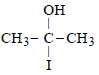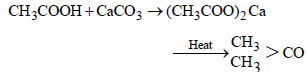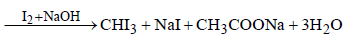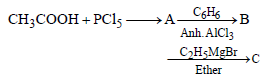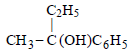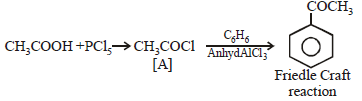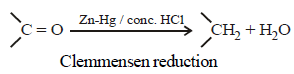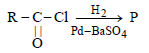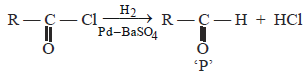All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Aldehydes, Ketones and Carboxylic Acids for NEET Exam
CH3CHO and C6H5CH2CHO can be distinguished chemically by: [2012]
- a)Benedict test
- b)Iodoform test
- c)Tollen’s reagent test
- d)Fehling solution test
Correct answer is option 'B'. Can you explain this answer?
CH3CHO and C6H5CH2CHO can be distinguished chemically by: [2012]
a)
Benedict test
b)
Iodoform test
c)
Tollen’s reagent test
d)
Fehling solution test

|
Ayush Choudhury answered |
CH3CHO and C6H5CH2CHO both are aldehydes so they can give the test of Tollen’s reagent, Fehling's solution and Benedict’s solution.
The carbonyl compounds with the structure R-CO-CH3 can only give the Iodoform test.
CH3CHO is the only aldehyde which reacts with NaOH and I2 to give yellow crystals of Iodoform while C6H5CH2CHO doesn’t react with it.
So, the iodoform test is used to distinguish between CH3CHO and C6H5CH2CHO compounds.
CH3CHO + 3I2 + 4NaOH ⟶ CHI3 + HCOONa + 3NaI +3H2O
C6H5CH2CHO + I2 + 4NaOH⟶ No reaction.
Propionic acid with Br2|P yields a dibromo product. Its structure would be: [2009]- a)
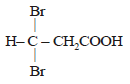
- b)CH2Br – CH2 – COBr
- c)
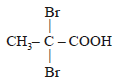
- d)CH2 Br – CHBr – COOH
Correct answer is option 'C'. Can you explain this answer?
Propionic acid with Br2|P yields a dibromo product. Its structure would be: [2009]
a)
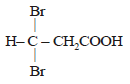
b)
CH2Br – CH2 – COBr
c)
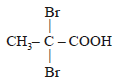
d)
CH2 Br – CHBr – COOH

|
Anand Jain answered |
This reaction is an example of Hell - Volhard
Zelinsky reaction. In this reaction acids
containing α– H on treatment with X2 /P
give di-halo substituted acid.
Zelinsky reaction. In this reaction acids
containing α– H on treatment with X2 /P
give di-halo substituted acid.
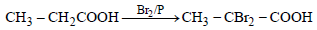
Self condensation of two moles of ethyl acetatein presence of sodium ethoxide yields [2006]- a)acetoacetic ester
- b)methyl acetoacetate
- c)ethyl propionate
- d)ethyl butyrate
Correct answer is option 'A'. Can you explain this answer?
Self condensation of two moles of ethyl acetatein presence of sodium ethoxide yields [2006]
a)
acetoacetic ester
b)
methyl acetoacetate
c)
ethyl propionate
d)
ethyl butyrate

|
Ishani Nambiar answered |
It is an example of Claisen condensation. The
product is acetoacetic ester.
product is acetoacetic ester.

Iodoform test is not given by [1999]- a)2-Pentanone
- b)Ethanol
- c)Ethanal
- d)3-Pentanone
Correct answer is option 'D'. Can you explain this answer?
Iodoform test is not given by [1999]
a)
2-Pentanone
b)
Ethanol
c)
Ethanal
d)
3-Pentanone

|
Priyanka Iyer answered |
Iodoform test is exhibited by ethyl alcohol acetaldehyde, acetone methyl ketones and those alcohols which possess CH3CH(OH)- group. As 3-pentanone does not contain
CH3CO-group as therefore it does not give iodoform test.
CH3CO-group as therefore it does not give iodoform test.
Which of the following is incorrect? [2001]- a)NaHSO3 is used in detection of carbonylcompound
- b)FeCl3 is used in detection of phenolic group
- c)Tollen reagent is used in detection ofunsaturation
- d)Fehling solution is used in detection ofglucose
Correct answer is option 'C'. Can you explain this answer?
Which of the following is incorrect? [2001]
a)
NaHSO3 is used in detection of carbonylcompound
b)
FeCl3 is used in detection of phenolic group
c)
Tollen reagent is used in detection ofunsaturation
d)
Fehling solution is used in detection ofglucose

|
Priyanka Iyer answered |
Tollen's reagent is used to detect of
aldehydes. Tollen's reagent is an ammonical
solution of silver nitrate. When aldehyde is
added to Tollen's reagent, silver oxide is
reduced to metallic silver which deposits as
mirror.
aldehydes. Tollen's reagent is an ammonical
solution of silver nitrate. When aldehyde is
added to Tollen's reagent, silver oxide is
reduced to metallic silver which deposits as
mirror.
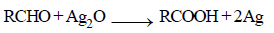
Which one of the following compounds will be most readily dehydrated? [2010]- a)
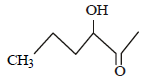
- b)
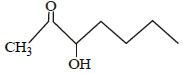
- c)
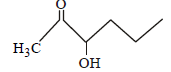
- d)
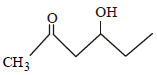
Correct answer is option 'D'. Can you explain this answer?
Which one of the following compounds will be most readily dehydrated? [2010]
a)

b)

c)

d)


|
Raghav Khanna answered |
The intermediate is carbocation which is
destabilised by C = O group (present on ��-
carbon to the –OH group) in the first three
cases. In (d), α–hydrogen is more acidic which
can be removed as water. Moreover, the
positive charge on the intermediate carbocation
is relatively away from the C = O group.
destabilised by C = O group (present on ��-
carbon to the –OH group) in the first three
cases. In (d), α–hydrogen is more acidic which
can be removed as water. Moreover, the
positive charge on the intermediate carbocation
is relatively away from the C = O group.
∴ Correct choice : (d)
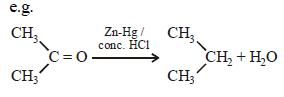
Clemmensen reduction of a ketone is carried outin the presence of which of the following ? [2011]- a)Glycol with KOH
- b)Zn-Hg with HCl
- c)Li Al H4
- d)H2 and Pt as catalyst
Correct answer is option 'B'. Can you explain this answer?
Clemmensen reduction of a ketone is carried outin the presence of which of the following ? [2011]
a)
Glycol with KOH
b)
Zn-Hg with HCl
c)
Li Al H4
d)
H2 and Pt as catalyst
|
|
Sankar Banerjee answered |
Clemmensen Reduction and Its Mechanism:
The Clemmensen reduction is a chemical reaction that involves the conversion of a carbonyl group in a ketone to a methylene group using zinc amalgam and hydrochloric acid. This process is particularly effective for reducing ketones to alkanes.
Role of Zn-Hg with HCl:
- In the Clemmensen reduction, zinc amalgam (Zn-Hg) serves as the reducing agent while hydrochloric acid (HCl) acts as a catalyst.
- The reaction occurs at high temperatures, typically refluxing conditions, to facilitate the reduction of the carbonyl group.
- The zinc amalgam reacts with the carbonyl group in the ketone, leading to the formation of an alkyl zinc intermediate.
- The alkyl zinc intermediate is unstable and undergoes protonation by HCl to form the corresponding alkane.
Comparison with Other Reagents:
- Glycol with KOH is commonly used for cleaving ethers, not for the Clemmensen reduction of ketones.
- LiAlH4 is a powerful reducing agent that can reduce ketones to alcohols, but it is not typically used in the Clemmensen reduction.
- H2 and Pt as a catalyst are commonly used for hydrogenation reactions, not for the Clemmensen reduction.
In conclusion, the Clemmensen reduction of a ketone is carried out in the presence of zinc amalgam and hydrochloric acid. These reagents work together to reduce the carbonyl group in the ketone to a methylene group, producing the desired alkane product.
The cyanohydrin of a compound on hydrolysisgives an optically active α-hydroxy acid. Thecompound is [1999]- a)Diethyl ketone
- b)Formaldehyde
- c)Acetaldehyde
- d)Acetone
Correct answer is option 'C'. Can you explain this answer?
The cyanohydrin of a compound on hydrolysisgives an optically active α-hydroxy acid. Thecompound is [1999]
a)
Diethyl ketone
b)
Formaldehyde
c)
Acetaldehyde
d)
Acetone

|
Ritika Khanna answered |
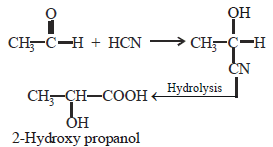
(As it has a chiral C-atom thus it is optically active)
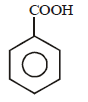
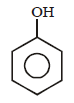
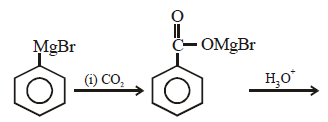
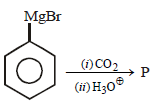 In the above reaction product 'P' is [2002]
In the above reaction product 'P' is [2002]- a)
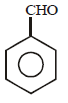
- b)
- c)
- d)
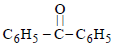
Correct answer is option 'B'. Can you explain this answer?
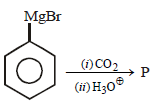
In the above reaction product 'P' is [2002]
a)

b)

c)

d)
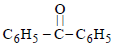

|
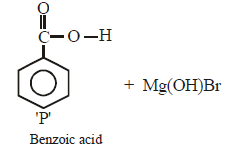
Ishani Nambiar answered |
Grignard reagent forms addition product with
bubbled carbondioxide which on hydrolysis
with HCl yields benzoic acid.
bubbled carbondioxide which on hydrolysis
with HCl yields benzoic acid.
Which of the following reactions will not resultin the formation of carbon-carbon bonds?- a)Reimer-Tieman reaction [2010]
- b)Cannizaro reaction
- c)Wurtz reaction
- d)Friedel-Crafts acylation
Correct answer is option 'B'. Can you explain this answer?
Which of the following reactions will not resultin the formation of carbon-carbon bonds?
a)
Reimer-Tieman reaction [2010]
b)
Cannizaro reaction
c)
Wurtz reaction
d)
Friedel-Crafts acylation

|
Aashna Mukherjee answered |
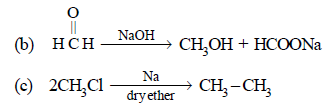
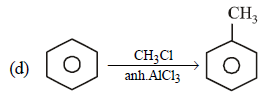
Note that new C–C bond is formed is a, c and d.
Which one of the following can be oxidised tothe corresponding carbonyl compound? [2004]- a)2-hydroxy-propane
- b)Ortho-nitro-phenol
- c)Phenol
- d)2-methyl-2 hydroxy-propane
Correct answer is option 'A'. Can you explain this answer?
Which one of the following can be oxidised tothe corresponding carbonyl compound? [2004]
a)
2-hydroxy-propane
b)
Ortho-nitro-phenol
c)
Phenol
d)
2-methyl-2 hydroxy-propane

|
Snehal Shah answered |
Carbonyl compounds (aldehydes and
ketones) are obtained by the oxidation of 1°
and 2° alcohols respectively. Among the
given options, only (a) is 2° alcohol hence it
can be oxidized to ketone.
ketones) are obtained by the oxidation of 1°
and 2° alcohols respectively. Among the
given options, only (a) is 2° alcohol hence it
can be oxidized to ketone.
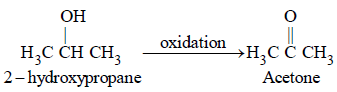
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]- a)phosphorus pentachloride
- b)hypochlorous acid
- c)chlorine
- d)hydrochloric acid
Correct answer is option 'A'. Can you explain this answer?
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]
a)
phosphorus pentachloride
b)
hypochlorous acid
c)
chlorine
d)
hydrochloric acid

|
Pragati Dey answered |
Phosphorus Pentachloride as a Reagent for Substitution Reactions
Phosphorus pentachloride (PCl5) is commonly used as a reagent for substitution reactions involving the replacement of hydroxyl (-OH) groups with chlorine. This reaction is especially common in the conversion of alcohols to alkyl chlorides.
Mechanism of the Reaction
- In the presence of PCl5, the lone pairs on the oxygen atom of the alcohol or carboxylic acid attack the electrophilic phosphorus atom in PCl5.
- This leads to the formation of an intermediate complex which then undergoes elimination of HCl to form the desired alkyl chloride or acyl chloride product.
Advantages of Using Phosphorus Pentachloride
- Phosphorus pentachloride is a cost-effective and readily available reagent.
- It allows for a rapid and efficient conversion of alcohols and carboxylic acids to their corresponding chlorides.
- The reaction conditions are relatively mild compared to other chlorinating agents.
Alternative Reagents
- Hypochlorous acid (HOCl) is not a suitable reagent for this type of substitution reaction as it is more commonly used as a bleaching agent.
- Chlorine gas and hydrochloric acid are not typically used for the direct substitution of hydroxyl groups with chlorine due to the harsh reaction conditions and potential side reactions that may occur.
In conclusion, phosphorus pentachloride is the most suitable reagent for replacing the -OH group of an alcohol or the -COOH group of a carboxylic acid with chlorine due to its efficiency, mild reaction conditions, and availability.
Phosphorus pentachloride (PCl5) is commonly used as a reagent for substitution reactions involving the replacement of hydroxyl (-OH) groups with chlorine. This reaction is especially common in the conversion of alcohols to alkyl chlorides.
Mechanism of the Reaction
- In the presence of PCl5, the lone pairs on the oxygen atom of the alcohol or carboxylic acid attack the electrophilic phosphorus atom in PCl5.
- This leads to the formation of an intermediate complex which then undergoes elimination of HCl to form the desired alkyl chloride or acyl chloride product.
Advantages of Using Phosphorus Pentachloride
- Phosphorus pentachloride is a cost-effective and readily available reagent.
- It allows for a rapid and efficient conversion of alcohols and carboxylic acids to their corresponding chlorides.
- The reaction conditions are relatively mild compared to other chlorinating agents.
Alternative Reagents
- Hypochlorous acid (HOCl) is not a suitable reagent for this type of substitution reaction as it is more commonly used as a bleaching agent.
- Chlorine gas and hydrochloric acid are not typically used for the direct substitution of hydroxyl groups with chlorine due to the harsh reaction conditions and potential side reactions that may occur.
In conclusion, phosphorus pentachloride is the most suitable reagent for replacing the -OH group of an alcohol or the -COOH group of a carboxylic acid with chlorine due to its efficiency, mild reaction conditions, and availability.
Ketones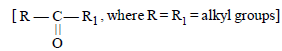 can be obtained in one step by [1998]
can be obtained in one step by [1998]- a)oxidation of primary alcohols
- b)hydrolysis of esters
- c)oxidation of tertiary alcohols
- d)reaction of acid halides with alcohols
Correct answer is option 'C'. Can you explain this answer?
Ketones
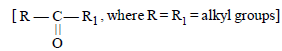 can be obtained in one step by [1998]
can be obtained in one step by [1998]a)
oxidation of primary alcohols
b)
hydrolysis of esters
c)
oxidation of tertiary alcohols
d)
reaction of acid halides with alcohols

|
Roshni Chavan answered |
By oxidation of tertiary alcohol with stronger oxidising agents ketones may be formed along with carboxylic acid.
Which of the following is correct? [2001]- a)Diastase is an enzyme
- b)Acetophenone is an ether
- c)Cycloheptane is an aromatic compound
- d)All the above
Correct answer is option 'A'. Can you explain this answer?
Which of the following is correct? [2001]
a)
Diastase is an enzyme
b)
Acetophenone is an ether
c)
Cycloheptane is an aromatic compound
d)
All the above

|
Ruchi Chopra answered |
Acetophenone is a ketone, cyclopentane
doesn’t contain (4n + 2)π electron hence is
not aromatic. Diastase is an enzyme used in
the preparation of Maltose (Malt sugar, C12
H22 O11) through hydrolysis of starch.
doesn’t contain (4n + 2)π electron hence is
not aromatic. Diastase is an enzyme used in
the preparation of Maltose (Malt sugar, C12
H22 O11) through hydrolysis of starch.
The relative reactivities of acyl compounds towardsnucleophilic substitution are in the order of : [2008]- a)Acyl chloride > Acid anhydride > Ester >Amide
- b)Ester > Acyl chloride > Amide > Acidanhydride
- c)Acid anhydride > Amide > Ester > Acylchloride
- d)Acyl chloride > Ester > Acid anhydride >Amide
Correct answer is option 'A'. Can you explain this answer?
The relative reactivities of acyl compounds towardsnucleophilic substitution are in the order of : [2008]
a)
Acyl chloride > Acid anhydride > Ester >Amide
b)
Ester > Acyl chloride > Amide > Acidanhydride
c)
Acid anhydride > Amide > Ester > Acylchloride
d)
Acyl chloride > Ester > Acid anhydride >Amide

|
Diya Datta answered |
The relative reactivities of acyl compounds
towards nucleophilic substitution follow
the order Acyl halides > Acid anhydride >
Ester > Amide. Thus the correct answer is
(a).
towards nucleophilic substitution follow
the order Acyl halides > Acid anhydride >
Ester > Amide. Thus the correct answer is
(a).
Which one of the following esters cannotundergo Claisen self-condensation? [1998]- a)CH3 —CH2 —CH2 —CH2 —COOC2H5
- b)C6H5COOC2H5
- c)C6H5CH2COOC2H5
- d)C6H11CH2COOC2H5
Correct answer is option 'B'. Can you explain this answer?
Which one of the following esters cannotundergo Claisen self-condensation? [1998]
a)
CH3 —CH2 —CH2 —CH2 —COOC2H5
b)
C6H5COOC2H5
c)
C6H5CH2COOC2H5
d)
C6H11CH2COOC2H5

|
Surbhi Das answered |
The ester having α hydrogen atom show
Claisen condensation reaction. We know that ethyl benzoate (C6H5COOC2H5) does not contain α-hydrogen. Therefore
C6H5COOC2H5 does not undergo Claisen self condensation.
Claisen condensation reaction. We know that ethyl benzoate (C6H5COOC2H5) does not contain α-hydrogen. Therefore
C6H5COOC2H5 does not undergo Claisen self condensation.
An organic compound ‘A’ on treatment with NH3 gives ‘B’ which on heating gives ‘C’, ‘C’ whentreated with Br2 in the presence of KOH produces ethylamine. Compound ‘A’ is:[2011 M]- a)CH3COOH
- b)CH3 CH2 CH2 COOH
- c)
- d)CH3CH2COOH
Correct answer is option 'D'. Can you explain this answer?
An organic compound ‘A’ on treatment with NH3 gives ‘B’ which on heating gives ‘C’, ‘C’ whentreated with Br2 in the presence of KOH produces ethylamine. Compound ‘A’ is:[2011 M]
a)
CH3COOH
b)
CH3 CH2 CH2 COOH
c)
d)
CH3CH2COOH

|
Surbhi Das answered |
Since, C when heated with Br2 in presence
of KOH produces ethylamine, hence it must
be propanamide and hence the organic
compound (A) will be propanoic acid. The
reactions follows.
of KOH produces ethylamine, hence it must
be propanamide and hence the organic
compound (A) will be propanoic acid. The
reactions follows.
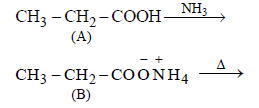
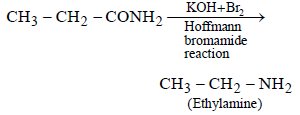
Which of the following represents the correctorder of the acidity in the given compounds? [2007]- a)FCH2COOH > CH3COOH > BrCH2COOH >ClCH2COOH
- b)BrCH2COOH > ClCH2COOH > FCH2COOH> CH3COOH
- c)FCH2COOH > ClCH2COOH > BrCH2COOH> CH3COOH
- d)CH3 COOH > BrCH2COOH > ClCH2COOH >FCH2COOH
Correct answer is option 'C'. Can you explain this answer?
Which of the following represents the correctorder of the acidity in the given compounds? [2007]
a)
FCH2COOH > CH3COOH > BrCH2COOH >ClCH2COOH
b)
BrCH2COOH > ClCH2COOH > FCH2COOH> CH3COOH
c)
FCH2COOH > ClCH2COOH > BrCH2COOH> CH3COOH
d)
CH3 COOH > BrCH2COOH > ClCH2COOH >FCH2COOH

|
Sneha Basak answered |
Electron withdrawing substituent (like
halogen, —NO2, C6H5 etc.) would disperse
the negative charge and hence stabilise the
carboxylate ion and thus increase acidity
of the parent acid. On the other hand,
electron-releasing substituents would
intensify the negative charge, destabilise
the carboxylate ion and thus decrease
acidity of the parent acid.
Electronegativity decreases in order
F > Cl > Br
and hence –I effect also decreases in the
same order, therefore the correct option is
halogen, —NO2, C6H5 etc.) would disperse
the negative charge and hence stabilise the
carboxylate ion and thus increase acidity
of the parent acid. On the other hand,
electron-releasing substituents would
intensify the negative charge, destabilise
the carboxylate ion and thus decrease
acidity of the parent acid.
Electronegativity decreases in order
F > Cl > Br
and hence –I effect also decreases in the
same order, therefore the correct option is
FCH2COOH > ClCH2COOH > BrCH2COOH> CH3COOH
Which of the following compounds will give ayellow precipitate with iodine and alkali ?[2012 M]- a)Acetophenone
- b)Acetamide
- c)Methyl acetate
- d)2-Hydroxypropane
Correct answer is option 'A,D'. Can you explain this answer?
Which of the following compounds will give ayellow precipitate with iodine and alkali ?[2012 M]
a)
Acetophenone
b)
Acetamide
c)
Methyl acetate
d)
2-Hydroxypropane

|
Nayanika Reddy answered |
It is iodoform reaction. Acetophenone
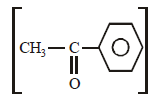 and 2-Hydroxypropane
and 2-Hydroxypropane 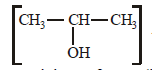 both give a yellow
both give a yellowprecipitate of CHI3 (iodoform) with iodine
and alkali.
The product formed in Aldol condensation is [2007]- a)a beta-hydroxy aldehyde or a beta-hydroxyketone
- b)an alpha-hydroxy aldehyde or ketone
- c)an alpha, beta unsaturated ester
- d)a beta-hydroxy acid
Correct answer is option 'A'. Can you explain this answer?
The product formed in Aldol condensation is [2007]
a)
a beta-hydroxy aldehyde or a beta-hydroxyketone
b)
an alpha-hydroxy aldehyde or ketone
c)
an alpha, beta unsaturated ester
d)
a beta-hydroxy acid

|
Priyanka Iyer answered |
Aldehydes and ketones having at least one
α-hydrogen atom in presence of dilute
alkali give β-hydroxy aldehyde or β-
hydroxy ketone
α-hydrogen atom in presence of dilute
alkali give β-hydroxy aldehyde or β-
hydroxy ketone
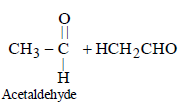
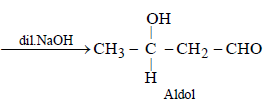
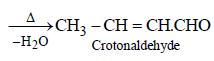
The order of stability of the following tautomeric compounds is :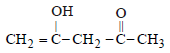
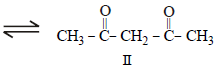
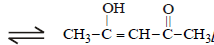
- a)III > II > I
- b)II > I > III
- c)II > III > I
- d)I > II > III
Correct answer is option 'A'. Can you explain this answer?
The order of stability of the following tautomeric compounds is :
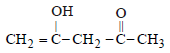
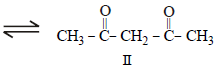
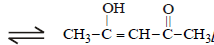
a)
III > II > I
b)
II > I > III
c)
II > III > I
d)
I > II > III

|
Prisha Singh answered |
Enolic form predominates in compounds
containing two carbonyl groups separated
by a – CH2 group. This is due to
following two factors.
(i) Presence of conjugation which
increases stability.
(ii) Formation of intramolecular hydrogen
bond between enolic hydroxyl
group and second carbonyl group
which leads to stablisation of the
molecule. Hence the correct answer is
III > II > I.
containing two carbonyl groups separated
by a – CH2 group. This is due to
following two factors.
(i) Presence of conjugation which
increases stability.
(ii) Formation of intramolecular hydrogen
bond between enolic hydroxyl
group and second carbonyl group
which leads to stablisation of the
molecule. Hence the correct answer is
III > II > I.
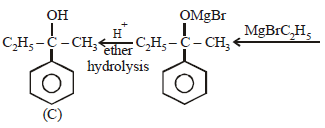
Phenylmethyl ketone can be converted intoethylbezene in one step by which of the followingreagents? [1999]- a)LiAlH4
- b)Zn-Hg/HCl
- c)NaBH4
- d)CH3MgI
Correct answer is option 'B'. Can you explain this answer?
Phenylmethyl ketone can be converted intoethylbezene in one step by which of the followingreagents? [1999]
a)
LiAlH4
b)
Zn-Hg/HCl
c)
NaBH4
d)
CH3MgI
|
|
Roshni Desai answered |
Conversion of Phenylmethyl ketone to Ethylbenzene
Introduction:
Phenylmethyl ketone, also known as acetophenone, can be converted into ethylbenzene through a one-step reaction using specific reagents. This conversion involves reducing the carbonyl group of the ketone to an alcohol and then further reducing it to a hydrocarbon.
Reagents:
The correct reagent for this one-step conversion is option 'B', which is Zn-Hg/HCl.
Explanation:
The Zn-Hg/HCl reagent is commonly referred to as Clemmensen reduction. It is a powerful reducing agent that is capable of converting carbonyl groups (such as ketones) into hydrocarbons.
Reaction mechanism:
The conversion of phenylmethyl ketone to ethylbenzene using Zn-Hg/HCl reagent can be explained through the following reaction mechanism:
1. Deprotonation:
The Zn-Hg/HCl reagent serves as a source of H- ions. In the presence of acidic conditions (HCl), the H- ions are generated, which can abstract a proton from the ketone.
2. Formation of carbanion:
The deprotonation of the ketone leads to the formation of a carbanion intermediate. This carbanion is stabilized by resonance with the aromatic ring.
3. Addition of Zn-Hg:
The carbanion intermediate then adds to the Zn-Hg, forming a new carbon-carbon bond. This step results in the formation of an alkyl zinc intermediate.
4. Acidic workup:
The alkyl zinc intermediate is then treated with an acidic workup, which involves the addition of HCl. This acidic environment helps in the removal of the zinc atom and the formation of the desired product, ethylbenzene.
Overall reaction:
The overall reaction can be summarized as follows:
Phenylmethyl ketone + Zn-Hg/HCl → Ethylbenzene
Conclusion:
In conclusion, the conversion of phenylmethyl ketone to ethylbenzene can be achieved in one step using Zn-Hg/HCl reagent. This reagent acts as a powerful reducing agent, allowing for the transformation of the carbonyl group into a hydrocarbon.
Introduction:
Phenylmethyl ketone, also known as acetophenone, can be converted into ethylbenzene through a one-step reaction using specific reagents. This conversion involves reducing the carbonyl group of the ketone to an alcohol and then further reducing it to a hydrocarbon.
Reagents:
The correct reagent for this one-step conversion is option 'B', which is Zn-Hg/HCl.
Explanation:
The Zn-Hg/HCl reagent is commonly referred to as Clemmensen reduction. It is a powerful reducing agent that is capable of converting carbonyl groups (such as ketones) into hydrocarbons.
Reaction mechanism:
The conversion of phenylmethyl ketone to ethylbenzene using Zn-Hg/HCl reagent can be explained through the following reaction mechanism:
1. Deprotonation:
The Zn-Hg/HCl reagent serves as a source of H- ions. In the presence of acidic conditions (HCl), the H- ions are generated, which can abstract a proton from the ketone.
2. Formation of carbanion:
The deprotonation of the ketone leads to the formation of a carbanion intermediate. This carbanion is stabilized by resonance with the aromatic ring.
3. Addition of Zn-Hg:
The carbanion intermediate then adds to the Zn-Hg, forming a new carbon-carbon bond. This step results in the formation of an alkyl zinc intermediate.
4. Acidic workup:
The alkyl zinc intermediate is then treated with an acidic workup, which involves the addition of HCl. This acidic environment helps in the removal of the zinc atom and the formation of the desired product, ethylbenzene.
Overall reaction:
The overall reaction can be summarized as follows:
Phenylmethyl ketone + Zn-Hg/HCl → Ethylbenzene
Conclusion:
In conclusion, the conversion of phenylmethyl ketone to ethylbenzene can be achieved in one step using Zn-Hg/HCl reagent. This reagent acts as a powerful reducing agent, allowing for the transformation of the carbonyl group into a hydrocarbon.
Consider the reaction :
RCHO + NH2NH2 → RCH = N – NH2What sort of reaction is it ? [2012 M]- a)Electrophilic addition – eliminationreaction
- b)Free radical addition – elimination reaction
- c)Electrophilic substitution – eliminationreaction
- d)Nucleophilic addition – elimination reaction
Correct answer is option 'D'. Can you explain this answer?
Consider the reaction :
RCHO + NH2NH2 → RCH = N – NH2
RCHO + NH2NH2 → RCH = N – NH2
What sort of reaction is it ? [2012 M]
a)
Electrophilic addition – eliminationreaction
b)
Free radical addition – elimination reaction
c)
Electrophilic substitution – eliminationreaction
d)
Nucleophilic addition – elimination reaction

|
Pallabi Reddy answered |
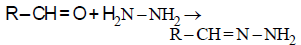
Such reactions take place in slightly acidic
medium and involve nucleophilic addition
of the ammonia derivative.
medium and involve nucleophilic addition
of the ammonia derivative.
Which one of the following on treatment with 50% aqueous sodium hydroxide yields the corresponding alcohol and acid? [2007]- a)C6H5CHO
- b)CH3CH2CH2CHO
- c)
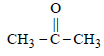
- d)C6H5CH2CHO
Correct answer is option 'A'. Can you explain this answer?
Which one of the following on treatment with 50% aqueous sodium hydroxide yields the corresponding alcohol and acid? [2007]
a)
C6H5CHO
b)
CH3CH2CH2CHO
c)
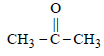
d)
C6H5CH2CHO

|
Ambition Institute answered |
- Aldehydes containing no α-hydrogen atom on warming with 50% NaOH or KOH undergo disproportionation i.e. self oxidation - reduction known as cannizzaro’s reaction.
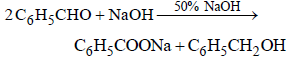
Following compounds are given:(a) CH3CH2OH(b) CH3COCH3(c) 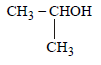 (d) CH3OHWhich of the above compound(s), on being warmed with iodine solution and NaOH, will give iodoform? [2010]
(d) CH3OHWhich of the above compound(s), on being warmed with iodine solution and NaOH, will give iodoform? [2010]- a)(a) and (b)
- b)(a), (c) and (d)
- c)only (b)
- d)(a), (b) and (c)
Correct answer is option 'D'. Can you explain this answer?
Following compounds are given:
(a) CH3CH2OH
(b) CH3COCH3
(c) 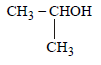
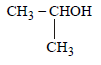
(d) CH3OH
Which of the above compound(s), on being warmed with iodine solution and NaOH, will give iodoform? [2010]
a)
(a) and (b)
b)
(a), (c) and (d)
c)
only (b)
d)
(a), (b) and (c)

|
Charvi Shah answered |
Among the given compounds only
CH3OH does not give iodoform reaction.
∴ Correct choice : (d)
CH3OH does not give iodoform reaction.
∴ Correct choice : (d)
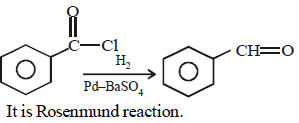
The catalyst used in Rosenmund's reduction is [2000]- a)HgSO4
- b)Pd/BaSO4
- c)anhydrous AlCl3
- d)anhydrous ZnCl2
Correct answer is option 'B'. Can you explain this answer?
The catalyst used in Rosenmund's reduction is [2000]
a)
HgSO4
b)
Pd/BaSO4
c)
anhydrous AlCl3
d)
anhydrous ZnCl2

|
Snehal Shah answered |
Catalyst used in Rosenmund reduction is Pd/
BaSO4. Rosenmund Reduction is used for
reduction of acid chloride.
BaSO4. Rosenmund Reduction is used for
reduction of acid chloride.
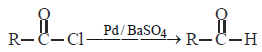
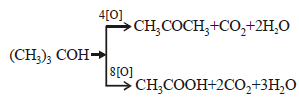
(CH3)3C—CHO does not undergo Aldol condensation due to [1996]- a)three electron donating methyl groups
- b)cleavage taking place between —C— CHObond
- c)absence of alpha hydrogen atom in themolecule
- d)bulky (CH3)3 C—group
Correct answer is option 'C'. Can you explain this answer?
(CH3)3C—CHO does not undergo Aldol condensation due to [1996]
a)
three electron donating methyl groups
b)
cleavage taking place between —C— CHObond
c)
absence of alpha hydrogen atom in themolecule
d)
bulky (CH3)3 C—group

|
Rounak Desai answered |
Aldol condensation is given by the
compounds which contain α hydrogen
atom. As the given compound does not
contain α hydrogen atom. Hence it does not undergo aldol condensation.
compounds which contain α hydrogen
atom. As the given compound does not
contain α hydrogen atom. Hence it does not undergo aldol condensation.
The correct order of decreasing acid strength oftrichloroacetic acid (A), trifluoroacetic acid (A),acetic acid (C) and formic acid (D) is : [2012]- a)B > A > D > C
- b)B > D > C > A
- c)A > B > C > D
- d)A > C > B > D
Correct answer is option 'A'. Can you explain this answer?
The correct order of decreasing acid strength oftrichloroacetic acid (A), trifluoroacetic acid (A),acetic acid (C) and formic acid (D) is : [2012]
a)
B > A > D > C
b)
B > D > C > A
c)
A > B > C > D
d)
A > C > B > D

|
Arindam Khanna answered |
CF3 COOH > CCl3 COOH > HCOOH >
CH3COOH (Ka order)
The halogenated fatty acids are much
stronger acids than the parent fatty acid
and more over the acidity among the
halogenated fatty acid is increased almost
proportionately with the increase in
electronegativity of the halogen present.
Further formic acid having no alky group is
more acidic than acetic acid.
CH3COOH (Ka order)
The halogenated fatty acids are much
stronger acids than the parent fatty acid
and more over the acidity among the
halogenated fatty acid is increased almost
proportionately with the increase in
electronegativity of the halogen present.
Further formic acid having no alky group is
more acidic than acetic acid.
Nucleophilic addition reaction will be mostfavoured in [2006]- a)(CH3)2C = O
- b)CH3CH2CHO
- c)CH3CHO
- d)
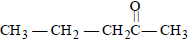
Correct answer is option 'C'. Can you explain this answer?
Nucleophilic addition reaction will be mostfavoured in [2006]
a)
(CH3)2C = O
b)
CH3CH2CHO
c)
CH3CHO
d)
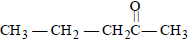

|
Naveen Menon answered |
Aldehydes are more reactive than ketones
due to +I effect of –CH3 group. There are
two – CH3 group in acetone which reduces
+ve charge density on carbon atom of
carbonyl group. More hindered carbonyl
group too becomes less reactive. So in the
give case CH3CHO is the right choice.
due to +I effect of –CH3 group. There are
two – CH3 group in acetone which reduces
+ve charge density on carbon atom of
carbonyl group. More hindered carbonyl
group too becomes less reactive. So in the
give case CH3CHO is the right choice.
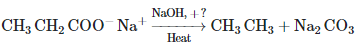
Consider the above reaction and identify the missing reagent/chemical. [2021]- a)CaO
- b)DIBAL-H
- c)B2H6
- d)Red Phosphorus
Correct answer is option 'A'. Can you explain this answer?
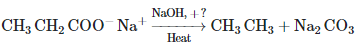
Consider the above reaction and identify the missing reagent/chemical. [2021]
a)
CaO
b)
DIBAL-H
c)
B2H6
d)
Red Phosphorus
|
|
Ajay Yadav answered |
Decarboxylation takes place by soda-lime (NaOH + CaO)
Acetone is treated with excess of ethanol in the presence of hydrochloric acid. The product obtained is : [2012]- a)
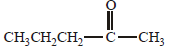
- b)
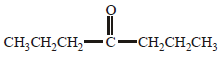
- c)
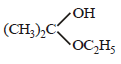
- d)
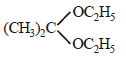
Correct answer is option 'D'. Can you explain this answer?
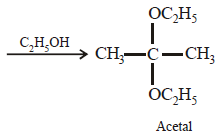
Acetone is treated with excess of ethanol in the presence of hydrochloric acid. The product obtained is : [2012]
a)
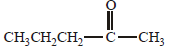
b)
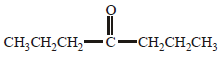
c)
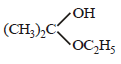
d)
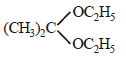

|
Krish Saha answered |
Anhydrous alcohols add to the carbonyl
group of aldehydes in the presence of
anhydrous hydrogen chloride to form
acetals via hemiacetals.
group of aldehydes in the presence of
anhydrous hydrogen chloride to form
acetals via hemiacetals.
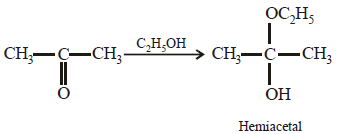
A carbonyl compound reacts with hydrogencyanide to form cyanohydrin which on hydrolysisforms a racemic mixture of α-hydroxy acid. Thecarbonyl compound is [2006]- a)acetone
- b)diethyl ketone
- c)formaldehyde
- d)acetaldehyde
Correct answer is option 'D'. Can you explain this answer?
A carbonyl compound reacts with hydrogencyanide to form cyanohydrin which on hydrolysisforms a racemic mixture of α-hydroxy acid. Thecarbonyl compound is [2006]
a)
acetone
b)
diethyl ketone
c)
formaldehyde
d)
acetaldehyde

|
Ayush Choudhury answered |
Out of given compound only acetaldehyde
can form optical active hydroxy acid as it
contains one asymmetric carbon atom as
marked below :
can form optical active hydroxy acid as it
contains one asymmetric carbon atom as
marked below :
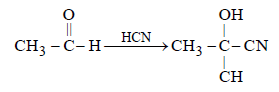
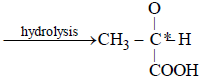
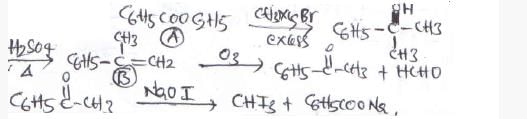
An ester (A) with molecular fomula, C9H10O2 wastreated with excess of CH3MgBr and the complexso formed was treated with H2SO4 to give anolefin (B). Ozonolysis of (B) gave a ketone withmolecular formula C8H8O which shows +veiodoform test. The structure of (A) is [1998]- a)C6H5COOC2H5
- b)C2H5COOC6H5
- c)H3COCH2COC6H5
- d)p—H3CO—C6H4 —COCH3
Correct answer is option 'A'. Can you explain this answer?
An ester (A) with molecular fomula, C9H10O2 wastreated with excess of CH3MgBr and the complexso formed was treated with H2SO4 to give anolefin (B). Ozonolysis of (B) gave a ketone withmolecular formula C8H8O which shows +veiodoform test. The structure of (A) is [1998]
a)
C6H5COOC2H5
b)
C2H5COOC6H5
c)
H3COCH2COC6H5
d)
p—H3CO—C6H4 —COCH3

|
Mahi Shah answered |
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure. [2008]- a)
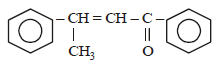
- b)
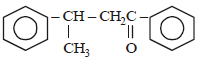
- c)
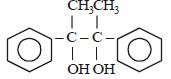
- d)
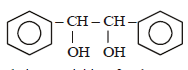
Correct answer is option 'A'. Can you explain this answer?
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure. [2008]
a)
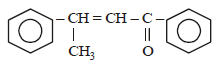
b)
c)

d)
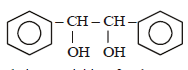

|
Srishti Sen answered |
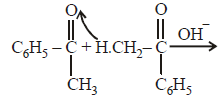
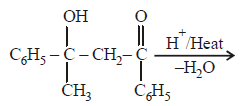
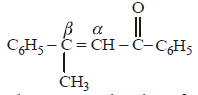
Thus two molecules of acetophenone
condense to form a β-hydroxy ketone which
gets dehydrated in the presence of acid
upon heating to form α β -unsaturated
compound.
i.e., option (a) is correct.
[Note: It is aldol condensation].
condense to form a β-hydroxy ketone which
gets dehydrated in the presence of acid
upon heating to form α β -unsaturated
compound.
i.e., option (a) is correct.
[Note: It is aldol condensation].
Reaction by which Benzaldehyde cannot be prepared : [NEET 2013]- a)
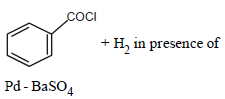
- b)
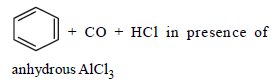
- c)
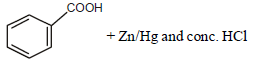
- d)
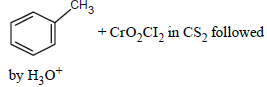
Correct answer is option 'C'. Can you explain this answer?
Reaction by which Benzaldehyde cannot be prepared : [NEET 2013]
a)
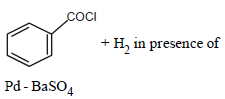
b)
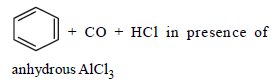
c)
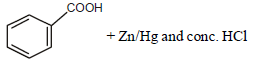
d)
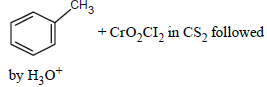

|
Shanaya Rane answered |
Zn/Hg and HCl reduce carboxyl group to
methylene group (Clemmensen reduction).
methylene group (Clemmensen reduction).
The order of reactivity of phenyl magnesium bromide (PhMgBr) with the following compounds [2011 M]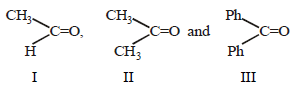
- a)III > II > I
- b)II > I > III
- c)I > III > II
- d)I > II > III
Correct answer is option 'D'. Can you explain this answer?
The order of reactivity of phenyl magnesium bromide (PhMgBr) with the following compounds [2011 M]
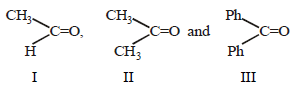
a)
III > II > I
b)
II > I > III
c)
I > III > II
d)
I > II > III

|
Nilanjan Chakraborty answered |
The reactivity of the carbonyl group toward
the nucleophilic addition reactions depend
upon the magnitude of the positive charge
on the carbonyl carbon atom (electronic
factor) and also on the crowding around
the carboxyl carbon atom in the transition
state (steric factor). Both these factors
predict the following order
the nucleophilic addition reactions depend
upon the magnitude of the positive charge
on the carbonyl carbon atom (electronic
factor) and also on the crowding around
the carboxyl carbon atom in the transition
state (steric factor). Both these factors
predict the following order
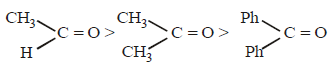
(due to steric crowding).
Chapter doubts & questions for Aldehydes, Ketones and Carboxylic Acids - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aldehydes, Ketones and Carboxylic Acids - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup

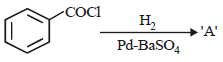

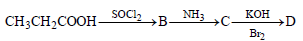

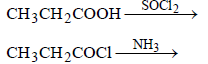
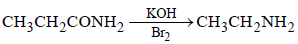


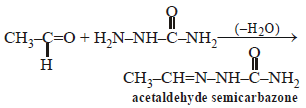

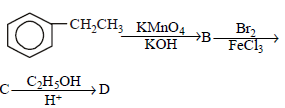
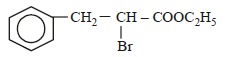
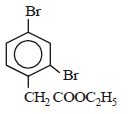
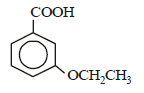
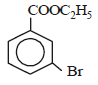

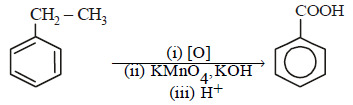
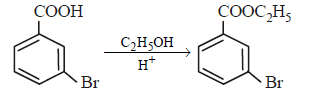
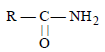

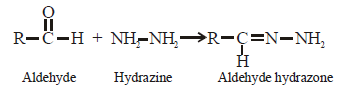




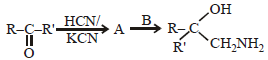
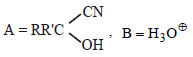
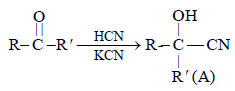
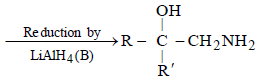
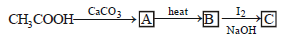
 is [1996]
is [1996]