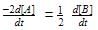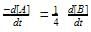All Exams >
NEET >
Physical Chemistry for NEET >
All Questions
All questions of Chemical Kinetics for NEET Exam
Direction (Q. Nos. 14 and 15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
The decomposition of NO2 at 400 K proceeds at a of rate of 5.4 x 10 -5 mol L-1 s-1 when [NO2] = 0.01 mol-1
2 NO2(g) → 2NO(g ) + O2(g).
Q. What is the rate law when observed rate is 1.35 x 10-5 mol L-1 s-1 at [NO2] = 0.005 mol L-1?
- a)k[NO2]
- b)k[NO2]0
- c)k[NO2]3
- d)k[NO2]2
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 14 and 15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
The decomposition of NO2 at 400 K proceeds at a of rate of 5.4 x 10 -5 mol L-1 s-1 when [NO2] = 0.01 mol-1
2 NO2(g) → 2NO(g ) + O2(g).
Q. What is the rate law when observed rate is 1.35 x 10-5 mol L-1 s-1 at [NO2] = 0.005 mol L-1?
a)
k[NO2]
b)
k[NO2]0
c)
k[NO2]3
d)
k[NO2]2
|
|
Geetika Shah answered |
Given unit of rate = mol L-1 s-1
General formula for unit of rate = mol n-1 Ln-1 s -1
On equating,
1-n = 1 ⇒ n = 0
n-1 = -1 ⇒ n = 0
General formula for unit of rate = mol n-1 Ln-1 s -1
On equating,
1-n = 1 ⇒ n = 0
n-1 = -1 ⇒ n = 0
Hence, it is a zero order reaction.
t1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value.
If the rate constant for a first order reaction is K, t1/4 can be written as – [AIEEE-2005]- a)0.29/K
- b)0.10/K
- c)0.75/K
- d)0.69/K
Correct answer is option 'A'. Can you explain this answer?
t1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value.
If the rate constant for a first order reaction is K, t1/4 can be written as – [AIEEE-2005]
If the rate constant for a first order reaction is K, t1/4 can be written as – [AIEEE-2005]
a)
0.29/K
b)
0.10/K
c)
0.75/K
d)
0.69/K

|
Aadhar Academy answered |
The correct answer is option A
Let initial concentration be ao
After concentration decreases by
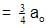
Using first order kinetic equation,
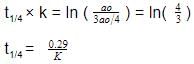
Let initial concentration be ao
After concentration decreases by

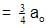
Using first order kinetic equation,
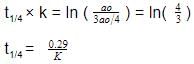
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is- a)43.85 kJ/mol
- b)55.3 kJ/mol
- c)11.97 kJ/mol
- d)6.65 kJ/mol
Correct answer is option 'B'. Can you explain this answer?
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is
a)
43.85 kJ/mol
b)
55.3 kJ/mol
c)
11.97 kJ/mol
d)
6.65 kJ/mol
|
|
Tanuja Kapoor answered |
t1/2 = 20 min at 300 K
t’1/2 = 5 min at 320 K
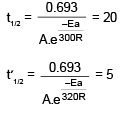
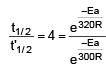
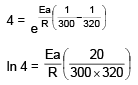
On solving, Ea = 55303.12 J
= 55.3 KJ
t’1/2 = 5 min at 320 K
On solving, Ea = 55303.12 J
= 55.3 KJ
The ratio of the rate constant of a reaction at two temperatures differing by __________0C is called temperature coefficient of reaction.- a)2
- b)10
- c)100
- d)50
Correct answer is option 'B'. Can you explain this answer?
The ratio of the rate constant of a reaction at two temperatures differing by __________0C is called temperature coefficient of reaction.
a)
2
b)
10
c)
100
d)
50

|
Devika Banerjee answered |
The ratio of the rate constant of a reaction at two temperatures differing by 100C is called temperature coefficient of reaction.
The initial rates of reaction for the equation, 2A + B → Products.Products were determined under various initial concentrations of reactants. Thus, rate law is equal to
Thus, rate law is equal to- a)k[A][B]
- b)k[A][B]2
- c) k[A]
- d) k[B]
Correct answer is option 'C'. Can you explain this answer?
The initial rates of reaction for the equation, 2A + B → Products.
Products were determined under various initial concentrations of reactants.
Thus, rate law is equal to
a)
k[A][B]
b)
k[A][B]2
c)
k[A]
d)
k[B]
|
|
Ritu Singh answered |
Let order w.r.t. A = a, order w.r.t. B = b
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about [AIEEE 2011]a)64 timesb)32 timesc)24 timesd)10 timesCorrect answer is option 'B'. Can you explain this answer?
|
|
Vijay Bansal answered |
Rate at 50 degree C/(Rate at T1 degree C)
= (2)^(ΔT/T1)
= (2)^(50/10)
= 2^5
= 32
Hence, the rate of the reaction increases by 32 times.
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?- a)0.25 h
- b)1 h
- c)4 h
- d)0.5 h
Correct answer is option 'A'. Can you explain this answer?
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?
a)
0.25 h
b)
1 h
c)
4 h
d)
0.5 h

|
Shashi Kumar answered |
For a zero order reaction,
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
Rate of reaction can be expressed by Arrhenius equation as k = Ae–E/RT , In this equation, E represents[AIEEE 2006]- a)the energy below which colliding molecules will not react
- b)the total energy of the reacting molecule at a temperature, T
- c)the fraction of molecules with energy greater than the activation energy of the reaction
- d)the energy above which all the colliding molecules will react
Correct answer is option 'A'. Can you explain this answer?
Rate of reaction can be expressed by Arrhenius equation as k = Ae–E/RT , In this equation, E represents
[AIEEE 2006]
a)
the energy below which colliding molecules will not react
b)
the total energy of the reacting molecule at a temperature, T
c)
the fraction of molecules with energy greater than the activation energy of the reaction
d)
the energy above which all the colliding molecules will react
|
|
Priyanka Sharma answered |
The correct answer is Option A.
E represents the energy of activation which implies it is the energy below which colliding molecules will not react Arrhenius equation gives the dependence of the rate constant k of a chemical reaction on the absolute temperature T (in Kelvin), where A is the pre-exponential factor (or simply the prefactor), Ea is the activation energy, and R is the Universal gas constant:
By Arrhenius equation, k=Ae-Ea/RT
By Arrhenius equation, k=Ae-Ea/RT
The effect of temperature on reaction rate is given by- a)Arrhenius equation
- b)Kirchoff’s Equation
- c)Clauius Claperyron equation
- d)Gibb’s Helmholtz equation
Correct answer is option 'A'. Can you explain this answer?
The effect of temperature on reaction rate is given by
a)
Arrhenius equation
b)
Kirchoff’s Equation
c)
Clauius Claperyron equation
d)
Gibb’s Helmholtz equation
|
|
Shanaya Choudhary answered |
Effect of Temperature on Reaction Rate - Arrhenius Equation
The Arrhenius equation explains the effect of temperature on the reaction rate. It is a mathematical formula that relates the rate constant of a chemical reaction to the temperature and activation energy of the reaction.
The equation is given by:
k = A * e^(-Ea/RT)
Where,
k - rate constant
A - pre-exponential factor
Ea - activation energy
R - gas constant
T - temperature
Explanation of the Equation:
Pre-Exponential Factor (A): It is a constant that reflects the frequency at which reactant molecules collide with each other. A higher value of A means that more collisions occur, thus increasing the reaction rate.
Activation Energy (Ea): It is the minimum energy required to initiate a chemical reaction. A higher value of Ea means that more energy is required to initiate the reaction, thus decreasing the reaction rate.
Gas Constant (R): It is a constant that relates the energy of a system to its temperature.
Temperature (T): It is the measure of the average kinetic energy of the molecules in a system. As the temperature increases, the kinetic energy of the molecules increases, leading to more collisions and thus increasing the reaction rate.
Application of the Equation:
The Arrhenius equation is widely used in chemical kinetics to predict the effect of temperature on the reaction rate. It is also used to determine the activation energy of a reaction by measuring the rate constant at different temperatures.
Conclusion:
In conclusion, the Arrhenius equation is a useful tool to understand the effect of temperature on the reaction rate. It helps in predicting the rate of a chemical reaction at different temperatures and determining the activation energy of a reaction.
The Arrhenius equation explains the effect of temperature on the reaction rate. It is a mathematical formula that relates the rate constant of a chemical reaction to the temperature and activation energy of the reaction.
The equation is given by:
k = A * e^(-Ea/RT)
Where,
k - rate constant
A - pre-exponential factor
Ea - activation energy
R - gas constant
T - temperature
Explanation of the Equation:
Pre-Exponential Factor (A): It is a constant that reflects the frequency at which reactant molecules collide with each other. A higher value of A means that more collisions occur, thus increasing the reaction rate.
Activation Energy (Ea): It is the minimum energy required to initiate a chemical reaction. A higher value of Ea means that more energy is required to initiate the reaction, thus decreasing the reaction rate.
Gas Constant (R): It is a constant that relates the energy of a system to its temperature.
Temperature (T): It is the measure of the average kinetic energy of the molecules in a system. As the temperature increases, the kinetic energy of the molecules increases, leading to more collisions and thus increasing the reaction rate.
Application of the Equation:
The Arrhenius equation is widely used in chemical kinetics to predict the effect of temperature on the reaction rate. It is also used to determine the activation energy of a reaction by measuring the rate constant at different temperatures.
Conclusion:
In conclusion, the Arrhenius equation is a useful tool to understand the effect of temperature on the reaction rate. It helps in predicting the rate of a chemical reaction at different temperatures and determining the activation energy of a reaction.
The reactions with low activation energy are- a)Slow
- b)Non-spontaneous
- c)Fast
- d)Always spontaneous
Correct answer is option 'C'. Can you explain this answer?
The reactions with low activation energy are
a)
Slow
b)
Non-spontaneous
c)
Fast
d)
Always spontaneous
|
|
Vivek Godara answered |
Low activation energy mean energy reguire for reaction to occur is low so product making is fast
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about- a)64 times
- b)10 times
- c)24 times
- d)32 times
Correct answer is option 'D'. Can you explain this answer?
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about
a)
64 times
b)
10 times
c)
24 times
d)
32 times

|
Mohit Rajpoot answered |
For every 10°C rise of temperature, the rate is doubled. Thus, the temperature coefficient of the reaction = 2
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
A foreign substance that increase the speed of a chemical reaction is called- a)promotor
- b)catalyst
- c)moderator
- d)inhibitor
Correct answer is option 'B'. Can you explain this answer?
A foreign substance that increase the speed of a chemical reaction is called
a)
promotor
b)
catalyst
c)
moderator
d)
inhibitor
|
|
Nandini Patel answered |
Catalyst: Substances which alter the rate of a chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction are known as catalysts and the phenomenon is known as catalysis.
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in- a)24 min
- b)4 min
- c)16 min
- d)8 min
Correct answer is option 'C'. Can you explain this answer?
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in
a)
24 min
b)
4 min
c)
16 min
d)
8 min

|
Avi Chawla answered |
75% completion means 2 half lifes so 50% completion means only one half life.
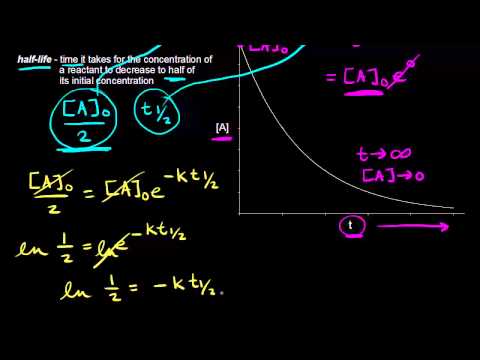
Read the passage given below and answer the following questions:The half-life of a reaction is the time required for the concentration of reactant to decrease by half, i.e.,[A]t = [A]/2For first order reaction,t1/2 = 0.693/kThis means t1/2 is independent of initial concentration. Figure shows that typical variation of concentration of reactant exhibiting first order kinetics. It may be noted that though the major portion of the first order kinetics may be over in a finite time, but the reaction will never cease as the concentration of reactant will be zero only at infinite time.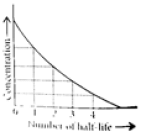 The following questions are multiple choice questions. Choose the most appropriate answer:Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?
The following questions are multiple choice questions. Choose the most appropriate answer:Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?- a)2.303 s
- b)23.03 s
- c)230.3 s
- d)2303 s
Correct answer is option 'C'. Can you explain this answer?
Read the passage given below and answer the following questions:
The half-life of a reaction is the time required for the concentration of reactant to decrease by half, i.e.,
[A]t = [A]/2
For first order reaction,
t1/2 = 0.693/k
This means t1/2 is independent of initial concentration. Figure shows that typical variation of concentration of reactant exhibiting first order kinetics. It may be noted that though the major portion of the first order kinetics may be over in a finite time, but the reaction will never cease as the concentration of reactant will be zero only at infinite time.

The following questions are multiple choice questions. Choose the most appropriate answer:
Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?
a)
2.303 s
b)
23.03 s
c)
230.3 s
d)
2303 s

|
Ambition Institute answered |
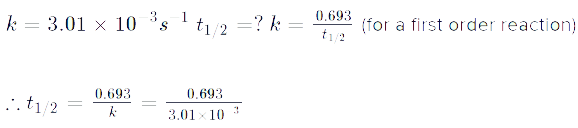
The following mechanism has been proposed for the exothermic catalyzed complex reaction.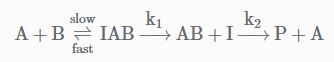 If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
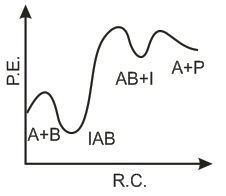
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.- a)
- b)
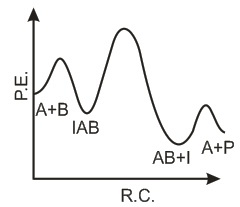
- c)
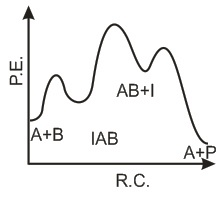
- d)
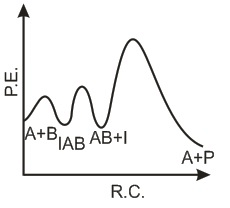
Correct answer is option 'C'. Can you explain this answer?
The following mechanism has been proposed for the exothermic catalyzed complex reaction.
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
a)

b)

c)

d)


|
Imk Pathsala answered |
Since
K1 <<< K2 = most Imp. peack will be higher
The rate law for a reaction between the substances A and 8 is given byrate = k[A]n [B]mIf concentration of A is doubled and that of 8 is halved, the new rate as compared to the earlier rate would be - a)
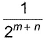
- b)(m + n)
- c)(n - m)
- d)2(n-m)
Correct answer is option 'D'. Can you explain this answer?
The rate law for a reaction between the substances A and 8 is given by
rate = k[A]n [B]m
If concentration of A is doubled and that of 8 is halved, the new rate as compared to the earlier rate would be
a)
b)
(m + n)
c)
(n - m)
d)
2(n-m)

|
Top Rankers answered |
The energies of activation for forward and reverse reactions for A2 + B2  2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) – [AIEEE 2007]
2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) – [AIEEE 2007]- a)300
- b)120
- c)280
- d)20
Correct answer is option 'D'. Can you explain this answer?
The energies of activation for forward and reverse reactions for A2 + B2  2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) – [AIEEE 2007]
2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) – [AIEEE 2007]
a)
300
b)
120
c)
280
d)
20
|
|
Naina Bansal answered |
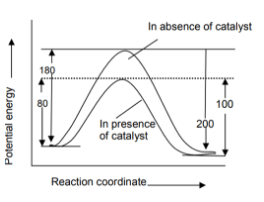
So, ∆ =− HReaction Ef- Eb
= 80 – 100 = -20
A reaction is 50% completed in 2 hours and 75% completed in 4 hours. The order of reaction is [NEET Kar. 2013]- a)0
- b)1
- c)2
- d)3
Correct answer is option 'B'. Can you explain this answer?
A reaction is 50% completed in 2 hours and 75% completed in 4 hours. The order of reaction is [NEET Kar. 2013]
a)
0
b)
1
c)
2
d)
3

|
Naveen Menon answered |
For a first order reaction, t75% = 2 × t50%
During the kinetic study of the reaction, 2A + B → C + D, following results were obtained:
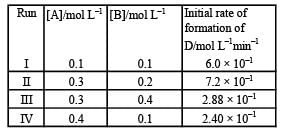 Based on the above data which one of the following is correct? [2010]
Based on the above data which one of the following is correct? [2010]
- a)rate = k [A]2 [B]
- b)rate = k[A] [B]
- c)rate = k [A]2 [B]2
- d)rate = k [A] [B]2
Correct answer is option 'D'. Can you explain this answer?
During the kinetic study of the reaction, 2A + B → C + D, following results were obtained:

Based on the above data which one of the following is correct? [2010]
a)
rate = k [A]2 [B]
b)
rate = k[A] [B]
c)
rate = k [A]2 [B]2
d)
rate = k [A] [B]2

|
Krish Khanna answered |
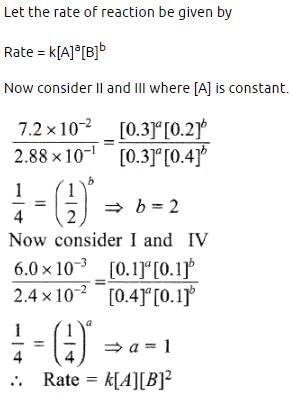
The unit of rate constant for a first order reaction is- a)Mol/L
- b)Mol2 / L2 / S2
- c)S-1
- d)Mol/L/S
Correct answer is option 'C'. Can you explain this answer?
The unit of rate constant for a first order reaction is
a)
Mol/L
b)
Mol2 / L2 / S2
c)
S-1
d)
Mol/L/S
|
|
Nikita Singh answered |
The correct answer is Option C.
Let R be the rate of reaction.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
The rate is independent of the concentration of the reactants in- a)First order
- b)Second order
- c)Third order
- d)Zero order
Correct answer is option 'D'. Can you explain this answer?
The rate is independent of the concentration of the reactants in
a)
First order
b)
Second order
c)
Third order
d)
Zero order
|
|
Naiham Difoesa answered |
Bcz rate is proportional to the zeroth power of the concentration of reactant.
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correctQ. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?4NH3 + 502 → 4NO + 6H2O- a)O2
- b)NO
- c)H2O
- d)Equal
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?
4NH3 + 502 → 4NO + 6H2O
a)
O2
b)
NO
c)
H2O
d)
Equal

|
Nabanita Basu answered |
Understanding the Reaction
The given reaction is:
4NH3 + 5O2 → 4NO + 6H2O
This reaction involves the disappearance of ammonia (NH3) and the appearance of the products NO and H2O.
Rate of Reaction
The rate of a chemical reaction can be expressed in terms of the rate of disappearance of reactants or the rate of appearance of products.
Stoichiometry of the Reaction
- According to the stoichiometry:
- 4 moles of NH3 produce 4 moles of NO and 6 moles of H2O.
- The coefficients in the balanced equation indicate the relative rates of disappearance and appearance.
Rate of Disappearance
- The rate of disappearance of NH3 is given by:
Rate = - (1/4) * d[NH3]/dt
- The rates for O2, NO, and H2O can be expressed similarly:
- O2: Rate = - (1/5) * d[O2]/dt
- NO: Rate = (1/4) * d[NO]/dt
- H2O: Rate = (1/6) * d[H2O]/dt
Comparison of Rates
To find the maximum rate of disappearance, we can compare the rates derived from the balanced equation:
- NH3: - (1/4) (for every 1 mole of disappearance)
- O2: - (1/5) (for every 1 mole of disappearance)
- NO: (1/4) (for every 1 mole of appearance)
- H2O: (1/6) (for every 1 mole of appearance)
The fractions reveal how many moles of each substance are involved in the reaction. The lower the denominator, the higher the rate of disappearance or appearance.
Conclusion
- Among the reactants and products, H2O has the highest coefficient when considering the rate of disappearance of NH3.
- Therefore, the maximum rate of disappearance is related to H2O's formation.
Thus, the correct answer is option 'C' (H2O).
The given reaction is:
4NH3 + 5O2 → 4NO + 6H2O
This reaction involves the disappearance of ammonia (NH3) and the appearance of the products NO and H2O.
Rate of Reaction
The rate of a chemical reaction can be expressed in terms of the rate of disappearance of reactants or the rate of appearance of products.
Stoichiometry of the Reaction
- According to the stoichiometry:
- 4 moles of NH3 produce 4 moles of NO and 6 moles of H2O.
- The coefficients in the balanced equation indicate the relative rates of disappearance and appearance.
Rate of Disappearance
- The rate of disappearance of NH3 is given by:
Rate = - (1/4) * d[NH3]/dt
- The rates for O2, NO, and H2O can be expressed similarly:
- O2: Rate = - (1/5) * d[O2]/dt
- NO: Rate = (1/4) * d[NO]/dt
- H2O: Rate = (1/6) * d[H2O]/dt
Comparison of Rates
To find the maximum rate of disappearance, we can compare the rates derived from the balanced equation:
- NH3: - (1/4) (for every 1 mole of disappearance)
- O2: - (1/5) (for every 1 mole of disappearance)
- NO: (1/4) (for every 1 mole of appearance)
- H2O: (1/6) (for every 1 mole of appearance)
The fractions reveal how many moles of each substance are involved in the reaction. The lower the denominator, the higher the rate of disappearance or appearance.
Conclusion
- Among the reactants and products, H2O has the highest coefficient when considering the rate of disappearance of NH3.
- Therefore, the maximum rate of disappearance is related to H2O's formation.
Thus, the correct answer is option 'C' (H2O).
Reaction kinetics deals with the study of- a)Rate of reaction
- b)Mechanism of reaction
- c)Factors which affects the rate of reaction
- d)All of the mentioned
Correct answer is option 'D'. Can you explain this answer?
Reaction kinetics deals with the study of
a)
Rate of reaction
b)
Mechanism of reaction
c)
Factors which affects the rate of reaction
d)
All of the mentioned
|
|
Om Desai answered |
Reaction kinetics deals with the study of rate of reaction, their mechanism and the factors which affects the rate of reaction. It specifies all the general characteristics of a chemical reaction.
Chapter doubts & questions for Chemical Kinetics - Physical Chemistry for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Kinetics - Physical Chemistry for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Physical Chemistry for NEET
117 videos|226 docs|237 tests
|


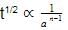

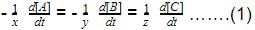
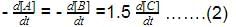
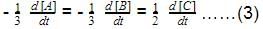
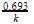
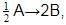 rate of disappearance of A is related to rate of appearance of B by the expression:
rate of disappearance of A is related to rate of appearance of B by the expression: