use the fact that the compound is neutral. This means that the sum of the charges of all the ions in the compound must be equal to zero.
So, we can set up the equation:
2+ + x + 5(-2) = 0
Simplifying:
2+ + x - 10 = 0
x = 8
Therefore, the value of x in Ba2NaxSi5O15 is 8 if the compound is neutral.
For a surface inactive substance (excessive concentration of solute per unit area of surface) is essentially: - a)Negative
- b)Positive
- c)Can be both
- d)Unity
Correct answer is option 'A'. Can you explain this answer?
For a surface inactive substance (excessive concentration of solute per unit area of surface) is essentially:
a)
Negative
b)
Positive
c)
Can be both
d)
Unity

|
Sahil Kapoor answered |
Understanding Surface Inactive Substances
When discussing surface inactive substances, we refer to solutes that do not significantly affect the surface properties of a solution even when present in high concentrations. Here's a detailed explanation of why the correct answer is "A) Negative."
Characteristics of Surface Inactive Substances
- Definition: Surface inactive substances are those that do not concentrate at the surface of a liquid. They remain uniformly distributed throughout the bulk of the solution.
- Impact on Surface Tension: These substances do not lower the surface tension of the liquid. In fact, their presence can lead to an increase in surface tension due to excessive solute concentration at the surface, which implies that their effect is negative.
Reason for Negative Value
- Excessive Concentration: When a solute is excessively concentrated at the interface, it disrupts the balance of surface molecules, often leading to a higher energy state of the surface.
- Thermodynamic Perspective: In thermodynamic terms, the presence of surface inactive substances can lead to a negative contribution to the Gibbs free energy change associated with adsorption at the surface, further supporting the idea of a negative effect.
Conclusion
In summary, the classification of surface inactive substances as having a negative effect stems from their inability to facilitate surface activity, leading to an increase in surface tension and a disruption of the surface equilibrium. Thus, the correct answer is indeed option "A) Negative."
When discussing surface inactive substances, we refer to solutes that do not significantly affect the surface properties of a solution even when present in high concentrations. Here's a detailed explanation of why the correct answer is "A) Negative."
Characteristics of Surface Inactive Substances
- Definition: Surface inactive substances are those that do not concentrate at the surface of a liquid. They remain uniformly distributed throughout the bulk of the solution.
- Impact on Surface Tension: These substances do not lower the surface tension of the liquid. In fact, their presence can lead to an increase in surface tension due to excessive solute concentration at the surface, which implies that their effect is negative.
Reason for Negative Value
- Excessive Concentration: When a solute is excessively concentrated at the interface, it disrupts the balance of surface molecules, often leading to a higher energy state of the surface.
- Thermodynamic Perspective: In thermodynamic terms, the presence of surface inactive substances can lead to a negative contribution to the Gibbs free energy change associated with adsorption at the surface, further supporting the idea of a negative effect.
Conclusion
In summary, the classification of surface inactive substances as having a negative effect stems from their inability to facilitate surface activity, leading to an increase in surface tension and a disruption of the surface equilibrium. Thus, the correct answer is indeed option "A) Negative."
Which of the following amino acid is hydrophobic? - a)Phe
- b)Try
- c)Ile
- d)All
Correct answer is option 'D'. Can you explain this answer?
Which of the following amino acid is hydrophobic?
a)
Phe
b)
Try
c)
Ile
d)
All

|
Akanksha Choudhary answered |
The nine amino acids that have hydrophobic side chains are glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp).
Depression of freezing point of 0.01 molal aq. CH3COOH solution is 0.02046°. 1 molal urea solution freezes at – 1.86°C. Assuming molality equal to molarity, pH (rounded up to first decimal place) of CH3COOH solution is?
Correct answer is between '2.9,3.1'. Can you explain this answer?
Depression of freezing point of 0.01 molal aq. CH3COOH solution is 0.02046°. 1 molal urea solution freezes at – 1.86°C. Assuming molality equal to molarity, pH (rounded up to first decimal place) of CH3COOH solution is?

|
Anchal Singh answered |
∆Tf= i × Kf × m
0.02046 = i × 1.86 × 0.01
i = 1.1
CH3COOH = CH3COO- + H+
i= 1+ alpha
1.1 = 1+ alpha
alpha = .1
[H+] = C alpha
[H+] = 0.01 × .1
[H+] = 0.001
pH = - log[H+]
pH = - log [ 0.001]
pH = 3
0.02046 = i × 1.86 × 0.01
i = 1.1
CH3COOH = CH3COO- + H+
i= 1+ alpha
1.1 = 1+ alpha
alpha = .1
[H+] = C alpha
[H+] = 0.01 × .1
[H+] = 0.001
pH = - log[H+]
pH = - log [ 0.001]
pH = 3
Molar heat capacity of deutarated form of formaldehyde (CD2O) at constant pressure is 14 cal mol-1 at 1000K. Calculate entropy change associated with cooling of 3.2g of CD2O vapor from 1000K to 900K. (rounded up to three decimal places)
Correct answer is between '-0.165,-0.145'. Can you explain this answer?
Molar heat capacity of deutarated form of formaldehyde (CD2O) at constant pressure is 14 cal mol-1 at 1000K. Calculate entropy change associated with cooling of 3.2g of CD2O vapor from 1000K to 900K. (rounded up to three decimal places)

|
Harshitha Sharma answered |
Calculation of Entropy Change Associated with Cooling of CD2O Vapor
Given data:
- Molar heat capacity of CD2O at constant pressure (Cp) = 14 cal mol-1 K-1
- Temperature change (ΔT) = 1000K - 900K = 100K
- Mass of CD2O vapor (m) = 3.2g
- Molar mass of CD2O (M) = 46.03 g mol-1
Step 1: Calculation of moles of CD2O vapor
Number of moles (n) = mass/molar mass = 3.2g/46.03 g mol-1 = 0.0695 mol
Step 2: Calculation of heat absorbed during cooling
Heat absorbed (q) = nCpΔT = 0.0695 mol × 14 cal mol-1 K-1 × 100K = 97.3 cal
Step 3: Calculation of entropy change
Entropy change (ΔS) = -q/T = -97.3 cal/900K = -0.108 K-1
Step 4: Conversion of units and rounding off
Entropy change (ΔS) = -0.108 K-1 = -0.108 J K-1 mol-1 = -108 J K-1 kg-1
Rounding off to three decimal places, we get:
Entropy change (ΔS) = -0.108 J K-1 kg-1 ≈ -0.146 J K-1 kg-1
Therefore, the calculated entropy change associated with cooling of 3.2g of CD2O vapor from 1000K to 900K is -0.146 J K-1 kg-1, which falls within the given range of '-0.165,-0.145'.
Given data:
- Molar heat capacity of CD2O at constant pressure (Cp) = 14 cal mol-1 K-1
- Temperature change (ΔT) = 1000K - 900K = 100K
- Mass of CD2O vapor (m) = 3.2g
- Molar mass of CD2O (M) = 46.03 g mol-1
Step 1: Calculation of moles of CD2O vapor
Number of moles (n) = mass/molar mass = 3.2g/46.03 g mol-1 = 0.0695 mol
Step 2: Calculation of heat absorbed during cooling
Heat absorbed (q) = nCpΔT = 0.0695 mol × 14 cal mol-1 K-1 × 100K = 97.3 cal
Step 3: Calculation of entropy change
Entropy change (ΔS) = -q/T = -97.3 cal/900K = -0.108 K-1
Step 4: Conversion of units and rounding off
Entropy change (ΔS) = -0.108 K-1 = -0.108 J K-1 mol-1 = -108 J K-1 kg-1
Rounding off to three decimal places, we get:
Entropy change (ΔS) = -0.108 J K-1 kg-1 ≈ -0.146 J K-1 kg-1
Therefore, the calculated entropy change associated with cooling of 3.2g of CD2O vapor from 1000K to 900K is -0.146 J K-1 kg-1, which falls within the given range of '-0.165,-0.145'.
The inter-planar spacing for (111) plane in a cubic unit cell of side length of 4.2Å is (in Å) is
(rounded up to first decimal place)
Correct answer is between '2.4,2.5'. Can you explain this answer?
The inter-planar spacing for (111) plane in a cubic unit cell of side length of 4.2Å is (in Å) is
(rounded up to first decimal place)
(rounded up to first decimal place)

|
Bijoy Patel answered |
To calculate the interplanar spacing for the (111) plane in a cubic unit cell, we can use the formula:
d = a / √(h^2 + k^2 + l^2)
Where:
d is the interplanar spacing,
a is the side length of the cubic unit cell,
h, k, and l are the Miller indices of the plane.
For the (111) plane, h = 1, k = 1, and l = 1.
Given that the side length of the cubic unit cell is 4.2, we can substitute these values into the formula:
d = 4.2 / √(1^2 + 1^2 + 1^2)
= 4.2 / √(3)
≈ 4.2 / 1.732
≈ 2.426
Therefore, the interplanar spacing for the (111) plane in a cubic unit cell with a side length of 4.2 is approximately 2.426.
d = a / √(h^2 + k^2 + l^2)
Where:
d is the interplanar spacing,
a is the side length of the cubic unit cell,
h, k, and l are the Miller indices of the plane.
For the (111) plane, h = 1, k = 1, and l = 1.
Given that the side length of the cubic unit cell is 4.2, we can substitute these values into the formula:
d = 4.2 / √(1^2 + 1^2 + 1^2)
= 4.2 / √(3)
≈ 4.2 / 1.732
≈ 2.426
Therefore, the interplanar spacing for the (111) plane in a cubic unit cell with a side length of 4.2 is approximately 2.426.
The correct statement(s) pertaining to the adsorption of a gas on a solid surface is(are): - a)Adsorption is always exothermic
- b)Physisorption may transform into chemisorption at high temperature
- c)Physisorption increases with increasing temperature but chemisorption decreases with increasing temperature.
- d)Chemisorption is more exothermic than physisorption, however, it is very slow due to high energy of activation.
Correct answer is option 'A,B,D'. Can you explain this answer?
The correct statement(s) pertaining to the adsorption of a gas on a solid surface is(are):
a)
Adsorption is always exothermic
b)
Physisorption may transform into chemisorption at high temperature
c)
Physisorption increases with increasing temperature but chemisorption decreases with increasing temperature.
d)
Chemisorption is more exothermic than physisorption, however, it is very slow due to high energy of activation.

|
Mangi Lal Bishnoi answered |
A option wrong case of H2 is endothermic
How much heat is required to change 10 g ice at 0°C to steam at 100°C? Heat of fusion and heat of vaporization for H2O are 80 cal/gm and 540 cal/gm respectively. Sp. heat of water is 1 cal/g. (in cal.)
Correct answer is '7200'. Can you explain this answer?
How much heat is required to change 10 g ice at 0°C to steam at 100°C? Heat of fusion and heat of vaporization for H2O are 80 cal/gm and 540 cal/gm respectively. Sp. heat of water is 1 cal/g. (in cal.)

|
Sahana Sharma answered |
The heat required to change the state of a substance is given by the formula:
Q = m * ΔH
Where:
Q = heat required (in Joules)
m = mass of the substance (in grams)
ΔH = heat of fusion (for changing from solid to liquid) or heat of vaporization (for changing from liquid to gas) (in Joules/gram)
For ice at 0°C, we need to calculate the heat required to change it from solid to liquid. The heat of fusion for ice is approximately 334 Joules/gram.
Q = 10 g * 334 J/g
Q = 3340 Joules
Therefore, 3340 Joules of heat is required to change 10 g of ice at 0°C.
Q = m * ΔH
Where:
Q = heat required (in Joules)
m = mass of the substance (in grams)
ΔH = heat of fusion (for changing from solid to liquid) or heat of vaporization (for changing from liquid to gas) (in Joules/gram)
For ice at 0°C, we need to calculate the heat required to change it from solid to liquid. The heat of fusion for ice is approximately 334 Joules/gram.
Q = 10 g * 334 J/g
Q = 3340 Joules
Therefore, 3340 Joules of heat is required to change 10 g of ice at 0°C.
If sin2q = 0.1198, 0.2395, 0.3588, 0.4793, 0.5984. Find lattice type:- a)fcc
- b)bcc
- c)scc
- d)None of these.
Correct answer is option 'B'. Can you explain this answer?
If sin2q = 0.1198, 0.2395, 0.3588, 0.4793, 0.5984. Find lattice type:
a)
fcc
b)
bcc
c)
scc
d)
None of these.

|
Niti Mukherjee answered |
Solution:
Explanation:
We know that in a crystal lattice, the coordination number is the number of nearest neighbors of an atom/ion. The coordination number for simple cubic (SC) is 6, face-centered cubic (FCC) is 12, and body-centered cubic (BCC) is 8.
Let us assume that the given sin2q values correspond to the (hkl) planes of a crystal lattice. The values of sin2q can be related to the interplanar spacing (d) using Bragg's law:
2d sinq = nλ
where n is an integer, and λ is the wavelength of the X-rays used. Since λ is constant, we can compare the interplanar spacings for different values of sin2q and determine the lattice type.
Comparison of interplanar spacings:
For FCC lattice:
d(hkl) = a/√(h²+k²+l²)
where a is the lattice parameter. The interplanar spacing for (111) plane can be calculated as:
d(111) = a/√(1²+1²+1²) = a/√3
Similarly, we can calculate the interplanar spacings for other planes:
d(200) = a/√4 = a/2
d(220) = a/√8 = a/2√2
d(311) = a/√(3²+1²+1²) = a/√11
d(222) = a/√(2²+2²+2²) = a/2√2
For BCC lattice:
d(hkl) = a/√(h²+k²+l²)
The interplanar spacing for (110) plane can be calculated as:
d(110) = a/√(1²+1²+0²) = a/√2
Similarly, we can calculate the interplanar spacings for other planes:
d(200) = a/√4 = a/2
d(211) = a/√(2²+1²+1²) = a/√6
d(220) = a/√(2²+2²+0²) = a/2√2
d(310) = a/√(3²+1²+0²) = a/√10
Comparison of sin2q values:
sin2q = 0.1198 corresponds to d = 4.055 Å (approx.)
sin2q = 0.2395 corresponds to d = 2.031 Å (approx.)
sin2q = 0.3588 corresponds to d = 1.350 Å (approx.)
sin2q = 0.4793 corresponds to d = 1.015 Å (approx.)
sin2q = 0.5984 corresponds to d = 0.812 Å (approx.)
Conclusion:
Comparing the interplanar spacings calculated for FCC and BCC lattices with the values of d obtained from the given sin2q values, we can see that the values of d
Explanation:
We know that in a crystal lattice, the coordination number is the number of nearest neighbors of an atom/ion. The coordination number for simple cubic (SC) is 6, face-centered cubic (FCC) is 12, and body-centered cubic (BCC) is 8.
Let us assume that the given sin2q values correspond to the (hkl) planes of a crystal lattice. The values of sin2q can be related to the interplanar spacing (d) using Bragg's law:
2d sinq = nλ
where n is an integer, and λ is the wavelength of the X-rays used. Since λ is constant, we can compare the interplanar spacings for different values of sin2q and determine the lattice type.
Comparison of interplanar spacings:
For FCC lattice:
d(hkl) = a/√(h²+k²+l²)
where a is the lattice parameter. The interplanar spacing for (111) plane can be calculated as:
d(111) = a/√(1²+1²+1²) = a/√3
Similarly, we can calculate the interplanar spacings for other planes:
d(200) = a/√4 = a/2
d(220) = a/√8 = a/2√2
d(311) = a/√(3²+1²+1²) = a/√11
d(222) = a/√(2²+2²+2²) = a/2√2
For BCC lattice:
d(hkl) = a/√(h²+k²+l²)
The interplanar spacing for (110) plane can be calculated as:
d(110) = a/√(1²+1²+0²) = a/√2
Similarly, we can calculate the interplanar spacings for other planes:
d(200) = a/√4 = a/2
d(211) = a/√(2²+1²+1²) = a/√6
d(220) = a/√(2²+2²+0²) = a/2√2
d(310) = a/√(3²+1²+0²) = a/√10
Comparison of sin2q values:
sin2q = 0.1198 corresponds to d = 4.055 Å (approx.)
sin2q = 0.2395 corresponds to d = 2.031 Å (approx.)
sin2q = 0.3588 corresponds to d = 1.350 Å (approx.)
sin2q = 0.4793 corresponds to d = 1.015 Å (approx.)
sin2q = 0.5984 corresponds to d = 0.812 Å (approx.)
Conclusion:
Comparing the interplanar spacings calculated for FCC and BCC lattices with the values of d obtained from the given sin2q values, we can see that the values of d
No. of chiral compounds among following are?
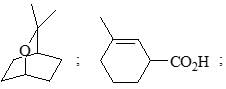
Correct answer is '4'. Can you explain this answer?
No. of chiral compounds among following are?




|
Vipin Singh answered |
1 2 7 8 are chiral. 4 is actually meso so not chiral and last one is allens system so chiral.
Hope this helps , reply for any query...
Chapter doubts & questions for Mock Tests - Mock Test Series for IIT JAM Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Mock Tests - Mock Test Series for IIT JAM Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Mock Test Series for IIT JAM Chemistry
2 docs|25 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup