All Exams >
Class 6 >
Online MCQ Tests for Class 6 >
All Questions
All questions of Separation of Substances for Class 6 Exam
The process by which the unwanted solid particles are removed from the liquid is called- a)Filtration
- b)Decantation
- c)Sedimentation
- d)Loading
Correct answer is option 'A'. Can you explain this answer?
The process by which the unwanted solid particles are removed from the liquid is called
a)
Filtration
b)
Decantation
c)
Sedimentation
d)
Loading
|
|
Rajesh Khatri answered |
Filtration, the process in which solid particles in a liquid or gaseous fluid are removed by the use of a filter medium that permits the fluid to pass through but retains the solid particles. Either the clarified fluid or the solid particles removed from the fluid may be the desired product. In some processes used in the production of chemicals, both the fluid filtrate and the solid filter cake are recovered. Other media, such as electricity, light, and sound, also can be filtered.
The art of filtration was known to early humans, who obtained clear water from a muddy river by scooping a hole in the sand on a river bank to a depth below the river water level. Clear water filtered by the sand would trickle into the hole. The same process on a larger scale and with refinements is commonly used to purify water for cities.
This harmful microbe in water causes typhoid and jaundice- a)Virus
- b)Bacteria
- c)Algae
- d)Fungi
Correct answer is option 'B'. Can you explain this answer?
This harmful microbe in water causes typhoid and jaundice
a)
Virus
b)
Bacteria
c)
Algae
d)
Fungi
|
|
Meera Singh answered |
Harmful diseases like jaundice and typhoid are caused by bacteria.
Separating the insoluble suspended solids of various sizes from a liquid is called- a)Filtration
- b)Crystallization
- c)Evaporation
- d)Condensation
Correct answer is option 'A'. Can you explain this answer?
Separating the insoluble suspended solids of various sizes from a liquid is called
a)
Filtration
b)
Crystallization
c)
Evaporation
d)
Condensation

|
Jhanvi Banerjee answered |
Filtration is the process of separating suspended solid matter from a liquid, by causing the latter to pass through the pores of some substance, called a filter. The liquid which has passed through the filter is called the filtrate.
The properties used to separate two solids from a mixture by winnowing is- a)Attraction by magnet.
- b)Difference in weight
- c)Difference is colour
- d)Difference in size
Correct answer is option 'B'. Can you explain this answer?
The properties used to separate two solids from a mixture by winnowing is
a)
Attraction by magnet.
b)
Difference in weight
c)
Difference is colour
d)
Difference in size
|
|
Manish Singh answered |
This method of separating components of a mixture is calledwinnowing. Winnowing is used to separate heavier and lighter components of a mixture by wind or by blowing air. threshing is done with the help of bullocks. Ans is B
A saturated solution can dissolve more of a substance on- a)Cooling
- b)Heating
- c)Condensing
- d)Evaporating
Correct answer is option 'B'. Can you explain this answer?
A saturated solution can dissolve more of a substance on
a)
Cooling
b)
Heating
c)
Condensing
d)
Evaporating

|
Swara Mukherjee answered |
If saturated solution is heated the solution become unsaturated because solubility is directly depends upon temperature, so on heating solubility of the solution increase and its become unsaturated or in other words the solution is now capable to take some more solute into it.
Paneer is separated from curdled milk
- a)Condensation
- b)Filtration
- c)Evaporation
- d)Sedimentation
Correct answer is option 'B'. Can you explain this answer?
Paneer is separated from curdled milk
a)
Condensation
b)
Filtration
c)
Evaporation
d)
Sedimentation

|
Swara Mukherjee answered |
Explanation:
Paneer is separated from curdled milk through the process of filtration. When milk is curdled by adding an acid like lemon juice or vinegar, the solid curds (which become paneer) are separated from the liquid whey. Filtration is used to separate the solid curds from the liquid, leaving you with paneer.
How are grain seeds removed from their stalks?- a)Sieving
- b)Winnowing
- c)Threshing
- d)All of the above
Correct answer is option 'C'. Can you explain this answer?
How are grain seeds removed from their stalks?
a)
Sieving
b)
Winnowing
c)
Threshing
d)
All of the above

|
Shruti Mishra answered |
By threshing (i.e., beating the stalks on a slab) grain seeds are separated from their stalks.
Most of the substances that we see around us are:
- a)Element
- b)Compound
- c)Mixture
- d)Pure solution
Correct answer is option 'C'. Can you explain this answer?
Most of the substances that we see around us are:

a)
Element
b)
Compound
c)
Mixture
d)
Pure solution
|
|
Eduskill Classes answered |
Most of the substances that we see around us are a mixture of two or more substances.
Which of the following statements about a mixture is TRUE?- a)It is a pure substance.
- b)Its constituents are not combined chemically.
- c)Its constituents do not retain their individual properties.
- d)It is always homogeneous.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements about a mixture is TRUE?
a)
It is a pure substance.
b)
Its constituents are not combined chemically.
c)
Its constituents do not retain their individual properties.
d)
It is always homogeneous.

|
Upasana Basak answered |
In a mixture, the constituent substances are mixed only physically but not combined chemically. A mixture is not a pure substance as its constituents retain their properties. So, a mixture can be either homogeneous or heterogeneous.
A solution which cannot dissolve more of a given substance at a given temperature is- a)Solution
- b)Filtrate
- c)Saturated solution
- d)Unsaturated solution
Correct answer is option 'C'. Can you explain this answer?
A solution which cannot dissolve more of a given substance at a given temperature is
a)
Solution
b)
Filtrate
c)
Saturated solution
d)
Unsaturated solution
|
|
Birendra Pani answered |
The maximum amount of solute dissolved in solvent at any concentration is called Saturated solutions.in these solutions ,there is no solute can be added after the completion of the reaction.
The following flow chart which gives the techniques a student adopted to separate the constituents of a mixture.

What could R be?
- a)Sugar
- b)Chalk powder
- c)Glass
- d)Oxygen
Correct answer is option 'A'. Can you explain this answer?
The following flow chart which gives the techniques a student adopted to separate the constituents of a mixture.

What could R be?

What could R be?
a)
Sugar
b)
Chalk powder
c)
Glass
d)
Oxygen

|
Amrita Roy answered |
The correct answer is A: Sugar.
- The separation technique shown is crystallization, used to separate solids from a solution.
- Sugar can be separated from a sugar-water solution through crystallization.
- In this process, the sugar is dissolved in water, heated to dissolve more sugar, then cooled to allow sugar crystals to form and separate.
- Chalk powder, glass, and oxygen cannot be separated using crystallization.
R should be a substance which is soluble in water i.e., sugar.
- The separation technique shown is crystallization, used to separate solids from a solution.
- Sugar can be separated from a sugar-water solution through crystallization.
- In this process, the sugar is dissolved in water, heated to dissolve more sugar, then cooled to allow sugar crystals to form and separate.
- Chalk powder, glass, and oxygen cannot be separated using crystallization.
R should be a substance which is soluble in water i.e., sugar.
Evaporation followed by condensation occurs in:- a)Filtration
- b)Sublimation
- c)Evaporation
- d)Distillation
Correct answer is option 'D'. Can you explain this answer?
Evaporation followed by condensation occurs in:
a)
Filtration
b)
Sublimation
c)
Evaporation
d)
Distillation

|
Dr Manju Sen answered |
Distillation is a process that combines evaporation and condensation to separate liquids. Here’s how it works:
- The liquid is heated, causing it to evaporate into vapour.
- The vapour is then cooled, leading to condensation back into liquid form.
- This method is effective for separating mixtures based on their boiling points.
For example, in the distillation of water:
- Water is boiled to produce steam.
- The steam is cooled and collected as pure water.
This process is widely used in laboratories and industries for various applications.
A mixture of sugar and water can be separated by- a)Filtration
- b)Evaporation
- c)Separating funnel
- d)decantation
Correct answer is option 'B'. Can you explain this answer?
A mixture of sugar and water can be separated by
a)
Filtration
b)
Evaporation
c)
Separating funnel
d)
decantation
|
|
Amit Sharma answered |
The easiest method to separate mixture of sugar and water is evaporation as it will evaporate water and will leave behind the sugar molecules.
The process of settling down of particles of a solid in a liquid is known as- a)sublimation
- b)decantation
- c)sedimentation
- d)precipitation
Correct answer is option 'C'. Can you explain this answer?
The process of settling down of particles of a solid in a liquid is known as
a)
sublimation
b)
decantation
c)
sedimentation
d)
precipitation

|
Sahil Desai answered |
The process of settling down of particles of a solid in a liquid is known as sedimentation.
X is a separation technique based on the difference in weights of the solids in a solid-solid mixture. What is X?- a)Sieving
- b)Handpicking
- c)Threshing
- d)Winnowing
Correct answer is option 'D'. Can you explain this answer?
X is a separation technique based on the difference in weights of the solids in a solid-solid mixture. What is X?
a)
Sieving
b)
Handpicking
c)
Threshing
d)
Winnowing

|
Anjana Rane answered |
Correct Answer: D
Solution :
X is winnowing, a process which uses the difference in weights of solids in a solid- solid mixture for separating the components
What technique is used when the particles are too small to be separated by hand and the quantity is large?
- a)Handpicking
- b)Threshing
- c)Sieving
- d)Winnowing
Correct answer is option 'C'. Can you explain this answer?
What technique is used when the particles are too small to be separated by hand and the quantity is large?
a)
Handpicking
b)
Threshing
c)
Sieving
d)
Winnowing

|
Vrinda Jenna answered |
Sieving is used when the particles are too small to be separated by hand and the quantity is large.
Salt is obtained from sea water by using which of the following process.- a)Sedimentation
- b)Condensation
- c)Evaporation
- d)Centrifugation
Correct answer is option 'C'. Can you explain this answer?
Salt is obtained from sea water by using which of the following process.
a)
Sedimentation
b)
Condensation
c)
Evaporation
d)
Centrifugation
|
|
Aruna sharma answered |
Seawater contains a large amount of common salt and the salts of other metals dissolved in it. Near the sea-shore, the sea water is collected in shallow pits and allowed to evaporate in the sunshine. In a few days, the water evaporates, leaving behind salt. Hence it is obtained by evapouration
When preparing lemonade by mixing lemon juice and sugar in water, should you add ice before or after dissolving the sugar to dissolve more sugar?- a)Add ice before dissolving sugar
- b)Add ice after dissolving sugar
- c)It doesn't matter when you add ice
- d)Ice should not be added
Correct answer is option 'B'. Can you explain this answer?
a)
Add ice before dissolving sugar
b)
Add ice after dissolving sugar
c)
It doesn't matter when you add ice
d)
Ice should not be added

|
Vp Classes answered |
You should add ice after dissolving the sugar to dissolve more sugar in lemonade. When sugar is dissolved in warm water, it dissolves more readily due to higher solubility at warmer temperatures. Adding ice before dissolving sugar can reduce the temperature of the mixture, making it harder for the sugar to dissolve. Therefore, it is best to dissolve the sugar in warm or room-temperature water first and then add ice to cool the lemonade. This approach ensures that the maximum amount of sugar is dissolved.
Which method can be used to separate sugar mixed with wheat flour?- a)Filtration
- b)Decantation
- c)Sieving
- d)Evaporation
Correct answer is option 'C'. Can you explain this answer?
a)
Filtration
b)
Decantation
c)
Sieving
d)
Evaporation

|
Torcia Education answered |
Sieving is the method used to separate sugar from wheat flour. Since sugar and flour have different particle sizes, a sieve with appropriate mesh can separate them. This method is commonly used in cooking and baking to ensure that flour and sugar are evenly mixed or to remove lumps from the ingredients.
State whether the following statement is True or FalseEvaporation is a process where a liquid changes into a solid.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
State whether the following statement is True or False
Evaporation is a process where a liquid changes into a solid.
a)
True
b)
False
|
|
Pritam Kulkarni answered |
Understanding Evaporation
Evaporation is a key process in the water cycle and involves the transformation of a liquid into a gas. Here’s a detailed breakdown of why the statement is false:
Definition of Evaporation
- Evaporation is the process where molecules at the surface of a liquid gain enough energy to transition into the gaseous state.
- This process occurs at any temperature, as long as the liquid has a surface.
Transition of States
- The correct transition for evaporation is:
- Liquid → Gas
- This means that evaporation cannot be a process where a liquid changes into a solid.
Solidification vs. Evaporation
- When a liquid changes into a solid, the process is called freezing or solidification.
- For example, water turns into ice when it freezes.
Examples of Evaporation
- Common examples include:
- Puddles drying up on a sunny day
- Wet clothes drying in the air
Conclusion
In summary, evaporation is indeed the process of liquid turning into gas, not solid. Therefore, the statement "Evaporation is a process where a liquid changes into a solid" is false. The correct answer is option 'B'. Understanding these processes helps us grasp essential concepts in science and the environment.
Evaporation is a key process in the water cycle and involves the transformation of a liquid into a gas. Here’s a detailed breakdown of why the statement is false:
Definition of Evaporation
- Evaporation is the process where molecules at the surface of a liquid gain enough energy to transition into the gaseous state.
- This process occurs at any temperature, as long as the liquid has a surface.
Transition of States
- The correct transition for evaporation is:
- Liquid → Gas
- This means that evaporation cannot be a process where a liquid changes into a solid.
Solidification vs. Evaporation
- When a liquid changes into a solid, the process is called freezing or solidification.
- For example, water turns into ice when it freezes.
Examples of Evaporation
- Common examples include:
- Puddles drying up on a sunny day
- Wet clothes drying in the air
Conclusion
In summary, evaporation is indeed the process of liquid turning into gas, not solid. Therefore, the statement "Evaporation is a process where a liquid changes into a solid" is false. The correct answer is option 'B'. Understanding these processes helps us grasp essential concepts in science and the environment.
To obtain salt from seawater, which method is used?- a)Filtration
- b)Evaporation
- c)Sedimentation
- d)Decantation
Correct answer is option 'B'. Can you explain this answer?
To obtain salt from seawater, which method is used?
a)
Filtration
b)
Evaporation
c)
Sedimentation
d)
Decantation

|
EduRev Class 6 answered |
To obtain salt from seawater, the method used is evaporation.
This process involves the following steps:
- Seawater is heated, which causes the water to evaporate.
- As the water evaporates, it leaves behind the salt as a solid residue.
- This method is effective because water has a lower boiling point than salt.
In summary, evaporation is a reliable way to extract salt from seawater by taking advantage of the different boiling points of water and salt.
The process of obtaining clear water from muddy water using alum is called:- a)Decantation
- b)Filtration
- c)Sedimentation
- d)Loading
Correct answer is option 'D'. Can you explain this answer?
The process of obtaining clear water from muddy water using alum is called:
a)
Decantation
b)
Filtration
c)
Sedimentation
d)
Loading
|
|
Nishtha Banerjee answered |
Understanding the Process of Purification
When we talk about obtaining clear water from muddy water, we refer to a series of processes aimed at separating impurities. One of the most effective methods involves the use of alum, a chemical compound that helps in this purification process.
What is Loading?
- Loading is the process where alum is added to muddy water.
- The alum causes tiny particles in the muddy water to clump together, forming larger particles called flocs.
- This process enhances sedimentation, where these heavier flocs settle at the bottom of the container, leaving behind clearer water.
Steps Involved in the Process
1. Addition of Alum:
- Alum is dissolved in water and mixed with muddy water.
2. Clumping of Particles:
- The positively charged ions in alum attract the negatively charged particles in the muddy water, causing them to stick together.
3. Formation of Flocs:
- The clumped particles form larger flocs that are easier to remove from the water.
4. Sedimentation:
- Over time, these flocs settle down to the bottom of the container due to gravity.
5. Separation:
- The clear water on top can then be carefully decanted or siphoned off, leaving the sediment behind.
Conclusion
In conclusion, while decantation, filtration, and sedimentation are related processes, the term "loading" specifically refers to the addition of alum to initiate the purification process. This crucial step is what makes the transition from muddy water to clear water possible.
When we talk about obtaining clear water from muddy water, we refer to a series of processes aimed at separating impurities. One of the most effective methods involves the use of alum, a chemical compound that helps in this purification process.
What is Loading?
- Loading is the process where alum is added to muddy water.
- The alum causes tiny particles in the muddy water to clump together, forming larger particles called flocs.
- This process enhances sedimentation, where these heavier flocs settle at the bottom of the container, leaving behind clearer water.
Steps Involved in the Process
1. Addition of Alum:
- Alum is dissolved in water and mixed with muddy water.
2. Clumping of Particles:
- The positively charged ions in alum attract the negatively charged particles in the muddy water, causing them to stick together.
3. Formation of Flocs:
- The clumped particles form larger flocs that are easier to remove from the water.
4. Sedimentation:
- Over time, these flocs settle down to the bottom of the container due to gravity.
5. Separation:
- The clear water on top can then be carefully decanted or siphoned off, leaving the sediment behind.
Conclusion
In conclusion, while decantation, filtration, and sedimentation are related processes, the term "loading" specifically refers to the addition of alum to initiate the purification process. This crucial step is what makes the transition from muddy water to clear water possible.
Which of the following does NOT belong to the group formed by the others?- a)Brass
- b)Water
- c)Butter-milk
- d)Steel
Correct answer is option 'B'. Can you explain this answer?
Which of the following does NOT belong to the group formed by the others?
a)
Brass
b)
Water
c)
Butter-milk
d)
Steel

|
Vaibhav Shah answered |
Water is a pure substance and all others are solid-in-solid and solid-in-liquid mixtures.
Tea leaves are separated from the liquid with a ______ while preparing tea.- a)cup
- b)sieve
- c)plate
- d)spoon
Correct answer is option 'B'. Can you explain this answer?
Tea leaves are separated from the liquid with a ______ while preparing tea.
a)
cup
b)
sieve
c)
plate
d)
spoon

|
Torcia Education answered |
When making tea, tea leaves are separated from the liquid using a sieve. The sieve helps to keep the tea leaves out of the cup, so you can enjoy a nice, smooth cup of tea without any pieces of leaves in it.
Which method is used to separate a mixture of wheat and rice?- a)Winnowing
- b)Handpicking
- c)Sieving
- d)Sedimentation
Correct answer is option 'C'. Can you explain this answer?
Which method is used to separate a mixture of wheat and rice?
a)
Winnowing
b)
Handpicking
c)
Sieving
d)
Sedimentation

|
EduRev Class 6 answered |
The method used to separate a mixture of wheat and rice is sieving. This process involves:
- Using a sieve with a mesh of suitable size.
- Allowing smaller particles, like rice, to pass through.
- Retaining larger particles, such as wheat.
Sieving is effective for separating mixtures based on particle size. It is commonly used in various applications, including:
- Removing impurities from flour.
- Separating pebbles and stones from sand.
In summary, sieving is a practical method for sorting materials with different sizes, making it an essential technique in both cooking and construction.
What term describes the process of converting water vapor into its liquid form?- a)Evaporation
- b)Sublimation
- c)Condensation
- d)Precipitation
Correct answer is option 'C'. Can you explain this answer?
What term describes the process of converting water vapor into its liquid form?
a)
Evaporation
b)
Sublimation
c)
Condensation
d)
Precipitation
|
|
Sagar Gupta answered |
Condensation
Condensation is the process of converting water vapor into its liquid form. This occurs when water vapor in the air comes into contact with a surface or air that is cooler than the dew point temperature of the water vapor. This causes the water vapor to lose heat energy, slow down, and eventually turn into liquid water droplets.
Key Points:
- Condensation is the opposite of evaporation, where liquid water turns into water vapor.
- The formation of dew on grass in the morning is a common example of condensation.
- Condensation is an important part of the water cycle, where water is constantly being circulated between the atmosphere, land, and oceans.
- Cloud formation is also a result of condensation, where water vapor in the air cools and forms visible water droplets or ice crystals.
Condensation is the process of converting water vapor into its liquid form. This occurs when water vapor in the air comes into contact with a surface or air that is cooler than the dew point temperature of the water vapor. This causes the water vapor to lose heat energy, slow down, and eventually turn into liquid water droplets.
Key Points:
- Condensation is the opposite of evaporation, where liquid water turns into water vapor.
- The formation of dew on grass in the morning is a common example of condensation.
- Condensation is an important part of the water cycle, where water is constantly being circulated between the atmosphere, land, and oceans.
- Cloud formation is also a result of condensation, where water vapor in the air cools and forms visible water droplets or ice crystals.
Given below are some methods of separation.X - Winnowing
Y - Threshing
Z - SievingWhich of the following methods of separation does not require air for the process of separation?- a) Only X
- b) Only Y, Z
- c)Only Z, X
- d)X, Y and Z
Correct answer is option 'B'. Can you explain this answer?
Given below are some methods of separation.
X - Winnowing
Y - Threshing
Z - Sieving
Y - Threshing
Z - Sieving
Which of the following methods of separation does not require air for the process of separation?
a)
Only X
b)
Only Y, Z
c)
Only Z, X
d)
X, Y and Z
|
|
Nishanth Majumdar answered |
Understanding the Methods of Separation
In the context of separating mixtures, let's explore the three methods mentioned: Winnowing, Threshing, and Sieving, to determine which does not require air.
1. Winnowing
- Definition: Winnowing is a method used to separate lighter particles from heavier ones, commonly used for grains.
- Air Requirement: This process employs wind or air currents to blow away the lighter chaff, making air essential for its operation.
2. Threshing
- Definition: Threshing is the process of separating grains from the husks and straw.
- Air Requirement: While it can involve mechanical means to beat the grains, it often uses air to help separate the lighter husks from the heavier grains. Thus, air plays a role in this method as well.
3. Sieving
- Definition: Sieving is a simple method of separation that involves passing a mixture through a sieve or mesh to separate particles based on size.
- Air Requirement: This method does NOT rely on air; it purely depends on the size of the particles being separated.
Conclusion
From the explanations:
- Winnowing requires air.
- Threshing also involves air in many cases.
- Sieving is the only method that operates independently of air.
Thus, the correct answer is B) Only Y, Z, as Threshing (Y) and Sieving (Z) can be performed without the need for air, while Winnowing (X) cannot.
In the context of separating mixtures, let's explore the three methods mentioned: Winnowing, Threshing, and Sieving, to determine which does not require air.
1. Winnowing
- Definition: Winnowing is a method used to separate lighter particles from heavier ones, commonly used for grains.
- Air Requirement: This process employs wind or air currents to blow away the lighter chaff, making air essential for its operation.
2. Threshing
- Definition: Threshing is the process of separating grains from the husks and straw.
- Air Requirement: While it can involve mechanical means to beat the grains, it often uses air to help separate the lighter husks from the heavier grains. Thus, air plays a role in this method as well.
3. Sieving
- Definition: Sieving is a simple method of separation that involves passing a mixture through a sieve or mesh to separate particles based on size.
- Air Requirement: This method does NOT rely on air; it purely depends on the size of the particles being separated.
Conclusion
From the explanations:
- Winnowing requires air.
- Threshing also involves air in many cases.
- Sieving is the only method that operates independently of air.
Thus, the correct answer is B) Only Y, Z, as Threshing (Y) and Sieving (Z) can be performed without the need for air, while Winnowing (X) cannot.
State whether the following statement is True or False:Tea leaves are separated from the liquid with a strainer while preparing tea.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
State whether the following statement is True or False:
Tea leaves are separated from the liquid with a strainer while preparing tea.
a)
True
b)
False

|
Torcia Education answered |
When we make tea, we use a strainer to separate the tea leaves from the liquid. This is true because we don't want the leaves in our tea when we drink it. The strainer helps keep the leaves out, so we only get the tasty tea to drink!
What method can be used to separate salt from water in a mixture?- a)Sieving
- b)Decantation
- c)Evaporation
- d)Filtration
Correct answer is option 'C'. Can you explain this answer?
What method can be used to separate salt from water in a mixture?
a)
Sieving
b)
Decantation
c)
Evaporation
d)
Filtration

|
Torcia Education answered |
- When we want to separate salt from water in a mixture, we can use a method called evaporation.
- This means heating the mixture until the water boils away, leaving the salt behind.
- It's like when water in a pot boils and turns into steam, leaving things like salt or sugar behind.
- So, to separate salt from water, we can use evaporation to make the water disappear, and then we'll find the salt left behind.
A mixture of chalk and water can be separated by: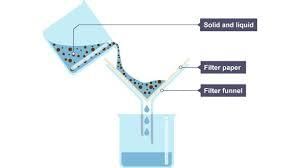
- a)Filtration
- b)Evaporation
- c)Condensation
- d)Decantation
Correct answer is option 'A'. Can you explain this answer?
A mixture of chalk and water can be separated by:

a)
Filtration
b)
Evaporation
c)
Condensation
d)
Decantation

|
Gunjan Lakhani answered |
- Chalk powder is a carbonate compound, insoluble in water.
- Because of this property of chalk powder, it is easy to separate it from water.
- The mixture containing chalk powder and water is allowed to get filtered.
- (Filter paper can be used for filtration) After filtration, the retentate obtained is chalk and the filtrate obtained is water without chalk.
- The retentate is allowed to get dried and hence, chalk is obtained in powder form.
What is the process by which a gas changes into a liquid?- a)Decantation
- b)Sublimation
- c)Condensation
- d)Sedimentation
Correct answer is option 'C'. Can you explain this answer?
What is the process by which a gas changes into a liquid?
a)
Decantation
b)
Sublimation
c)
Condensation
d)
Sedimentation

|
Sagar Mehra answered |
When a gas changes into a liquid, the process is known as condensation.
The salt can be easily obtained from the salty water by the process of:- a)Evaporation
- b)Condensation
- c)Filtration
- d)Both A and B
Correct answer is option 'A'. Can you explain this answer?
The salt can be easily obtained from the salty water by the process of:
a)
Evaporation
b)
Condensation
c)
Filtration
d)
Both A and B

|
Dr Manju Sen answered |
The process of evaporation involves converting water into vapour by heating. This method effectively separates salt from salty water. When the water is boiled, it evaporates, leaving the salt behind.
Key points about evaporation:
- Evaporation occurs continuously wherever water is present.
- Sea water contains various salts, including common salt.
- When sea water is placed in shallow pits, sunlight heats it, causing the water to evaporate.
- After several days, the water evaporates completely, leaving behind solid salts.
- Common salt can be obtained from these salts through further purification.
In summary, evaporation is a simple and effective method for extracting salt from water.
A solution of sugar in which some more sugar could be dissolved without changing its temperature is called a/an :- a)unsaturated solution
- b)saturated solution
- c)solid solution
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?
A solution of sugar in which some more sugar could be dissolved without changing its temperature is called a/an :
a)
unsaturated solution
b)
saturated solution
c)
solid solution
d)
none of the above
|
|
Pritam Kumar answered |
Understanding Solutions
In chemistry, solutions can be classified based on their solute concentration. When discussing sugar solutions, it's important to differentiate between unsaturated and saturated solutions.
Unsaturated Solution
- An unsaturated solution is one where the solvent can still dissolve more solute at a given temperature.
- For example, if you have a glass of water with sugar dissolved in it and you can keep adding more sugar without any leftover at the bottom, this solution is unsaturated.
- The temperature remains constant in this scenario, indicating that the solution can accommodate more solute.
Saturated Solution
- A saturated solution occurs when no more solute can dissolve in the solvent at a specific temperature.
- In this case, if you add sugar and it does not dissolve, remaining at the bottom, the solution is saturated.
- The sugar particles are in equilibrium with the dissolved particles, meaning the solution has reached its maximum solute concentration.
Examples of Solutions
- Unsaturated Solution: A glass of warm water to which you can add more sugar easily.
- Saturated Solution: A glass of cold water where sugar starts to settle at the bottom after reaching the maximum dissolving point.
Conclusion
The correct answer to the question is option 'A' because an unsaturated solution allows for additional solute to dissolve without altering the temperature. Understanding these concepts helps in grasping the basic principles of solubility and solutions.
In chemistry, solutions can be classified based on their solute concentration. When discussing sugar solutions, it's important to differentiate between unsaturated and saturated solutions.
Unsaturated Solution
- An unsaturated solution is one where the solvent can still dissolve more solute at a given temperature.
- For example, if you have a glass of water with sugar dissolved in it and you can keep adding more sugar without any leftover at the bottom, this solution is unsaturated.
- The temperature remains constant in this scenario, indicating that the solution can accommodate more solute.
Saturated Solution
- A saturated solution occurs when no more solute can dissolve in the solvent at a specific temperature.
- In this case, if you add sugar and it does not dissolve, remaining at the bottom, the solution is saturated.
- The sugar particles are in equilibrium with the dissolved particles, meaning the solution has reached its maximum solute concentration.
Examples of Solutions
- Unsaturated Solution: A glass of warm water to which you can add more sugar easily.
- Saturated Solution: A glass of cold water where sugar starts to settle at the bottom after reaching the maximum dissolving point.
Conclusion
The correct answer to the question is option 'A' because an unsaturated solution allows for additional solute to dissolve without altering the temperature. Understanding these concepts helps in grasping the basic principles of solubility and solutions.
The essential condition for winnowing activity is:- a)water
- b)wind
- c)electricity
- d)machines
Correct answer is option 'B'. Can you explain this answer?
The essential condition for winnowing activity is:
a)
water
b)
wind
c)
electricity
d)
machines

|
Coachify answered |
Winnowing is a method used to separate chaff or husk from grains like wheat. This process relies on the application of wind to distinguish between heavier and lighter components.
Key points about winnowing:
- It is commonly employed by farmers to remove lighter husk particles from heavier seeds.
- The wind carries away the husk, allowing the seeds to form a heap.
- The separated husk can be repurposed, for example, as fodder for cattle.
In summary, winnowing effectively uses the natural force of wind to facilitate the separation of different components in a mixture.
The method used to separate a dissolved solid component from its solution- a)Evaporation
- b)Filtration
- c)sedimentation
- d)Decantation
Correct answer is option 'A'. Can you explain this answer?
The method used to separate a dissolved solid component from its solution
a)
Evaporation
b)
Filtration
c)
sedimentation
d)
Decantation

|
Anshu Nambiar answered |
Evaporation is a technique used to separate out homogenous mixtures where there is one or more dissolved solids. This method drives off the liquid components from the solid components. The process typically involves heating the mixture until no more liquid remains, Prior to using this method, the mixture should only contain one liquid component, unless it is not important to isolate the liquid components. This is because all liquid components will evaporate over time. This method is suitable to separate a soluble solid from a liquid.
Handpicking, winnowing, sieving, sedimentation, decantation, and filtration are methods of separating substances from their ______.- a)friends
- b)mixtures
- c)toys
- d)colors
Correct answer is option 'B'. Can you explain this answer?
Handpicking, winnowing, sieving, sedimentation, decantation, and filtration are methods of separating substances from their ______.
a)
friends
b)
mixtures
c)
toys
d)
colors
|
|
Harshad Goyal answered |
Explanation:
Methods of Separating Substances from Mixtures:
Handpicking:
- Involves manually picking out components of a mixture based on their physical properties.
- Useful for separating larger particles from a mixture.
Winnowing:
- Involves blowing air through a mixture to remove lighter components.
- Effective for separating grains from chaff or dirt.
Sieving:
- Involves passing a mixture through a sieve or mesh to separate particles based on size.
- Useful for separating solids of different sizes.
Sedimentation:
- Involves allowing particles to settle at the bottom of a container due to gravity.
- Useful for separating insoluble solids from liquids.
Decantation:
- Involves pouring off the liquid portion of a mixture while leaving the solid behind.
- Useful for separating liquids with different densities.
Filtration:
- Involves passing a mixture through a filter to separate solid particles from a liquid.
- Useful for separating insoluble solids from liquids.
These methods are essential in chemistry and everyday life for separating different substances from mixtures based on their physical properties.
Methods of Separating Substances from Mixtures:
Handpicking:
- Involves manually picking out components of a mixture based on their physical properties.
- Useful for separating larger particles from a mixture.
Winnowing:
- Involves blowing air through a mixture to remove lighter components.
- Effective for separating grains from chaff or dirt.
Sieving:
- Involves passing a mixture through a sieve or mesh to separate particles based on size.
- Useful for separating solids of different sizes.
Sedimentation:
- Involves allowing particles to settle at the bottom of a container due to gravity.
- Useful for separating insoluble solids from liquids.
Decantation:
- Involves pouring off the liquid portion of a mixture while leaving the solid behind.
- Useful for separating liquids with different densities.
Filtration:
- Involves passing a mixture through a filter to separate solid particles from a liquid.
- Useful for separating insoluble solids from liquids.
These methods are essential in chemistry and everyday life for separating different substances from mixtures based on their physical properties.
What is the process of separating solid particles suspended in a liquid by allowing them to settle down called?- a)Filtration
- b)Decantation
- c)Sedimentation
- d)Evaporation
Correct answer is option 'C'. Can you explain this answer?
What is the process of separating solid particles suspended in a liquid by allowing them to settle down called?
a)
Filtration
b)
Decantation
c)
Sedimentation
d)
Evaporation
|
|
Kunal Ghosh answered |
Understanding Sedimentation
Sedimentation is a natural process used to separate solid particles suspended in a liquid by allowing them to settle down due to gravity. This method is commonly applied in various fields including water treatment, mining, and environmental science.
How Sedimentation Works
- Gravity's Role: When a mixture of solid and liquid is left undisturbed, the denser solid particles gradually settle at the bottom of the container. This occurs because gravity pulls the particles downward.
- Time Factor: The rate at which particles settle depends on their size, shape, and density, as well as the viscosity of the liquid. Larger or denser particles settle faster than smaller or lighter ones.
Applications of Sedimentation
- Water Purification: Sedimentation is an essential step in water treatment processes where impurities and suspended solids are removed, leading to cleaner water.
- Industrial Processes: In mining, sedimentation helps in separating valuable minerals from waste material.
Difference from Other Processes
- Filtration: While filtration also separates solids from liquids, it uses a filter medium to physically block solid particles, whereas sedimentation relies on gravity.
- Decantation: This process follows sedimentation; once solids have settled, the clear liquid can be poured off, leaving the sediment behind.
- Evaporation: Evaporation involves converting liquid into vapor to separate dissolved solids, which is entirely different from the settling mechanism of sedimentation.
In summary, sedimentation is an efficient and straightforward method for separating solid particles from liquids, making it a fundamental concept in both science and practical applications.
Sedimentation is a natural process used to separate solid particles suspended in a liquid by allowing them to settle down due to gravity. This method is commonly applied in various fields including water treatment, mining, and environmental science.
How Sedimentation Works
- Gravity's Role: When a mixture of solid and liquid is left undisturbed, the denser solid particles gradually settle at the bottom of the container. This occurs because gravity pulls the particles downward.
- Time Factor: The rate at which particles settle depends on their size, shape, and density, as well as the viscosity of the liquid. Larger or denser particles settle faster than smaller or lighter ones.
Applications of Sedimentation
- Water Purification: Sedimentation is an essential step in water treatment processes where impurities and suspended solids are removed, leading to cleaner water.
- Industrial Processes: In mining, sedimentation helps in separating valuable minerals from waste material.
Difference from Other Processes
- Filtration: While filtration also separates solids from liquids, it uses a filter medium to physically block solid particles, whereas sedimentation relies on gravity.
- Decantation: This process follows sedimentation; once solids have settled, the clear liquid can be poured off, leaving the sediment behind.
- Evaporation: Evaporation involves converting liquid into vapor to separate dissolved solids, which is entirely different from the settling mechanism of sedimentation.
In summary, sedimentation is an efficient and straightforward method for separating solid particles from liquids, making it a fundamental concept in both science and practical applications.
What method is used to separate tea leaves from prepared tea?- a)Sedimentation
- b)Filtration
- c)Decantation
- d)Winnowing
Correct answer is option 'B'. Can you explain this answer?
What method is used to separate tea leaves from prepared tea?
a)
Sedimentation
b)
Filtration
c)
Decantation
d)
Winnowing
|
|
Priyanka Mukherjee answered |
Method to Separate Tea Leaves from Prepared Tea
When preparing tea, it is essential to separate the tea leaves from the liquid to enjoy a smooth beverage. The best method for this is filtration.
Understanding Filtration
- Definition: Filtration is a process that uses a barrier to separate solid particles from a liquid or gas.
- Application in Tea Preparation: In tea preparation, a filter (often a tea strainer or a fine mesh sieve) is used to catch the tea leaves while allowing the brewed liquid to pass through.
Why Filtration is the Correct Answer
- Efficiency:
- Filtration effectively separates small tea leaves from the liquid, ensuring that only the brewed tea is served.
- Clarity:
- This method helps achieve a clear liquid, free from unwanted particles, enhancing the drinking experience.
- Ease of Use:
- Using a strainer or filter is quick and practical, making it a convenient choice for most tea drinkers.
Other Methods Explained
- Sedimentation:
- This is the process of allowing heavier particles to settle at the bottom of a liquid. It is not effective for tea leaves, which do not settle quickly.
- Decantation:
- This involves pouring off the liquid from a settled solid. It is less precise since some leaves might still be mixed with the liquid.
- Winnowing:
- This method is used for separating grain from chaff, not applicable to tea leaves.
By using filtration, tea drinkers can enjoy their beverage free from any solid remnants, making it the most suitable method for separating tea leaves from prepared tea.
When preparing tea, it is essential to separate the tea leaves from the liquid to enjoy a smooth beverage. The best method for this is filtration.
Understanding Filtration
- Definition: Filtration is a process that uses a barrier to separate solid particles from a liquid or gas.
- Application in Tea Preparation: In tea preparation, a filter (often a tea strainer or a fine mesh sieve) is used to catch the tea leaves while allowing the brewed liquid to pass through.
Why Filtration is the Correct Answer
- Efficiency:
- Filtration effectively separates small tea leaves from the liquid, ensuring that only the brewed tea is served.
- Clarity:
- This method helps achieve a clear liquid, free from unwanted particles, enhancing the drinking experience.
- Ease of Use:
- Using a strainer or filter is quick and practical, making it a convenient choice for most tea drinkers.
Other Methods Explained
- Sedimentation:
- This is the process of allowing heavier particles to settle at the bottom of a liquid. It is not effective for tea leaves, which do not settle quickly.
- Decantation:
- This involves pouring off the liquid from a settled solid. It is less precise since some leaves might still be mixed with the liquid.
- Winnowing:
- This method is used for separating grain from chaff, not applicable to tea leaves.
By using filtration, tea drinkers can enjoy their beverage free from any solid remnants, making it the most suitable method for separating tea leaves from prepared tea.
To separate two immiscible liquids, we use:- a)Filtration
- b)Decantation
- c)Separating funnel
- d)Evaporation
Correct answer is option 'C'. Can you explain this answer?
To separate two immiscible liquids, we use:
a)
Filtration
b)
Decantation
c)
Separating funnel
d)
Evaporation
|
|
Sanskriti Bose answered |
Understanding Immiscible Liquids
Immiscible liquids are those that do not mix together, such as oil and water. When trying to separate these liquids, specific methods are required to achieve effective separation.
Why Use a Separating Funnel?
A separating funnel is the most efficient tool for separating immiscible liquids. Here’s how it works:
- Design: The funnel has a wide body and a narrow neck, allowing for easy separation of layers.
- Density Difference: Immiscible liquids typically have different densities. For example, oil is less dense than water and will float on top.
- Separation Process:
- Pour the mixture into the separating funnel.
- Allow the liquids to settle into distinct layers.
- Open the stopcock at the bottom to let the denser liquid (usually water) drain out, leaving the lighter liquid (like oil) in the funnel.
Other Methods Explained
- Filtration: This method is used to separate solids from liquids or gases. It is not suitable for immiscible liquids.
- Decantation: This involves pouring off one liquid from another after settling, but a separating funnel provides more precision, especially with smaller volumes.
- Evaporation: This technique is used to separate a soluble solid from a liquid, not for immiscible liquids.
Conclusion
The separating funnel is specifically designed for separating immiscible liquids due to its unique structure and functionality, making it the best choice among the options provided.
Immiscible liquids are those that do not mix together, such as oil and water. When trying to separate these liquids, specific methods are required to achieve effective separation.
Why Use a Separating Funnel?
A separating funnel is the most efficient tool for separating immiscible liquids. Here’s how it works:
- Design: The funnel has a wide body and a narrow neck, allowing for easy separation of layers.
- Density Difference: Immiscible liquids typically have different densities. For example, oil is less dense than water and will float on top.
- Separation Process:
- Pour the mixture into the separating funnel.
- Allow the liquids to settle into distinct layers.
- Open the stopcock at the bottom to let the denser liquid (usually water) drain out, leaving the lighter liquid (like oil) in the funnel.
Other Methods Explained
- Filtration: This method is used to separate solids from liquids or gases. It is not suitable for immiscible liquids.
- Decantation: This involves pouring off one liquid from another after settling, but a separating funnel provides more precision, especially with smaller volumes.
- Evaporation: This technique is used to separate a soluble solid from a liquid, not for immiscible liquids.
Conclusion
The separating funnel is specifically designed for separating immiscible liquids due to its unique structure and functionality, making it the best choice among the options provided.
Corn is separated from husk by the process of- a)Sieving
- b)Manual peeling or mechanical methods
- c)Churning
- d)Winnowing
Correct answer is option 'B'. Can you explain this answer?
Corn is separated from husk by the process of
a)
Sieving
b)
Manual peeling or mechanical methods
c)
Churning
d)
Winnowing

|
Dr Manju Sen answered |
Corn is separated from its husk by manually peeling it off or using mechanical methods specifically designed for this task. Unlike winnowing, which is used for separating lighter particles from grains using wind, corn requires a different approach as it involves removing the husk surrounding the cob.
The separation of grains from husk is done by the process of:

- a)Hand picking
- b)Threshing
- c)Winnowing
- d)Sieving
Correct answer is option 'C'. Can you explain this answer?
The separation of grains from husk is done by the process of:


a)
Hand picking
b)
Threshing
c)
Winnowing
d)
Sieving

|
Vp Classes answered |
- Winnowing is the method in which heavier components of the mixture are separated from the lighter substances with the help of wind.
- This method is used for separating grains from husk after the process of threshing.
The different processes used to separate sand, sawdust, and salt from the mixture dissolved in water are respectively:- a)sedimentation, filtration and evaporation
- b)filtration, evaporation and condensation
- c)decantation and evaporation
- d)hand picking and evaporation
Correct answer is option 'A'. Can you explain this answer?
The different processes used to separate sand, sawdust, and salt from the mixture dissolved in water are respectively:
a)
sedimentation, filtration and evaporation
b)
filtration, evaporation and condensation
c)
decantation and evaporation
d)
hand picking and evaporation

|
Praveen Kumar answered |
- Sedimentation is used to remove sand from water as sand particles settle down easily.
- Filtration is used to remove sawdust from water as the sawdust can be captured by the filter paper.
- Evaporation can be used to separate salt from water as the water evaporates and salt is left behind.
The process of separating butter from curd is called:- a)Filtration
- b)Decantation
- c)Churning
- d)Winnowing
Correct answer is option 'C'. Can you explain this answer?
The process of separating butter from curd is called:
a)
Filtration
b)
Decantation
c)
Churning
d)
Winnowing

|
Vp Classes answered |
The process of separating butter from curd is called churning. This method involves:
- Vigorously stirring or rotating the curd.
- Separating the butterfat from the liquid, known as buttermilk.
- Causing the butter to solidify and detach from the buttermilk.
Churning is a common technique used in dairy processing to obtain butter from curd.
Mixtures need to be separated because- a)to remove undesirable substances
- b)to get desirable substances
- c)to obtain highly pure substances
- d)all of the above
Correct answer is option 'D'. Can you explain this answer?
Mixtures need to be separated because
a)
to remove undesirable substances
b)
to get desirable substances
c)
to obtain highly pure substances
d)
all of the above
|
|
Aaditya Chawla answered |
Introduction:
Mixtures are combinations of two or more substances that are physically mixed together but can be separated through various methods. The need to separate mixtures arises due to several reasons, including the removal of undesirable substances, obtaining desirable substances, and obtaining highly pure substances. Consequently, the correct answer to the given question is option 'D', which encompasses all of the above reasons.
Explanation:
1. Removal of Undesirable Substances:
One of the primary reasons for separating mixtures is to remove undesirable substances. In many cases, mixtures contain impurities or unwanted components that need to be eliminated in order to obtain a desired product or to make the mixture safe for consumption. Separation techniques such as filtration, distillation, and chromatography are commonly employed for this purpose.
- Filtration: Filtration is used to separate a solid-liquid mixture, where the solid particles are larger than the pores of the filter paper or mesh, allowing only the liquid to pass through.
- Distillation: Distillation is used to separate a mixture of two or more liquids with different boiling points. The mixture is heated, and the component with the lower boiling point vaporizes first and can be collected separately.
- Chromatography: Chromatography is a technique that is used to separate a mixture into its individual components based on their different affinities to a mobile phase and a stationary phase.
2. Obtaining Desirable Substances:
Separating mixtures is also essential for obtaining desirable substances. Sometimes, mixtures contain valuable or useful substances that need to be isolated for further processing or utilization. By employing suitable separation methods, these substances can be obtained in their pure form.
- Evaporation: Evaporation is used to separate a mixture of a solid dissolved in a liquid. The mixture is heated, and the liquid component evaporates, leaving behind the solid residue.
- Crystallization: Crystallization is a technique used to separate a solid dissolved in a liquid by cooling the mixture. The dissolved solid forms crystals, which can be separated from the liquid.
- Sublimation: Sublimation is the process of separating a mixture where one component undergoes sublimation, i.e., it changes directly from a solid to a gaseous state without passing through the liquid phase. The sublimed substance can be collected separately.
3. Obtaining Highly Pure Substances:
Separating mixtures is also essential to obtain highly pure substances. In many scientific and industrial applications, it is necessary to isolate individual components of a mixture with a high degree of purity. This can be achieved through various separation techniques.
- Centrifugation: Centrifugation is used to separate mixtures based on the differences in density or particle size. The mixture is spun rapidly, causing the denser or larger particles to settle at the bottom, while the lighter or smaller particles remain in the liquid or on top.
- Decantation: Decantation is a simple method used to separate a mixture of a solid settled at the bottom of a container and a liquid. The liquid is carefully poured off, leaving the solid behind.
- Electrolysis: Electro
Mixtures are combinations of two or more substances that are physically mixed together but can be separated through various methods. The need to separate mixtures arises due to several reasons, including the removal of undesirable substances, obtaining desirable substances, and obtaining highly pure substances. Consequently, the correct answer to the given question is option 'D', which encompasses all of the above reasons.
Explanation:
1. Removal of Undesirable Substances:
One of the primary reasons for separating mixtures is to remove undesirable substances. In many cases, mixtures contain impurities or unwanted components that need to be eliminated in order to obtain a desired product or to make the mixture safe for consumption. Separation techniques such as filtration, distillation, and chromatography are commonly employed for this purpose.
- Filtration: Filtration is used to separate a solid-liquid mixture, where the solid particles are larger than the pores of the filter paper or mesh, allowing only the liquid to pass through.
- Distillation: Distillation is used to separate a mixture of two or more liquids with different boiling points. The mixture is heated, and the component with the lower boiling point vaporizes first and can be collected separately.
- Chromatography: Chromatography is a technique that is used to separate a mixture into its individual components based on their different affinities to a mobile phase and a stationary phase.
2. Obtaining Desirable Substances:
Separating mixtures is also essential for obtaining desirable substances. Sometimes, mixtures contain valuable or useful substances that need to be isolated for further processing or utilization. By employing suitable separation methods, these substances can be obtained in their pure form.
- Evaporation: Evaporation is used to separate a mixture of a solid dissolved in a liquid. The mixture is heated, and the liquid component evaporates, leaving behind the solid residue.
- Crystallization: Crystallization is a technique used to separate a solid dissolved in a liquid by cooling the mixture. The dissolved solid forms crystals, which can be separated from the liquid.
- Sublimation: Sublimation is the process of separating a mixture where one component undergoes sublimation, i.e., it changes directly from a solid to a gaseous state without passing through the liquid phase. The sublimed substance can be collected separately.
3. Obtaining Highly Pure Substances:
Separating mixtures is also essential to obtain highly pure substances. In many scientific and industrial applications, it is necessary to isolate individual components of a mixture with a high degree of purity. This can be achieved through various separation techniques.
- Centrifugation: Centrifugation is used to separate mixtures based on the differences in density or particle size. The mixture is spun rapidly, causing the denser or larger particles to settle at the bottom, while the lighter or smaller particles remain in the liquid or on top.
- Decantation: Decantation is a simple method used to separate a mixture of a solid settled at the bottom of a container and a liquid. The liquid is carefully poured off, leaving the solid behind.
- Electrolysis: Electro
Which method is used to separate soluble substances from a liquid?- a)Filtration
- b)Evaporation
- c)Sieving
- d)Handpicking
Correct answer is option 'B'. Can you explain this answer?
Which method is used to separate soluble substances from a liquid?
a)
Filtration
b)
Evaporation
c)
Sieving
d)
Handpicking

|
Praveen Kumar answered |
Evaporation is the method used to separate soluble substances from a liquid. This process involves the following steps:
- When a liquid containing a dissolved substance is heated, the liquid evaporates.
- This leaves behind the soluble substance, such as salt or sugar, as a solid residue.
- The effectiveness of this method is due to the fact that the solvent, typically water, has a lower boiling point than the dissolved substance.
For example, in the case of sea water:
- Sea water contains various salts, including common salt.
- When allowed to stand in shallow pits, sunlight heats the water, causing it to evaporate.
- After several days, the water completely evaporates, leaving behind solid salts.
- These salts can then be further purified to obtain common salt.
In summary, evaporation is a crucial method for separating soluble substances from liquids, making it widely used in various applications.
X is a separation technique based on the difference in weights of the solids in a solid-solid mixture. What is X?- a)Sieving
- b)Handpicking
- c)Threshing
- d)Winnowing
Correct answer is option 'D'. Can you explain this answer?
X is a separation technique based on the difference in weights of the solids in a solid-solid mixture. What is X?
a)
Sieving
b)
Handpicking
c)
Threshing
d)
Winnowing

|
Shiksha Academy answered |
X is winnowing, a process which uses the difference in weights of solids in a solid- solid mixture for separating the components.
A mixture contains two solids A and B. Solid A is light in weight while solid B is very heavy. Their sizes are almost the same. Here, A is the unwanted component. To get pure solid B, which separation method would you suggest?- a)Sieving
- b)Winnowing
- c)Magnetic separation
- d)Filtration
Correct answer is option 'B'. Can you explain this answer?
A mixture contains two solids A and B. Solid A is light in weight while solid B is very heavy. Their sizes are almost the same. Here, A is the unwanted component. To get pure solid B, which separation method would you suggest?
a)
Sieving
b)
Winnowing
c)
Magnetic separation
d)
Filtration

|
Dr Manju Sen answered |
Winnowing is a traditional farming technique used to separate lighter components from heavier ones in a mixture. It is particularly effective for separating grain from chaff.
Key points about winnowing:
- It involves using air or wind to blow away lighter particles.
- This method is ideal for heterogeneous solid-solid mixtures.
- Winnowing is commonly employed by farmers to separate husk from heavier seeds.
- The lighter husk is carried away by the wind, while the heavier seeds form a pile.
Winnowing is often preceded by threshing, which loosens the grain from its husks and straw. This method is not only effective for grains but can also be adapted for other mixtures.
Chapter doubts & questions for Separation of Substances - Online MCQ Tests for Class 6 2025 is part of Class 6 exam preparation. The chapters have been prepared according to the Class 6 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 6 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Separation of Substances - Online MCQ Tests for Class 6 in English & Hindi are available as part of Class 6 exam.
Download more important topics, notes, lectures and mock test series for Class 6 Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









