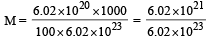All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Some Basic Concepts of Chemistry for NEET Exam
The maximum number of molecules is present in- a)15 L of H2 gas at STP [2004]
- b)5 L of N2 gas at STP
- c)0.5 g of H2 gas
- d)10 g of O2 gas
Correct answer is option 'A'. Can you explain this answer?
The maximum number of molecules is present in
a)
15 L of H2 gas at STP [2004]
b)
5 L of N2 gas at STP
c)
0.5 g of H2 gas
d)
10 g of O2 gas
|
|
Hansa Sharma answered |
No. of molecules in different cases:
(a) 22.4 litre at STP contains = 6.023×1023 molecule of H2
∴ 15 litre at STP contains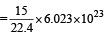 molecules
molecules
∴ 15 litre at STP contains
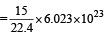 molecules
molecules(b) 22.4 litre at STP contains = 6.023×1023 molecule of N2
∴ 5 litre at STP contains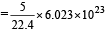 molecules
molecules
∴ 5 litre at STP contains
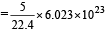 molecules
molecules(c) 2 gm of H2 = 6.023×1023 molecules of H2
∴ 0.5 gm of H2 contains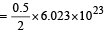 molecules
molecules
∴ 0.5 gm of H2 contains
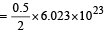 molecules
molecules(d) Similarly, 10 g of O2 gas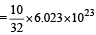 molecules
molecules
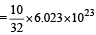 molecules
moleculesThus (a) will have maximum number of molecules
The number of atoms in 0.1 mol of a triatomic gas is :[2010](NA = 6.02 ×1023 mol–1)- a)6.026 × 1022
- b)1.806 × 1023
- c)3.600 × 1023
- d)1.800 × 1022
Correct answer is option 'B'. Can you explain this answer?
The number of atoms in 0.1 mol of a triatomic gas is :[2010](NA = 6.02 ×1023 mol–1)
a)
6.026 × 1022
b)
1.806 × 1023
c)
3.600 × 1023
d)
1.800 × 1022

|
Dipika Das answered |
The number of atoms in 0.1 mole of a triatomic gas = 0.1 × 3 × 6.023 × 1023.
= 1.806 × 1023
= 1.806 × 1023
Which has maximum number of molecules?- a)7 gm N2
- b)2 gm H2 [2002]
- c)16 gm NO2
- d)16 gm O2
Correct answer is option 'B'. Can you explain this answer?
Which has maximum number of molecules?
a)
7 gm N2
b)
2 gm H2 [2002]
c)
16 gm NO2
d)
16 gm O2

|
Subham Chavan answered |
2g of H2 means on e mole of H2, hence contains 6.023 × 1023 molecules. Others have less than one mole, so have less no. of molecules.
Boron has two stable isotopes, 10B (19%) and 11B (81%). Average atomic weight for boron in the periodic table is [1990]- a)10.8
- b)10.2
- c)11.2
- d)10.0
Correct answer is option 'A'. Can you explain this answer?
Boron has two stable isotopes, 10B (19%) and 11B (81%). Average atomic weight for boron in the periodic table is [1990]
a)
10.8
b)
10.2
c)
11.2
d)
10.0

|
Kajal Bose answered |
Average atomic mass
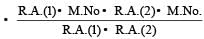
Where R.A. = relative abundance
M.No = Mass number
M.No = Mass number

10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be: [2009]- a)3 mol
- b)4 mol
- c)1 mol
- d)2 mol
Correct answer is option 'B'. Can you explain this answer?
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be: [2009]
a)
3 mol
b)
4 mol
c)
1 mol
d)
2 mol

|
Pallabi Reddy answered |
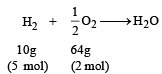
In this reaction oxygen is the limiting agent.
Hence amount of H2O produced depends on the amount of O2 taken 0.5 mole of O2 gives H2O = 1 mol
∴ 2 mole of O2 gives H2O = 4 mol
Hence amount of H2O produced depends on the amount of O2 taken 0.5 mole of O2 gives H2O = 1 mol
∴ 2 mole of O2 gives H2O = 4 mol
The weight of one molecule of a compound C 60 H122 is [1995]- a)1.2 x10 -20gram
- b)1.4 x10 -21gram
- c)5.025 x10 23gram
- d)6.023 x10 23gram
Correct answer is option 'B'. Can you explain this answer?
The weight of one molecule of a compound C 60 H122 is [1995]
a)
1.2 x10 -20gram
b)
1.4 x10 -21gram
c)
5.025 x10 23gram
d)
6.023 x10 23gram
|
|
Rohit Jain answered |
Molecular weight of C60H122 = (12 × 60) + 122 = 842.
Therefore weight of one molecule
Therefore weight of one molecule
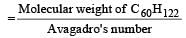
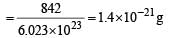
In an experiment it showed that 10 mL of 0.05 M solution of chloride required 10 mL of 0.1 M solution of AgNO3, which of the following will be the formula of the chloride (X stands for the symbol of the element other than chlorine): [NEET Kar. 2013]- a)X2Cl
- b)X2Cl2
- c)XCl2
- d)XCl4
Correct answer is option 'C'. Can you explain this answer?
In an experiment it showed that 10 mL of 0.05 M solution of chloride required 10 mL of 0.1 M solution of AgNO3, which of the following will be the formula of the chloride (X stands for the symbol of the element other than chlorine): [NEET Kar. 2013]
a)
X2Cl
b)
X2Cl2
c)
XCl2
d)
XCl4

|
Pallabi Reddy answered |
Millimoles of solution of chloride = 0.05 × 10 = 0.5
Millimoles of AgNO3 solution = 10 × 0.1 = 1
So, the millimoles of AgNO3 are double than the chloride solution
Millimoles of AgNO3 solution = 10 × 0.1 = 1
So, the millimoles of AgNO3 are double than the chloride solution
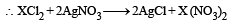
Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mL– 1. Volume of acid required to make one litre of 0.1MH2SO4 solution is [2007]- a)16.65 mL
- b)22.20 mL
- c)5.55 mL
- d)11.10 mL
Correct answer is option 'C'. Can you explain this answer?
Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mL– 1. Volume of acid required to make one litre of 0.1MH2SO4 solution is [2007]
a)
16.65 mL
b)
22.20 mL
c)
5.55 mL
d)
11.10 mL

|
Aman Sharma answered |
Molarity of H2SO4 solution
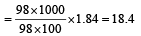
Suppose V ml of this H2SO4 is used to prepare 1 lit. of 0.1M H2SO4
∴ V x 18.02 = 1000 x 0.1
∴ V x 18.02 = 1000 x 0.1
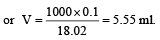
An organic compound contains 80% (by wt.) carbon and the remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of C is 12, H is 1] (2021)- a)CH3
- b)CH4
- c)CH
- d)CH2
Correct answer is option 'A'. Can you explain this answer?
An organic compound contains 80% (by wt.) carbon and the remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of C is 12, H is 1] (2021)
a)
CH3
b)
CH4
c)
CH
d)
CH2
|
|
Ajay Yadav answered |
An empirical formula represents the simplest whole number ratio of various atoms present in a compound, whereas, the molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
The number of moles of KMnO4 reduced by one mole of KI in alkaline medium is: [2 00 5]- a)one
- b)two
- c)five
- d)one fifth
Correct answer is option 'A'. Can you explain this answer?
The number of moles of KMnO4 reduced by one mole of KI in alkaline medium is: [2 00 5]
a)
one
b)
two
c)
five
d)
one fifth

|
Charvi Shah answered |
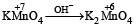
Change in oxidation number of Mn in basic medium is 1. Hence mole of KI is equal to mole of KMnO4.
In Haber process 30 litres of dihydrogen and 30 litres of dinitrogen were taken for reaction which yielded only 50% of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end? [2003]- a)20 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
- b)20 litres ammonia, 20 litres nitrogen, 20 litres hydrogen
- c)10 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
- d)20 litres ammonia, 10 litres nitrogen, 30 litres hydrogen
Correct answer is option 'C'. Can you explain this answer?
In Haber process 30 litres of dihydrogen and 30 litres of dinitrogen were taken for reaction which yielded only 50% of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end? [2003]
a)
20 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
b)
20 litres ammonia, 20 litres nitrogen, 20 litres hydrogen
c)
10 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
d)
20 litres ammonia, 10 litres nitrogen, 30 litres hydrogen

|
Pooja Choudhary answered |

It is given that only 50% of the expected product is formed hence only 10 litre of NH3 is formed N2 used = 5 litres, left = 30 – 5 = 25 litres H2 used = 15 litres, left = 30 – 15 = 15 litres
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is [2007]- a)4/5
- b)2/5
- c)1
- d)3/5
Correct answer is option 'B'. Can you explain this answer?
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is [2007]
a)
4/5
b)
2/5
c)
1
d)
3/5

|
Kunal Rane answered |
The balance chemical equation is :
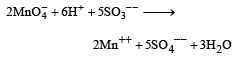
From the equation it is clear that Moles of MnO4– require to oxidise 5 moles of SO3– – are 2
Moles of MnO4– require to oxidise 1 mole of SO3– – are 2/5.
Moles of MnO4– require to oxidise 1 mole of SO3– – are 2/5.
An element , X has the following isotopic composition : [2007] 200X : 90% 199X : 8.0 % 202X : 2.0 %
The weighted average atomic mass of the naturally occuring element X is closest to- a)201 amu
- b)202 amu
- c)199 amu
- d)200 amu
Correct answer is option 'D'. Can you explain this answer?
An element , X has the following isotopic composition : [2007] 200X : 90% 199X : 8.0 % 202X : 2.0 %
The weighted average atomic mass of the naturally occuring element X is closest to
The weighted average atomic mass of the naturally occuring element X is closest to
a)
201 amu
b)
202 amu
c)
199 amu
d)
200 amu

|
Dipika Das answered |
Average isotopic mass of
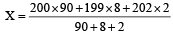
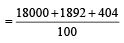
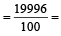 199.96 amu
199.96 amuAssuming fully decomposed, the volume of CO2 released at STP on heating 9.85 g of BaCO3 (Atomic mass, Ba = 137) will be [2000]- a)2.24 L
- b)4.96 L
- c)1.12 L
- d)0.84 L
Correct answer is option 'C'. Can you explain this answer?
Assuming fully decomposed, the volume of CO2 released at STP on heating 9.85 g of BaCO3 (Atomic mass, Ba = 137) will be [2000]
a)
2.24 L
b)
4.96 L
c)
1.12 L
d)
0.84 L

|
Shanaya Rane answered |
BaCO3 → BaO + CO2
197 gm
197 gm of BaCO3 released carbon dioxide = 22.4 litre at STP
∴ 1 gm of BaCO3 released carbon dioxide
197 gm
197 gm of BaCO3 released carbon dioxide = 22.4 litre at STP
∴ 1 gm of BaCO3 released carbon dioxide
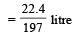
∴ 9.85 gm of BaCO3 released carbon dioxide
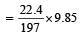 = 1.12 litre
= 1.12 litreHow many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g of HCl ? [2008]
- a)0.044
- b)0.333
- c)0.011
- d)0.029
Correct answer is option 'D'. Can you explain this answer?
How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g of HCl ? [2008]
a)
0.044
b)
0.333
c)
0.011
d)
0.029
|
|
Kaavya Joshi answered |
Given:
Mass of PbO = 6.5 g
Mass of HCl = 3.2 g
To find:
Moles of PbCl2 formed
Solution:
1. Write the balanced chemical equation for the reaction:
PbO + 2HCl → PbCl2 + H2O
2. Calculate the moles of PbO:
Molar mass of PbO = 207.2 g/mol
Number of moles of PbO = Mass/Molar mass = 6.5/207.2 = 0.0314 mol
3. Calculate the moles of HCl:
Molar mass of HCl = 36.5 g/mol
Number of moles of HCl = Mass/Molar mass = 3.2/36.5 = 0.0877 mol
4. Identify the limiting reagent:
From the balanced equation, 1 mole of PbO reacts with 2 moles of HCl to produce 1 mole of PbCl2. Therefore, the limiting reagent is PbO since it produces fewer moles of the product.
5. Calculate the moles of PbCl2 formed:
Number of moles of PbCl2 = Number of moles of limiting reagent × Ratio of moles of PbCl2 to moles of limiting reagent
Number of moles of PbCl2 = 0.0314 mol × 1 mol PbCl2/1 mol PbO = 0.0314 mol
Answer:
The number of moles of PbCl2 formed is 0.029 mol, which is closest to option (d) 0.029.
Mass of PbO = 6.5 g
Mass of HCl = 3.2 g
To find:
Moles of PbCl2 formed
Solution:
1. Write the balanced chemical equation for the reaction:
PbO + 2HCl → PbCl2 + H2O
2. Calculate the moles of PbO:
Molar mass of PbO = 207.2 g/mol
Number of moles of PbO = Mass/Molar mass = 6.5/207.2 = 0.0314 mol
3. Calculate the moles of HCl:
Molar mass of HCl = 36.5 g/mol
Number of moles of HCl = Mass/Molar mass = 3.2/36.5 = 0.0877 mol
4. Identify the limiting reagent:
From the balanced equation, 1 mole of PbO reacts with 2 moles of HCl to produce 1 mole of PbCl2. Therefore, the limiting reagent is PbO since it produces fewer moles of the product.
5. Calculate the moles of PbCl2 formed:
Number of moles of PbCl2 = Number of moles of limiting reagent × Ratio of moles of PbCl2 to moles of limiting reagent
Number of moles of PbCl2 = 0.0314 mol × 1 mol PbCl2/1 mol PbO = 0.0314 mol
Answer:
The number of moles of PbCl2 formed is 0.029 mol, which is closest to option (d) 0.029.
At S.T.P. the density of CCl4 vapours in g/L will be nearest to : [1988]- a)6.87
- b)3.42
- c)10.26
- d)4.57
Correct answer is option 'A'. Can you explain this answer?
At S.T.P. the density of CCl4 vapours in g/L will be nearest to : [1988]
a)
6.87
b)
3.42
c)
10.26
d)
4.57

|
Aman Sharma answered |
1 mol CCl4 vapour = 12 + 4 × 35.5 = 154 g ≡ 22.4 L at STP
∴ Density = 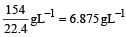
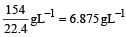
What is the [OH–] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl with 30.0 mL of 0.10 M Ba(OH)2? [2009]- a)0.40 M
- b)0.0050 M
- c)0.12 M
- d)0.10 M
Correct answer is option 'D'. Can you explain this answer?
What is the [OH–] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl with 30.0 mL of 0.10 M Ba(OH)2? [2009]
a)
0.40 M
b)
0.0050 M
c)
0.12 M
d)
0.10 M

|
Shanaya Rane answered |
No. of milli equivalent of HCl = 20 × 0.05
= 1.0
No. of milli equivalent of Br (OH)2 = 30 × 0.1 × 2 = 6.0
After neutralization, no. of milli equivalents in 50 ml. of solution = (6 – 1) = 5
Total volume of the solution = 20 + 30 = 50 ml
∴ No. of milli equivalent of OH– is 5 in 50 ml
= 1.0
No. of milli equivalent of Br (OH)2 = 30 × 0.1 × 2 = 6.0
After neutralization, no. of milli equivalents in 50 ml. of solution = (6 – 1) = 5
Total volume of the solution = 20 + 30 = 50 ml
∴ No. of milli equivalent of OH– is 5 in 50 ml
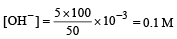
The number of gram molecules of oxygen in 6.02 × 1024 CO molecules is [1990]- a)10 gm molecules
- b)5 gm molecules
- c)1 gm molecules
- d)0.5 gm molelcules
Correct answer is option 'B'. Can you explain this answer?
The number of gram molecules of oxygen in 6.02 × 1024 CO molecules is [1990]
a)
10 gm molecules
b)
5 gm molecules
c)
1 gm molecules
d)
0.5 gm molelcules
|
|
Vijay Bansal answered |
6.02 x 10^23 molecules of CO = 1 mole of CO
6.02 x 10^2⁴ molecules of CO = 10 mole of CO
= 10 g atoms of O = 5 g molecules of O₂
In the reaction 4 NH3 (g) + 5O2(g) → 4NO(g) + 6H2O(l) When 1 mole of ammonia and 1 mole of O2 are made to react to completion, [1998]- a)1.0 mole of H2O is produced
- b)1.0 mole of NO will be produced
- c)all the oxygen will be consumed
- d)all the ammonia will be consumed
Correct answer is option 'C'. Can you explain this answer?
In the reaction 4 NH3 (g) + 5O2(g) → 4NO(g) + 6H2O(l) When 1 mole of ammonia and 1 mole of O2 are made to react to completion, [1998]
a)
1.0 mole of H2O is produced
b)
1.0 mole of NO will be produced
c)
all the oxygen will be consumed
d)
all the ammonia will be consumed

|
Rounak Desai answered |
It seems like the reaction equation you provided is incomplete. Could you please provide the complete reaction equation?
One litre hard water contains 12.00 mg Mg2+.Mili-equivalents of washing soda required to remove its hardness is : [1988]- a)1
- b)12. 16
- c)1 × 10–3
- d)12. 16×10–3
Correct answer is option 'A'. Can you explain this answer?
One litre hard water contains 12.00 mg Mg2+.Mili-equivalents of washing soda required to remove its hardness is : [1988]
a)
1
b)
12. 16
c)
1 × 10–3
d)
12. 16×10–3

|
Dipanjan Chawla answered |
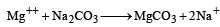
1 g eq. 1g eq.
1 g eq. of Mg2+ = 12 g of Mg2+ = 12000 mg
= 1000 milli eq. of Na2CO3
1 g eq. of Mg2+ = 12 g of Mg2+ = 12000 mg
= 1000 milli eq. of Na2CO3
∴ 12 mg Mg++ = 1 milli eq. Na2CO3
In the final answer of the expression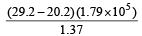 the number of significant figures is : [1994]
the number of significant figures is : [1994]- a)1
- b)2
- c)3
- d)4
Correct answer is option 'C'. Can you explain this answer?
In the final answer of the expression
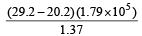
the number of significant figures is : [1994]
a)
1
b)
2
c)
3
d)
4
|
|
Preeti Iyer answered |
On calculation we find
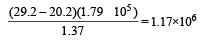
As the least precise number contains 3 significant figures therefore answers should also contains 3 significant figures.
The number of oxygen atoms in 4.4 g of CO2 is- a)1.2 × 1023
- b)6 × 1022 [1990]
- c)6 × 1023
- d)12 × 1023
Correct answer is option 'A'. Can you explain this answer?
The number of oxygen atoms in 4.4 g of CO2 is
a)
1.2 × 1023
b)
6 × 1022 [1990]
c)
6 × 1023
d)
12 × 1023

|
Diya Datta answered |
4.4 g CO2 =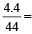 0.1 mol CO2
0.1 mol CO2
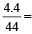 0.1 mol CO2
0.1 mol CO2(mol. wt. of CO2 = 44)
= 6 × 1022 molecules
= 2 × 6 × 1022 atoms of O
= 6 × 1022 molecules
= 2 × 6 × 1022 atoms of O
Haemoglobin contains 0.334% of iron by weight.The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe is 56) present in one molecule of haemoglobin are [1998]- a)1
- b)6
- c)4
- d)2
Correct answer is option 'C'. Can you explain this answer?
Haemoglobin contains 0.334% of iron by weight.The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe is 56) present in one molecule of haemoglobin are [1998]
a)
1
b)
6
c)
4
d)
2

|
Prashanth Dasgupta answered |
Given : Percentage of the iron = 0.334%; Molecular weight of the haemoglobin = 67200 and atomic weight of the iron = 56. We know that the number of iron atoms
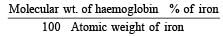
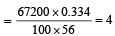
Liquid benzene (C6H6) burns in oxygen according to the equation 2C6H6(l ) + 15O2(g) —→ 12CO2(g) 6H2O(g) How many litres of O2 at STP are needed to complete the combustion of 39 g of liquid benzene?(Mol. wt. of O2 = 32, C6H6 = 78) [1996]- a)74 L
- b)11.2 L
- c)22.4 L
- d)84 L
Correct answer is option 'D'. Can you explain this answer?
Liquid benzene (C6H6) burns in oxygen according to the equation 2C6H6(l ) + 15O2(g) —→ 12CO2(g) 6H2O(g) How many litres of O2 at STP are needed to complete the combustion of 39 g of liquid benzene?(Mol. wt. of O2 = 32, C6H6 = 78) [1996]
a)
74 L
b)
11.2 L
c)
22.4 L
d)
84 L

|
Palak Khanna answered |
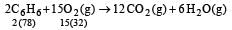
156 gm of benzene required oxygen = 15 × 22.4 litre
∴ 1 gm of benzene required oxygen
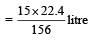
∴ 39 gm of Benzene required oxygen
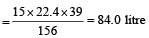
An organic compound containing C, H and O gave on analysis C – 40% and H – 6.66%. Its empirical formula would be [1999, 94]- a)C3H6O
- b)CHO
- c)CH2O
- d)CH4O
Correct answer is option 'C'. Can you explain this answer?
An organic compound containing C, H and O gave on analysis C – 40% and H – 6.66%. Its empirical formula would be [1999, 94]
a)
C3H6O
b)
CHO
c)
CH2O
d)
CH4O

|
Aashna Mukherjee answered |
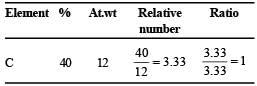
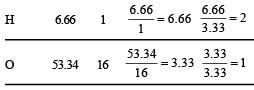
(% of O in organic compound Table for empirical formula : = 100 – (40 + 6.66 ) = 53.34 % )
Empirical formula of organic compound = CH2O.
Empirical formula of organic compound = CH2O.
The percentage weight of Zn in white vitriol [ZnSO4.7H2O] is approximately equal to ( Zn = 65, S = 32, O = 16 and H = 1) [1995]- a)33.65 %
- b)32.56 %
- c)23.65 %
- d)22.65 %
Correct answer is option 'D'. Can you explain this answer?
The percentage weight of Zn in white vitriol [ZnSO4.7H2O] is approximately equal to ( Zn = 65, S = 32, O = 16 and H = 1) [1995]
a)
33.65 %
b)
32.56 %
c)
23.65 %
d)
22.65 %

|
Shounak Nair answered |
Molecular weight of ZnSO4 .7H 2O = 65 + 32 + (4 × 16) + 7(2 × 1 + 16) = 287.
∴ percentage mass of zinc (Zn)
∴ percentage mass of zinc (Zn)
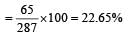
Percentage of Se in per oxidase anhydrase enzyme is 0.5% by weight (at. wt. = 78.4) then minimum molecular weight of peroxidase anhydrase enzyme is [2001]- a)1.568 × 103
- b)15.68
- c)2.136 × 104
- d)1.568 × 104
Correct answer is option 'D'. Can you explain this answer?
Percentage of Se in per oxidase anhydrase enzyme is 0.5% by weight (at. wt. = 78.4) then minimum molecular weight of peroxidase anhydrase enzyme is [2001]
a)
1.568 × 103
b)
15.68
c)
2.136 × 104
d)
1.568 × 104

|
Pooja Choudhary answered |
Suppose the mol. wt. of enzyme = x
Given 100g of enzyme wt of Se = 0.5 gm
Given 100g of enzyme wt of Se = 0.5 gm
∴ In xg of enzyme wt. of Se 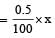
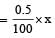
Hence 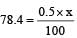
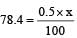
∴ x = 15680 = 1.568 × 104
Which one of the followings has maximum number of atoms? (2020)- a)1g of O2(g) (Atomic mass of O = 16)
- b)1g of Li(s) (Atomic mass of Li = 7)
- c)1g of Ag(s) (Atomic mass of Ag = 108)
- d)1g of Mg(s) (Atomic mass of Mg = 24)
Correct answer is option 'B'. Can you explain this answer?
Which one of the followings has maximum number of atoms? (2020)
a)
1g of O2(g) (Atomic mass of O = 16)
b)
1g of Li(s) (Atomic mass of Li = 7)
c)
1g of Ag(s) (Atomic mass of Ag = 108)
d)
1g of Mg(s) (Atomic mass of Mg = 24)
|
|
Ajay Yadav answered |
(a) no. of atoms
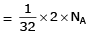
(b) no. of atoms
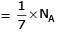
(c) no. of atoms
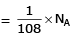
(d) no. of atoms
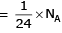
∴ 1 gm of Li is having maximum no. of atoms.
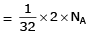
(b) no. of atoms
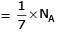
(c) no. of atoms
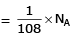
(d) no. of atoms
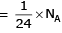
∴ 1 gm of Li is having maximum no. of atoms.
What is the weight of oxygen required for the complete combustion of 2.8 kg of ethylene?- a)2.8 kg
- b)6.4 kg
- c)9.6 kg
- d)96 kg
Correct answer is option 'C'. Can you explain this answer?
What is the weight of oxygen required for the complete combustion of 2.8 kg of ethylene?
a)
2.8 kg
b)
6.4 kg
c)
9.6 kg
d)
96 kg

|
Pankaj Kulkarni answered |
C2H4 + 3 O2 —→ 2CO2 + 2H2O 28 kg 96 kg
∵ 28 kg of C2H4 undergo complete combustion by = 96 kg of O2
∴ 2.8 kg of C2H4 undergo complete combustion by = 9.6 g of O2.
∵ 28 kg of C2H4 undergo complete combustion by = 96 kg of O2
∴ 2.8 kg of C2H4 undergo complete combustion by = 9.6 g of O2.
A metal oxide has the formula Z2O3. It can be reduced by hydrogen to give free metal and water. 0.1596 g of the metal oxide requires 6 mg of hydrogen for complete reduction. The atomic weight of the metal is [1989]- a)27.9
- b)159.6
- c)79.8
- d)55.8
Correct answer is option 'D'. Can you explain this answer?
A metal oxide has the formula Z2O3. It can be reduced by hydrogen to give free metal and water. 0.1596 g of the metal oxide requires 6 mg of hydrogen for complete reduction. The atomic weight of the metal is [1989]
a)
27.9
b)
159.6
c)
79.8
d)
55.8

|
Shounak Nair answered |
The reactioin may given as
Z2 O3 + 3H2 —→ 2Z + 3H2O
0.1596 g of Z2O3 react with H2
= 6 mg = 0.006 g
∴ 1 g of H2 react with
0.1596 g of Z2O3 react with H2
= 6 mg = 0.006 g
∴ 1 g of H2 react with
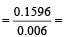 26.6g of Z2O3
26.6g of Z2O3∴ Eq. wt. of Z2O3 = 26.6 (from the elefinition of eq. wt.) Eq. wt. of Z + Eq. wt. of O = E + 8 = 26.6
⇒ Eq. wt. of Z = 26.6 – 8 = 18.6 Valency of metal in Z2O3 = 3
Eq. wt.of metal 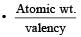
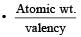
∴ At. wt. of Z = 18.6 × 3 = 55.8
What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1L of propane gas (C3H8) measured under the same conditions ? [2008]- a)7 L
- b)6 L
- c)5 L
- d)10 L
Correct answer is option 'C'. Can you explain this answer?
What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1L of propane gas (C3H8) measured under the same conditions ? [2008]
a)
7 L
b)
6 L
c)
5 L
d)
10 L

|
Srishti Sen answered |
Writing the equation of combustion of propane (C3H8), we get
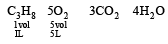
From the above equation we find that we need 5 L of oxygen at NTP to completely burn 1 L of propane at N.T.P.
If we change the conditions for both the gases from N.T.P. to same conditions of temperature and pressure. The same results are obtained. i.e. 5 L is the correct answer.
If we change the conditions for both the gases from N.T.P. to same conditions of temperature and pressure. The same results are obtained. i.e. 5 L is the correct answer.
If NA is Avogadro’s number then number of valence electrons in 4.2g of nitride ions (N3–) is- a)2.4 NA
- b)4.2 NA [1994]
- c)1.6 NA
- d)3.2 NA
Correct answer is option 'A'. Can you explain this answer?
If NA is Avogadro’s number then number of valence electrons in 4.2g of nitride ions (N3–) is
a)
2.4 NA
b)
4.2 NA [1994]
c)
1.6 NA
d)
3.2 NA
|
|
Yash Ghoshal answered |
If NA refers to Avogadro, then Avogadro's number is a fundamental constant in chemistry, equal to approximately 6.02214076 x 10^23. It represents the number of atoms or molecules in one mole of a substance.
An organic compound containing C, H and N gave the following analysis : C = 40% ; H = 13.33% ; N = 46.67%Its empirical formula would be [1998]- a)C2H7N2
- b)CH5N
- c)CH4N
- d)C2H7N
Correct answer is option 'C'. Can you explain this answer?
An organic compound containing C, H and N gave the following analysis : C = 40% ; H = 13.33% ; N = 46.67%Its empirical formula would be [1998]
a)
C2H7N2
b)
CH5N
c)
CH4N
d)
C2H7N
|
|
Anoushka Yadav answered |
To determine the empirical formula of the organic compound containing carbon (C), hydrogen (H), and nitrogen (N), we need to calculate the simplest ratio of the elements present in the compound.
1. Calculate the number of moles of each element:
Given:
C = 40%
H = 13.33%
N = 46.67%
Assume we have a 100g sample of the compound:
Mass of C = 40g (40% of 100g)
Mass of H = 13.33g (13.33% of 100g)
Mass of N = 46.67g (46.67% of 100g)
To calculate the number of moles of each element, we use the molar mass of each element:
The molar mass of C is 12.01 g/mol
The molar mass of H is 1.01 g/mol
The molar mass of N is 14.01 g/mol
Number of moles of C = Mass of C / Molar mass of C = 40g / 12.01 g/mol ≈ 3.33 mol
Number of moles of H = Mass of H / Molar mass of H = 13.33g / 1.01 g/mol ≈ 13.20 mol
Number of moles of N = Mass of N / Molar mass of N = 46.67g / 14.01 g/mol ≈ 3.33 mol
2. Determine the simplest whole number ratio of the elements:
Divide the number of moles of each element by the smallest number of moles to obtain the simplest whole number ratio.
Dividing all the moles by 3.33 (the smallest number of moles), we get:
C ≈ 3.33 mol / 3.33 mol = 1
H ≈ 13.20 mol / 3.33 mol ≈ 3.96 ≈ 4
N ≈ 3.33 mol / 3.33 mol = 1
The simplest whole number ratio of C, H, and N is 1:4:1.
3. Write the empirical formula:
The empirical formula represents the simplest whole number ratio of the elements. Therefore, the empirical formula of the organic compound is CH4N, which is option C.
1. Calculate the number of moles of each element:
Given:
C = 40%
H = 13.33%
N = 46.67%
Assume we have a 100g sample of the compound:
Mass of C = 40g (40% of 100g)
Mass of H = 13.33g (13.33% of 100g)
Mass of N = 46.67g (46.67% of 100g)
To calculate the number of moles of each element, we use the molar mass of each element:
The molar mass of C is 12.01 g/mol
The molar mass of H is 1.01 g/mol
The molar mass of N is 14.01 g/mol
Number of moles of C = Mass of C / Molar mass of C = 40g / 12.01 g/mol ≈ 3.33 mol
Number of moles of H = Mass of H / Molar mass of H = 13.33g / 1.01 g/mol ≈ 13.20 mol
Number of moles of N = Mass of N / Molar mass of N = 46.67g / 14.01 g/mol ≈ 3.33 mol
2. Determine the simplest whole number ratio of the elements:
Divide the number of moles of each element by the smallest number of moles to obtain the simplest whole number ratio.
Dividing all the moles by 3.33 (the smallest number of moles), we get:
C ≈ 3.33 mol / 3.33 mol = 1
H ≈ 13.20 mol / 3.33 mol ≈ 3.96 ≈ 4
N ≈ 3.33 mol / 3.33 mol = 1
The simplest whole number ratio of C, H, and N is 1:4:1.
3. Write the empirical formula:
The empirical formula represents the simplest whole number ratio of the elements. Therefore, the empirical formula of the organic compound is CH4N, which is option C.
The molecular weight of O2 and SO2 are 32 and 64 respectively. At 15°C and 150 mm Hg pressure, one litre of O2 contains ‘N’ molecules. The number of molecules in two litres of SO2 under the same conditions of temperature and pressure will be : [1990]- a)N/2
- b)N
- c)2N
- d)4N
Correct answer is option 'C'. Can you explain this answer?
The molecular weight of O2 and SO2 are 32 and 64 respectively. At 15°C and 150 mm Hg pressure, one litre of O2 contains ‘N’ molecules. The number of molecules in two litres of SO2 under the same conditions of temperature and pressure will be : [1990]
a)
N/2
b)
N
c)
2N
d)
4N

|
Mahesh Saini answered |
A ccording to Avogadro's law "equal volumes of all gases contain equal numbers of molecules under similar conditions of temperature and pressure". Thus if 1 L of one gas contains N molecules, 2 L of any gas under the same conditions will contain 2N molecules.
Specific volume of cylindrical virus particle is 6.02 × 10–2 cc/gm. whose radius and length 7 Å & 10 Å respectively. If NA = 6.02 × 1023, find molecular weight of virus [2001]- a)3.08 × 103 kg/mol
- b)3.08 × 104 kg/mol
- c)1.54 × 104 kg/mol
- d)15.4 kg/mol
Correct answer is option 'D'. Can you explain this answer?
Specific volume of cylindrical virus particle is 6.02 × 10–2 cc/gm. whose radius and length 7 Å & 10 Å respectively. If NA = 6.02 × 1023, find molecular weight of virus [2001]
a)
3.08 × 103 kg/mol
b)
3.08 × 104 kg/mol
c)
1.54 × 104 kg/mol
d)
15.4 kg/mol

|
Kajal Bose answered |
Specific volume (volume of 1 gm) of cylindrical virus particle = 6.02 × 10–2 cc/gm
Radius of virus (r) = 7 Å = 7 × 10–8 cm
Length of virus = 10 × 10–8 cm
Volume of virus
Radius of virus (r) = 7 Å = 7 × 10–8 cm
Length of virus = 10 × 10–8 cm
Volume of virus
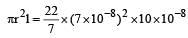
= 154 × 10–23 cc
Wt. of one virus particle 

∴ Mol. wt. of virus = Wt. of NA particle
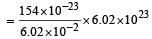
= 15400 g/mol = 15.4 kg/mole
A 5 molar solution of H2SO4 is diluted from 1 litre to a volume of 10 litres, the normality of the solution will be : [1991]- a)1N
- b)0.1N
- c)5N
- d)0.5N
Correct answer is option 'A'. Can you explain this answer?
A 5 molar solution of H2SO4 is diluted from 1 litre to a volume of 10 litres, the normality of the solution will be : [1991]
a)
1N
b)
0.1N
c)
5N
d)
0.5N

|
Prashanth Dasgupta answered |
5 MH2SO4 = 10 N H2SO4, (∵Basicity of H2SO4 = 2) N1V1 = N2V2,
10 × 1 = N2 × 10 or N2 = 1 N
10 × 1 = N2 × 10 or N2 = 1 N
Ratio of Cp and Cv of a gas ‘X’ is 1.4. The number of atoms of the gas ‘X’ present in 11.2 litres of it at NTP will be [1989]- a)6.02 ×1023
- b)1.2 × 1023
- c)3.01 × 1023
- d)2.01 × 1023
Correct answer is option 'A'. Can you explain this answer?
Ratio of Cp and Cv of a gas ‘X’ is 1.4. The number of atoms of the gas ‘X’ present in 11.2 litres of it at NTP will be [1989]
a)
6.02 ×1023
b)
1.2 × 1023
c)
3.01 × 1023
d)
2.01 × 1023

|
Dipika Das answered |
Cp / Cv = 1.4 shows that the gas is diatomic. 22.4 litre at NTP ≡ 6.02 × 1023 molecules 11.2 L at NTP = 3.01 × 1023 molecules
= 3.01 × 1023 × 2 atoms = 6.02 × 1023 atoms
= 3.01 × 1023 × 2 atoms = 6.02 × 1023 atoms
The number of significant figures for the three numbers 161 cm, 0.161 cm, 0.0161 cm are[1998]- a)3,4 and 5 respectively
- b)3,4 and 4 respectively
- c)3,3 and 4 respectively
- d)3,3 and 3 respectively
Correct answer is option 'D'. Can you explain this answer?
The number of significant figures for the three numbers 161 cm, 0.161 cm, 0.0161 cm are[1998]
a)
3,4 and 5 respectively
b)
3,4 and 4 respectively
c)
3,3 and 4 respectively
d)
3,3 and 3 respectively

|
Mrinalini Bajaj answered |
We know that all non -zero digits are significant and the zeros at the beginning of a number are not significant. Therefore number 161 cm, 0.161 cm and 0.0161cm have 3, 3 and 3 significant figures respectively.
Chapter doubts & questions for Some Basic Concepts of Chemistry - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Some Basic Concepts of Chemistry - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup




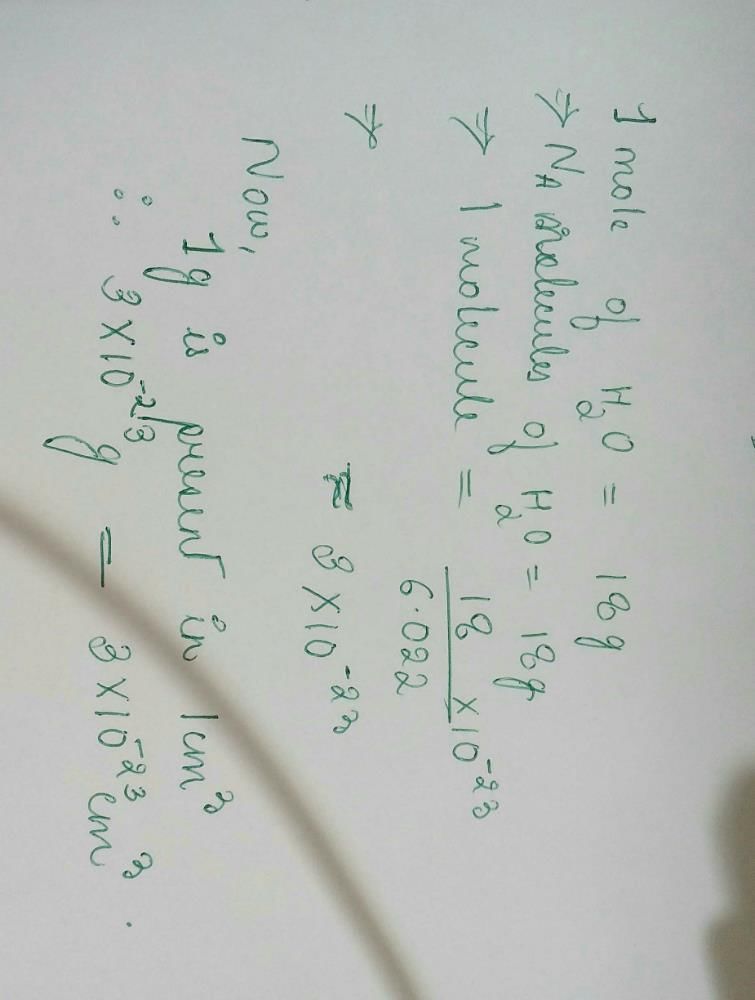

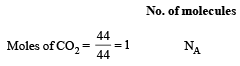
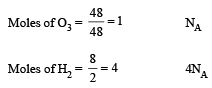
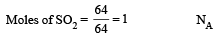
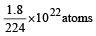
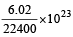 molecules
molecules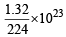 electrons
electrons