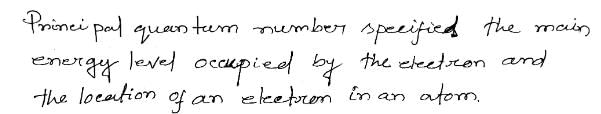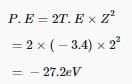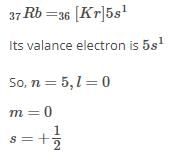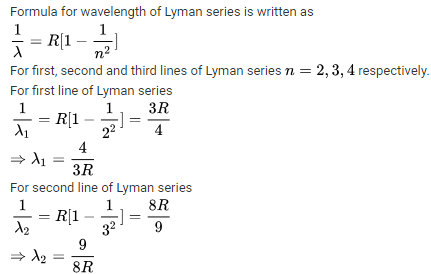All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Atomic Structure for JAMB Exam
Can you explain the answer of this question below:The number of radial nodes for 3p orbital is __________.A:3B:4C:2D:1The answer is d.
|
|
Krishna Iyer answered |
► Number of radial nodes = n - 1 – 1
► For 3p orbital, n = 3 – 1 – 1 = 1
► Number of radial nodes = 3 – 1 – 1 = 1.
► For 3p orbital, n = 3 – 1 – 1 = 1
► Number of radial nodes = 3 – 1 – 1 = 1.
The nucleus of a tritium atom, 3H, contains- a)three protons
- b)three neutrons
- c)two protons and one neutron
- d)two neutrons and one proton
Correct answer is option 'D'. Can you explain this answer?
The nucleus of a tritium atom, 3H, contains
a)
three protons
b)
three neutrons
c)
two protons and one neutron
d)
two neutrons and one proton
|
|
Hansa Sharma answered |
Tritium (3H) is a radioactive isotope of hydrogen. The nucleus decays (by emitting an electron and an antineutrino), changing from a triton (one proton and two neutrons) to a 3He nucleus (two protons and one neutron).
Thomson’s plum pudding model explained:
- a) Existence of electrons
- b)Electrical neutrality of an atom
- c)Existence of atoms
- d)Electrons move in fixed circular orbits
Correct answer is option 'B'. Can you explain this answer?
Thomson’s plum pudding model explained:
a)
Existence of electrons
b)
Electrical neutrality of an atom
c)
Existence of atoms
d)
Electrons move in fixed circular orbits
|
|
Raghav Bansal answered |
Postulates of Thomson’s atomic model
- An atom consists of a positively charged sphere with electrons filled into it. The negative and positive charge present inside an atom are equal and as a whole, an atom is electrically neutral.
- Thomson’s model of the atom was compared to plum pudding and watermelon. He compared the red edible part of the watermelon to positively charged sphere whereas the seeds of watermelon to negatively charged particles.
An element has 18 electrons, and 20 .neutrons. Its charge is —2. What is its mass number?- a)32
- b)38
- c)39
- d) 40
Correct answer is option 'C'. Can you explain this answer?
An element has 18 electrons, and 20 .neutrons. Its charge is —2. What is its mass number?
a)
32
b)
38
c)
39
d)
40

|
Knowledge Hub answered |
Number of orbitals in a shell = n2 = (5)2 = 25
Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom ?- a)n = 1 to n = 2
- b)n = 2 to n = 4
- c)n = 3 to n = 6
- d)n = 3 to n = ∞
Correct answer is option 'A'. Can you explain this answer?
Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom ?
a)
n = 1 to n = 2
b)
n = 2 to n = 4
c)
n = 3 to n = 6
d)
n = 3 to n = ∞
|
|
Naina Sharma answered |
Therefore, electronic transition (a) requires greatest quantity of energy.
Atomic mass of an element is equal to the sum of?- a) Electron and neutron
- b) Electron and proton
- c) Proton and neutron
- d) None of the above
Correct answer is option 'C'. Can you explain this answer?
Atomic mass of an element is equal to the sum of?
a)
Electron and neutron
b)
Electron and proton
c)
Proton and neutron
d)
None of the above
|
|
Neha Joshi answered |
Atomic Mass is the sum of no. of protons and neutrons.
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Which of the following is correct?- a)1H1 and 2He3 are isotopes
- b)6C14 and 7N14 are isotopes
- c)19K39 and 20Ca40 are isotones
- d)9F19 and 11Na24 are isodiaphers
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following is correct?
a)
1H1 and 2He3 are isotopes
b)
6C14 and 7N14 are isotopes
c)
19K39 and 20Ca40 are isotones
d)
9F19 and 11Na24 are isodiaphers
|
|
Raghav Bansal answered |
Because isotones means the same number of neutron, So, from the question, option c is right, number of neutron in k is,39-19= 20,and,the number of neutron in ca is also 40-20=20 so it is isotones.
Direction (Q. Nos. 16 - 19) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)Q. The energy of an electron in the first Bohr orbit of H-atom is -13.6 eV. What is the possible value of quantum number for the excited state to have energy -3.4 eV?
Correct answer is '2'. Can you explain this answer?
Direction (Q. Nos. 16 - 19) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. The energy of an electron in the first Bohr orbit of H-atom is -13.6 eV. What is the possible value of quantum number for the excited state to have energy -3.4 eV?
|
|
Pooja Shah answered |
For Bohr radius;
E = -13.6×Z2/ n2
For H atom, Z=1 and given energy = -3.4
So, we have, -3.4 = -13.6/n2
Or n = 2
E = -13.6×Z2/ n2
For H atom, Z=1 and given energy = -3.4
So, we have, -3.4 = -13.6/n2
Or n = 2
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Amrita Kumar answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
The nature of positive rays depends on?
- a)The nature of discharge tube.
- b)The nature of residual gas.
- c)The nature of electrode.
- d)All of above
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas.
c)
The nature of electrode.
d)
All of above
|
|
Om Desai answered |
- The nature of positive rays produced in a vacuum discharge tube depends upon the nature of the gas-filled.
- The positive rays consist of positive ions obtained by removing one or more electrons from gas molecules.
Increasing order of magnetic moment among the following species is __________ .Na+, Fe+3, Co2+, Cr+2- a)Na+<Fe+3<Co2+ <Cr+2
- b)Na+<Co2+<Cr2+ <Fe+3
- c)Na+<Cr2+<Co2+ <Fe+3
- d)Na+<Fe3+<Cr2+ <Co+2
Correct answer is option 'B'. Can you explain this answer?
Increasing order of magnetic moment among the following species is __________ .
Na+, Fe+3, Co2+, Cr+2
a)
Na+<Fe+3<Co2+ <Cr+2
b)
Na+<Co2+<Cr2+ <Fe+3
c)
Na+<Cr2+<Co2+ <Fe+3
d)
Na+<Fe3+<Cr2+ <Co+2
|
|
Nitin Khanna answered |
Na+, Co+2, Cr2+, Fe+3
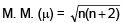
we get Na+, Co+2, Cr2+, Fe+3
we get Na+, Co+2, Cr2+, Fe+3
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?- a)Rutherford Model
- b)Bohr’s Model
- c)J.J Thomson Model
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
a)
Rutherford Model
b)
Bohr’s Model
c)
J.J Thomson Model
d)
None of the above
|
|
Suresh Reddy answered |
Bohr Model of atom:
- An atom is made up of three particles: Electrons, neutrons and protons.
- The protons and neutrons are located in a small nucleus at the centre of the atom.
- The electrons revolve rapidly around the nucleus at the centre of the atom.
- There is a limit to the number of electrons that each energy level can hold.
- Each energy level is associated with a fixed amount of energy.
- There is no change in the energy of electrons as long as they keep revolving in the same energy level.
Bohr explained the stability through the concept of revolution of electrons in different energy levels.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The nature of positive rays depends on?- a) The nature of discharge tube.
- b) The nature of residual gas
- c) All of above.
- d) The nature of electrode
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas
c)
All of above.
d)
The nature of electrode

|
Abhiram Choudhary answered |
The positive charges in these rays, other than negative cathode rays (which are electrons), depend on the gas that is used because they are cations - atoms with mostly one electron missing and thus one positive charge. So, if you accelerate, argon cations and protons over the same electric potential, the particles in the rays will have the same kinetic energy, but the argon ions will be much slower, as they are much heavier than the protons.
The first use of quantum theory to explain the structure of atom was made by :- a)Heisenberg
- b)Bohr
- c)Planck
- d)Einstein
Correct answer is option 'B'. Can you explain this answer?
The first use of quantum theory to explain the structure of atom was made by :
a)
Heisenberg
b)
Bohr
c)
Planck
d)
Einstein
|
|
Hansa Sharma answered |
Bohr's theory was based upon some postulations of classical physics and some postulations of the newly proposed quantum theory of Planck.
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is option 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Swara Saha answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
The charge on electron was determined by- a)Crooks
- b)Bohr
- c)Milliken
- d)Schrodinger
Correct answer is option 'C'. Can you explain this answer?
The charge on electron was determined by
a)
Crooks
b)
Bohr
c)
Milliken
d)
Schrodinger
|
|
Naina Bansal answered |
Millikan
The experiment helped earn Millikan a Nobel prize in 1923 but has been a source of some controversy over the years. J. J. Thomson discovered the electron in 1897 when he measured the charge-to-mass ratio for electrons in a beam. But the value of the charge and whether it was fundamental remained open questions.
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
- a)n=3, l=0
- b)n=3, l=1
- c)n=2, l=0
- d)n=2, l=1
Correct answer is option 'D'. Can you explain this answer?
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
a)
n=3, l=0
b)
n=3, l=1
c)
n=2, l=0
d)
n=2, l=1
|
|
Om Desai answered |
Given a set of quantum numbers, n=2,l=0 for a multi-electron atom refers to 2s orbital.
The next higher allowed set of 'n' and 'l' quantum numbers for this atom in the ground state is n=2,l=1. This corresponds to 2p orbital.
Note: The orbital with a higher value of the sum (n+l) has higher energy.
For 2s orbital (n+l)=(2+0)=2
For 2p orbital (n+l)=(2+1)=3
If in the hydrogen atom P.E. at ∞ is choosen to be 13.6 eV then the ratio of T.E. to K.E. for 1st orbit of H-atom is __________ .- a)Zero
- b)1
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
If in the hydrogen atom P.E. at ∞ is choosen to be 13.6 eV then the ratio of T.E. to K.E. for 1st orbit of H-atom is __________ .
a)
Zero
b)
1
c)
2
d)
3
|
|
Rahul Kumar answered |
Zero
Refrence level is Ist orbit
itself T. E. = 0
Ratio becomes zero
Refrence level is Ist orbit
itself T. E. = 0
Ratio becomes zero
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?a) Most of the space in the atom is empty.b) The radius of the atom is about 10^–10 m while that of nucleus is 10^–15 m.c) Electrons move in a circular path of fixed energy called orbits.d) Electrons and the nucleus are held together by electrostatic forces of attraction.Correct answer is 'C'. Can you explain this answer?
|
|
Preeti Iyer answered |
Conclusions of Rutherford's scattering experiment:
1. Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
2. Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
3. A very small fraction of α-particles were deflected by very large angles, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
4. From the data, he also calculated that the radius of the nucleus is about 10^5times less than the radius of the atom.
5. Electrons and the nucleus are held together by electrostatic forces of attraction.
Can you explain the answer of this question below:Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which is a possible set of quantum numbers for a valence unpaired electrons in ground state atom of phosphorus (Z = 15)?
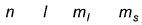
- A:
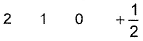
- B:
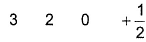
- C:
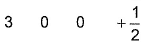
- D:
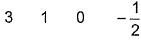
The answer is d.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which is a possible set of quantum numbers for a valence unpaired electrons in ground state atom of phosphorus (Z = 15)?
|
|
Dipika Singh answered |
Valence unpaired electrons are in 3p
Valence unpaired electrons are in 3p.
Thus, (d).
Can you explain the answer of this question below:Radiation of λ = 155 nm was irradiated on Li (work function = 5eV) plate. The stopping potential (in eV) is.
- A:
3eV
- B:
4eV
- C:
0.3eV
- D:
0.5 eV
The answer is a.
Radiation of λ = 155 nm was irradiated on Li (work function = 5eV) plate. The stopping potential (in eV) is.
3eV
4eV
0.3eV
0.5 eV

|
Ayush Joshi answered |
K.E.= hv − hv0 = hc/λ − W.F.= 6.6 x 10^−34 x 3 x 10^8/λ − 5 x 1.6 x 10^−19 and
K.Emax = eV0 where V0 is stopping potential so
V0 = 3eV
Given ΔH for the process Li(g) → Li+3(g) + 3e- is 19800 kJ/mole & IE1 for Li is 520 then IE2 & IE3 of Li+ are respectively (approx, value) :- a)11775, 7505
- b)19280, 520
- c)11775, 19280
- d)Data insufficient
Correct answer is option 'A'. Can you explain this answer?
Given ΔH for the process Li(g) → Li+3(g) + 3e- is 19800 kJ/mole & IE1 for Li is 520 then IE2 & IE3 of Li+ are respectively (approx, value) :
a)
11775, 7505
b)
19280, 520
c)
11775, 19280
d)
Data insufficient
|
|
Muskaan Kumar answered |
IE1 + IE2 + IE3 = 19800
IE2 + IE3 = 19800 – 520
IE2 + IE3 = 19280
IE2 + IE3 = 19800 – 520
IE2 + IE3 = 19280
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
Correct answer is '3'. Can you explain this answer?
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
|
|
Neha Sharma answered |
Number of waves = n(n - 1)/2 where n = Principal quantum number or number of orbit number of waves = 3(3 - 1)/2 = 3 * 2/2 = 3
ALTERNATIVE SOLUTIONS :
In general, the number of waves made by a Bohr electron in an orbit is equal to its quantum number.
According to Bohr’s postulate of angular momentum, in the 3rd orbit
Mur = n h/2π
Mur = 3 (h/2π) …..(i) [n = 3]
According to de Broglie relationship
λ = h/mu ….(ii)
Substituting (ii) in (i), we get
(h/λ) r = 3 (h/2π) or 3λ = 2πr
[∵ mu = h/λ]
Thus the circumference of the 3rd orbit is equal to 3 times the wavelength of electron i.e. the electron makes three revolution around the 3rd orbit.
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1 (Mass of proton = 1.67×10-27kg and h = 6.63×10-34Js)
- a)2.5 nm
- b)0.40 nm
- c) 14.0 nm
- d)32 nm
Correct answer is option 'B'. Can you explain this answer?
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1 (Mass of proton = 1.67×10-27kg and h = 6.63×10-34Js)
a)
2.5 nm
b)
0.40 nm
c)
14.0 nm
d)
32 nm
|
|
Siddharth Yadav answered |
To calculate the wavelength associated with a proton moving at 1.0 MeV, we can use the de Broglie wavelength equation:
λ = h / p
where λ is the wavelength, h is the Planck's constant (6.626 x 10^-34 J*s), and p is the momentum of the proton.
First, let's convert 1.0 MeV to joules:
1 MeV = 1.6 x 10^-13 J
Next, we need to calculate the momentum of the proton. The momentum (p) is given by:
p = sqrt(2 * m * KE)
Where m is the mass of the proton (1.6726219 x 10^-27 kg) and KE is the kinetic energy of the proton (1.0 MeV = 1.6 x 10^-13 J).
Now, let's calculate the momentum:
p = sqrt(2 * (1.6726219 x 10^-27 kg) * (1.6 x 10^-13 J))
p ≈ 1.29 x 10^-19 kg*m/s
Finally, we can calculate the wavelength:
λ = (6.626 x 10^-34 J*s) / (1.29 x 10^-19 kg*m/s)
λ ≈ 5.13 x 10^-16 m
To convert this wavelength to nanometers, we multiply by 10^9:
λ ≈ 5.13 x 10^-16 m * 10^9 nm/m
λ ≈ 5.13 x 10^-7 nm
Therefore, the wavelength associated with a proton moving at 1.0 MeV is approximately 5.13 x 10^-7 nanometers.
λ = h / p
where λ is the wavelength, h is the Planck's constant (6.626 x 10^-34 J*s), and p is the momentum of the proton.
First, let's convert 1.0 MeV to joules:
1 MeV = 1.6 x 10^-13 J
Next, we need to calculate the momentum of the proton. The momentum (p) is given by:
p = sqrt(2 * m * KE)
Where m is the mass of the proton (1.6726219 x 10^-27 kg) and KE is the kinetic energy of the proton (1.0 MeV = 1.6 x 10^-13 J).
Now, let's calculate the momentum:
p = sqrt(2 * (1.6726219 x 10^-27 kg) * (1.6 x 10^-13 J))
p ≈ 1.29 x 10^-19 kg*m/s
Finally, we can calculate the wavelength:
λ = (6.626 x 10^-34 J*s) / (1.29 x 10^-19 kg*m/s)
λ ≈ 5.13 x 10^-16 m
To convert this wavelength to nanometers, we multiply by 10^9:
λ ≈ 5.13 x 10^-16 m * 10^9 nm/m
λ ≈ 5.13 x 10^-7 nm
Therefore, the wavelength associated with a proton moving at 1.0 MeV is approximately 5.13 x 10^-7 nanometers.
If radius of second stationary orbit (in Bohr's atom) is R. Then radius of third orbit will be- a)R/3
- b)9R
- c)R/9
- d)2.25R
Correct answer is option 'D'. Can you explain this answer?
If radius of second stationary orbit (in Bohr's atom) is R. Then radius of third orbit will be
a)
R/3
b)
9R
c)
R/9
d)
2.25R
|
|
Shreya Singh answered |
R=0.529×n^2/Z.R is directly proportional to n^2.R1/R2=n1^2/n2^2.R/R2=2^2/3^2.R/R2=4/9.R2=9R/4.R2=2.25R.
The third line in Balmer series corresponds to an electronic transition between which Bohr's orbits in hydrogen :- a)5 → 3
- b)5 → 2
- c)4 → 3
- d)4 → 2
Correct answer is option 'B'. Can you explain this answer?
The third line in Balmer series corresponds to an electronic transition between which Bohr's orbits in hydrogen :
a)
5 → 3
b)
5 → 2
c)
4 → 3
d)
4 → 2

|
Krithika Kulkarni answered |
Balmer means transition to n = 2
1. line → 3 to 2
2. line → 4 to 2
3. line → 5 to 2
1. line → 3 to 2
2. line → 4 to 2
3. line → 5 to 2
Which quantum number is not related with Schrodinger equation :- a)Principal
- b)Azimuthal
- c)Magnetic
- d)Spin
Correct answer is option 'D'. Can you explain this answer?
Which quantum number is not related with Schrodinger equation :
a)
Principal
b)
Azimuthal
c)
Magnetic
d)
Spin
|
|
Nandini Patel answered |
Spin quantum number does not related with Schrodinger equation because they always show +1/2, -1/2 value.
The number of radial nodes in 3s and 2p respectively are- a)2 and 0
- b)0 and 2
- c)1 and 2
- d)2 and 1
Correct answer is option 'A'. Can you explain this answer?
The number of radial nodes in 3s and 2p respectively are
a)
2 and 0
b)
0 and 2
c)
1 and 2
d)
2 and 1
|
|
Gaurav Kumar answered |
Radial nodes = (n - l - 1)
Angular nodes = l, total nodes = (n - 1), nodal plane = l
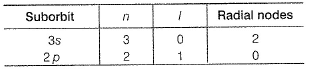
Angular nodes = l, total nodes = (n - 1), nodal plane = l
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in- a)1s
- b)2p
- c)3s
- d)3d
Correct answer is option 'C'. Can you explain this answer?
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in
a)
1s
b)
2p
c)
3s
d)
3d
|
|
Jyoti Kapoor answered |
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in. 1s.
Which of the following statements is/are correct?
- a)The electronic configuration Cr is [Ar] 3d5,4s1
- b)Spin quantum number of K (19) can have value - 1/2
- c)Any p-orbital can have maximum of two electrons
- d)A s-orbital can have maximum of two electron
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which of the following statements is/are correct?
a)
The electronic configuration Cr is [Ar] 3d5,4s1
b)
Spin quantum number of K (19) can have value - 1/2
c)
Any p-orbital can have maximum of two electrons
d)
A s-orbital can have maximum of two electron
|
|
Geetika Shah answered |
(a) Cr (24) = [Ar] 4s13d5 Correct
(b) K = 1s22s22 p63s23p64s1
(b) K = 1s22s22 p63s23p64s1
(c) p-suborbit has three orbitals, each orbital can have maximum of two electrons thus, correct.
(d) s-suborbit has one orbital and this orbital can have maximum of two electrons thus, correct.
Quantum Numbers are solutions of _____________- a)Heisenberg’s Uncertainty Principle
- b)Einstein’s mass energy relation
- c)Schrodinger’s Wave Equation
- d)Hamiltonian Operator
Correct answer is option 'C'. Can you explain this answer?
Quantum Numbers are solutions of _____________
a)
Heisenberg’s Uncertainty Principle
b)
Einstein’s mass energy relation
c)
Schrodinger’s Wave Equation
d)
Hamiltonian Operator
|
|
Arka Bose answered |
When the wave function for an atom is solved using the Schrodinger Wave Equation, the solutions obtained are called the Quantum Number which are basically n, l and m.
Direction (Q. Nos. 12-13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)Radius of Bohr’s orbit of H-atom is 52.9 pm. An emission in H-atom starts from the orbit having radius 1.3225 nm and ends at 211.6 pm.Q. Wavelength (in nm) associated with this emission is- a)434.17nm
- b)230.20 nm
- c)144.70nm
- d)289.40 nm
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 12-13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Radius of Bohr’s orbit of H-atom is 52.9 pm. An emission in H-atom starts from the orbit having radius 1.3225 nm and ends at 211.6 pm.
Q.
Wavelength (in nm) associated with this emission is
a)
434.17nm
b)
230.20 nm
c)
144.70nm
d)
289.40 nm
|
|
Satakshi Kumari answered |

Which quantum numbers gives the shell to which the electron belongs?- a)m
- b)n
- c)l
- d)s
Correct answer is option 'B'. Can you explain this answer?
Which quantum numbers gives the shell to which the electron belongs?
a)
m
b)
n
c)
l
d)
s
|
|
Sinjini Shah answered |
The quantum numbers in an atom are used to describe the energy levels and sublevels where electrons can be found. These quantum numbers include the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms).
- Principal Quantum Number (n): The principal quantum number determines the main energy level or shell in which an electron resides. It can have any positive integer value starting from 1. As the value of n increases, the energy level and distance from the nucleus also increase.
- Azimuthal Quantum Number (l): The azimuthal quantum number determines the sublevel or orbital shape within a particular energy level. It can have values ranging from 0 to (n-1). The sublevels are designated by letters: s, p, d, f, etc. The value of l also determines the number of orbitals within a sublevel. For example, when l = 0 (s sublevel), there is only one orbital; when l = 1 (p sublevel), there are three orbitals; when l = 2 (d sublevel), there are five orbitals, and so on.
- Magnetic Quantum Number (ml): The magnetic quantum number determines the orientation or spatial orientation of the orbitals within a sublevel. It can have values ranging from -l to +l, including zero. For example, when l = 1 (p sublevel), ml can have values of -1, 0, or +1, representing the three different orientations of the p orbitals.
- Spin Quantum Number (ms): The spin quantum number describes the spin orientation of an electron within an orbital. It can have two values: +1/2 (spin up) or -1/2 (spin down).
Out of these quantum numbers, the principal quantum number (n) determines the shell or energy level in which the electron resides. Therefore, the correct answer is option 'B' (n). The other quantum numbers (l, ml, and ms) provide further details about the specific sublevel, orbital orientation, and spin orientation of the electron within the shell.
- Principal Quantum Number (n): The principal quantum number determines the main energy level or shell in which an electron resides. It can have any positive integer value starting from 1. As the value of n increases, the energy level and distance from the nucleus also increase.
- Azimuthal Quantum Number (l): The azimuthal quantum number determines the sublevel or orbital shape within a particular energy level. It can have values ranging from 0 to (n-1). The sublevels are designated by letters: s, p, d, f, etc. The value of l also determines the number of orbitals within a sublevel. For example, when l = 0 (s sublevel), there is only one orbital; when l = 1 (p sublevel), there are three orbitals; when l = 2 (d sublevel), there are five orbitals, and so on.
- Magnetic Quantum Number (ml): The magnetic quantum number determines the orientation or spatial orientation of the orbitals within a sublevel. It can have values ranging from -l to +l, including zero. For example, when l = 1 (p sublevel), ml can have values of -1, 0, or +1, representing the three different orientations of the p orbitals.
- Spin Quantum Number (ms): The spin quantum number describes the spin orientation of an electron within an orbital. It can have two values: +1/2 (spin up) or -1/2 (spin down).
Out of these quantum numbers, the principal quantum number (n) determines the shell or energy level in which the electron resides. Therefore, the correct answer is option 'B' (n). The other quantum numbers (l, ml, and ms) provide further details about the specific sublevel, orbital orientation, and spin orientation of the electron within the shell.
Direction (Q. Nos. 16-17) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)Assume that azimuthal quantum number could have value of n also [in addition to normal value 0,1,2,..., (n - 1)].Q. Electronic configuration of vanadium (Z = 23) under the above condition would have been- a)
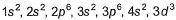
- b)
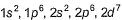
- c)

- d)
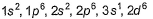
Correct answer is 'C'. Can you explain this answer?
Direction (Q. Nos. 16-17) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Assume that azimuthal quantum number could have value of n also [in addition to normal value 0,1,2,..., (n - 1)].
Q. Electronic configuration of vanadium (Z = 23) under the above condition would have been
a)
b)
c)
d)

|
Priyal answered |
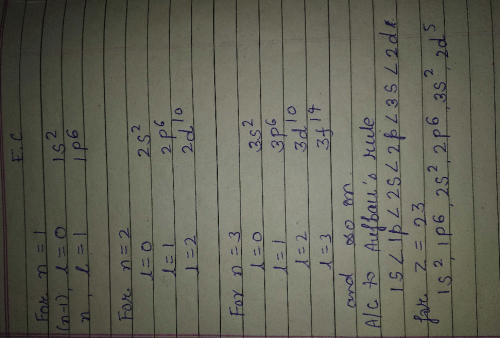
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?- a)Most of the space in the atom is empty.
- b)The radius of the atom is about 10–10 m while that of nucleus is 10–15 m.
- c)Electrons move in a circular path of fixed energy called orbits.
- d)Electrons and the nucleus are held together by electrostatic forces of attraction.
Correct answer is option 'C'. Can you explain this answer?
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?
a)
Most of the space in the atom is empty.
b)
The radius of the atom is about 10–10 m while that of nucleus is 10–15 m.
c)
Electrons move in a circular path of fixed energy called orbits.
d)
Electrons and the nucleus are held together by electrostatic forces of attraction.

|
Sparsh Sen answered |
Electrons revolve around the nucleus in a fixed circular path termed “orbits” or “shells” or “energy level.” The orbits are termed as “stationary orbit.” Everycircular orbit will have a certain amount of fixed energy and these circular orbitswere termed orbital shells.
In a multi-electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic and electric fields?

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C,D'. Can you explain this answer?
In a multi-electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic and electric fields?

a)
a
b)
b
c)
c
d)
d
|
|
Raghav Bansal answered |
(a) 2s (b) 2 p (c) 3d (d) 3d
Thus, (c) and (d), both 3d, have equal energy.
Thus, (c) and (d), both 3d, have equal energy.
Chapter doubts & questions for Atomic Structure - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Atomic Structure - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup