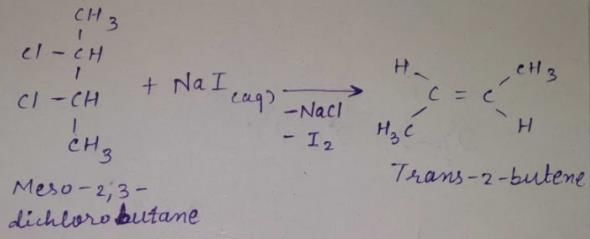All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Aliphatic Hydrocarbons for JAMB Exam
How many different isomeric alkynes on catalytic hydrogenation gives the same 3-ethyl hexane?- a)2
- b)3
- c)4
- d)6
Correct answer is option 'C'. Can you explain this answer?
How many different isomeric alkynes on catalytic hydrogenation gives the same 3-ethyl hexane?
a)
2
b)
3
c)
4
d)
6
|
|
Divya Menon answered |
Isomeric Alkynes and Catalytic Hydrogenation
Concept: Isomers are molecules that have the same molecular formula but different structural arrangements. Alkynes are hydrocarbons having at least one triple bond between two carbon atoms. Catalytic hydrogenation is the addition of hydrogen to an unsaturated organic compound in the presence of a catalyst. Catalytic hydrogenation of alkynes involves the addition of two hydrogen atoms across the triple bond, resulting in an alkane.
Explanation:
The given compound is 3-ethylhexane. The molecular formula of 3-ethylhexane is C8H18. The possible isomeric alkynes of C8H18 are:
1. Octyne-1
2. Octyne-2
3. Octyne-3
4. Octyne-4
5. Octyne-5
6. Octyne-6
Catalytic hydrogenation of octyne-1 and octyne-5 would result in the formation of the same product, i.e., 3-ethylhexane. Similarly, catalytic hydrogenation of octyne-2 and octyne-4 would also result in the formation of 3-ethylhexane. However, catalytic hydrogenation of octyne-3 and octyne-6 would result in the formation of different products.
Therefore, the total number of different isomeric alkynes that on catalytic hydrogenation gives the same 3-ethylhexane is 4.
Answer: The correct option is (C) 4.
Concept: Isomers are molecules that have the same molecular formula but different structural arrangements. Alkynes are hydrocarbons having at least one triple bond between two carbon atoms. Catalytic hydrogenation is the addition of hydrogen to an unsaturated organic compound in the presence of a catalyst. Catalytic hydrogenation of alkynes involves the addition of two hydrogen atoms across the triple bond, resulting in an alkane.
Explanation:
The given compound is 3-ethylhexane. The molecular formula of 3-ethylhexane is C8H18. The possible isomeric alkynes of C8H18 are:
1. Octyne-1
2. Octyne-2
3. Octyne-3
4. Octyne-4
5. Octyne-5
6. Octyne-6
Catalytic hydrogenation of octyne-1 and octyne-5 would result in the formation of the same product, i.e., 3-ethylhexane. Similarly, catalytic hydrogenation of octyne-2 and octyne-4 would also result in the formation of 3-ethylhexane. However, catalytic hydrogenation of octyne-3 and octyne-6 would result in the formation of different products.
Therefore, the total number of different isomeric alkynes that on catalytic hydrogenation gives the same 3-ethylhexane is 4.
Answer: The correct option is (C) 4.
Which of the following statements correctly describes the general reactivity of alkynes?- a)Alkynes are more reactive than alkenes
- b)Alkynes reacts as an electrophile
- c)Unlike alkenes, alkynes do not undergo electrophilic addition reaction
- d)An alkene is electron rich molecule, therefore react as a nucleophile
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements correctly describes the general reactivity of alkynes?
a)
Alkynes are more reactive than alkenes
b)
Alkynes reacts as an electrophile
c)
Unlike alkenes, alkynes do not undergo electrophilic addition reaction
d)
An alkene is electron rich molecule, therefore react as a nucleophile
|
|
Preeti Khanna answered |
The correct answer is option D
The clouds of electrons surrounding the sigma bond makes an alkyne an electron-rich molecule. They are therefore nucleophiles that react with electrophiles. Thus alkynes, like alkenes, undergo electrophilic addition reactions because of their weak pi bonds. When a proton adds to an alkyne, a vinylic cation is formed.
The clouds of electrons surrounding the sigma bond makes an alkyne an electron-rich molecule. They are therefore nucleophiles that react with electrophiles. Thus alkynes, like alkenes, undergo electrophilic addition reactions because of their weak pi bonds. When a proton adds to an alkyne, a vinylic cation is formed.
If 3-methyl-2-pentanol is heated with concentrated H2SO4 , in principle how many different alkenes result?
Correct answer is '5'. Can you explain this answer?
If 3-methyl-2-pentanol is heated with concentrated H2SO4 , in principle how many different alkenes result?
|
|
Krishna Iyer answered |
- methyl 1 pentene
- 3 methyl 2 butene
- 2 ethyl 1 butene
Hence 3 is the correct answer.
In which of the following reactions, reactants and products are correctly matched ?- a)
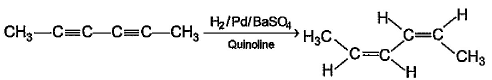
- b)
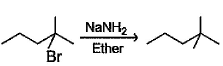
- c)
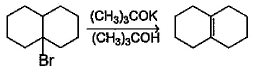
- d)
Correct answer is option 'A,B'. Can you explain this answer?
In which of the following reactions, reactants and products are correctly matched ?
a)
b)
c)
d)

|
Infinity Academy answered |
The correct answer is option A,B
With H2/Pd−BaSO4, cis hydrogenation will take place at both triple bonds.
With NaNH2, Sytzeff's elimination product will be the major one
(A) Cis addition of hydrogen
(B) Carbanion is more stable at secondary carbon so Saytzeff's elimination product will form.
(C) Carbanion should form at secondary carbon but given product is formed from tertiary unstable carbanion carbon so this is not possible.
(D) Complete reduction of alkyne to alkane in Pt reduction
With H2/Pd−BaSO4, cis hydrogenation will take place at both triple bonds.
With NaNH2, Sytzeff's elimination product will be the major one

(A) Cis addition of hydrogen
(B) Carbanion is more stable at secondary carbon so Saytzeff's elimination product will form.
(C) Carbanion should form at secondary carbon but given product is formed from tertiary unstable carbanion carbon so this is not possible.
(D) Complete reduction of alkyne to alkane in Pt reduction
Propene on ozonolysis forms:- a)Acetaldehyde
- b)Formaldehyde
- c)Both acetaldehyde and formaldehyde
- d)Acetone
Correct answer is option 'C'. Can you explain this answer?
Propene on ozonolysis forms:
a)
Acetaldehyde
b)
Formaldehyde
c)
Both acetaldehyde and formaldehyde
d)
Acetone
|
|
Raghav Bansal answered |
When propene on ozonolysis it yields a new structure called ozonide
and there cleavage takes place and it yields two products namely
1.acetaldehyde
2.formaldehyde
and there cleavage takes place and it yields two products namely
1.acetaldehyde
2.formaldehyde
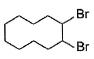
When vicinal dibromide is heated with KOH in ethanol (~ 200°C), double dehydrohalogenation takes place giving alkyne. Which of the following fails to give alkyne according to this procedure?- a)
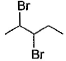
- b)
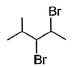
- c)
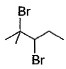
- d)
Correct answer is option 'C'. Can you explain this answer?
When vicinal dibromide is heated with KOH in ethanol (~ 200°C), double dehydrohalogenation takes place giving alkyne. Which of the following fails to give alkyne according to this procedure?
a)
b)
c)
d)
|
|
Rajesh Gupta answered |
The correct answer is option C
CH³)²BrC*–CHBr–CH²–CH³
C* has no H attached to it hence the next Bromine cannot perform dehydrohalogenation using this Carbon. Therefore, triple bond formation is not possible in this compound.
CH³)²BrC*–CHBr–CH²–CH³
C* has no H attached to it hence the next Bromine cannot perform dehydrohalogenation using this Carbon. Therefore, triple bond formation is not possible in this compound.
How many different heptenes result by partial hydrogenation of all possible, unbranched heptynes?
Correct answer is '5'. Can you explain this answer?
How many different heptenes result by partial hydrogenation of all possible, unbranched heptynes?
|
|
Neha Joshi answered |
The correct answer is 5.
C=C-C-C-C-C-C , no geometrical isomerism so 1
C-C=C-C-C-C-C 2 , geometrical cis trans
C-C-C=C-C-C-C , cis trans again so 2
∴ 2+2+1=5
C=C-C-C-C-C-C , no geometrical isomerism so 1
C-C=C-C-C-C-C 2 , geometrical cis trans
C-C-C=C-C-C-C , cis trans again so 2
∴ 2+2+1=5
Out of the following compounds , which will be have a zero dipole moment.- a)1,1 - dichloroethylene
- b)cis-1,2-dichlorethylene
- c)trans-1,2-dichlorethylene
- d)none of these.
Correct answer is option 'C'. Can you explain this answer?
Out of the following compounds , which will be have a zero dipole moment.
a)
1,1 - dichloroethylene
b)
cis-1,2-dichlorethylene
c)
trans-1,2-dichlorethylene
d)
none of these.
|
|
Hansa Sharma answered |
In case of trans-1,2-dichloroethylene
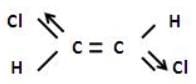
The net dipole moment is zero but in case of cis -1,2 -dicholorethylene.
There is some resultant dipole moment.
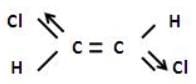
The net dipole moment is zero but in case of cis -1,2 -dicholorethylene.
There is some resultant dipole moment.
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many different alkenes are formed when 2 -chlorobutane is treated with ethanolic solution of KOH?
- a)1
- b)3
- c)2
- d)4
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many different alkenes are formed when 2 -chlorobutane is treated with ethanolic solution of KOH?
a)
1
b)
3
c)
2
d)
4
|
|
Arya Dasgupta answered |
The reaction of 2-chlorobutane with ethanolic solution of KOH is an example of an elimination reaction known as dehydrohalogenation. In this reaction, a hydrogen atom and a halogen atom are removed from adjacent carbon atoms to form a double bond.
The possible alkenes that can be formed are:
1. Butene
2. But-2-ene
3. But-1-ene
4. Isobutene
The number of different alkenes formed depends on the position of the double bond. In the case of 2-chlorobutane, the chlorine atom is attached to the second carbon atom, so the double bond can form between the first and second carbon atoms or between the second and third carbon atoms.
Hence, the correct answer is option (b) 2.
The possible alkenes that can be formed are:
1. Butene
2. But-2-ene
3. But-1-ene
4. Isobutene
The number of different alkenes formed depends on the position of the double bond. In the case of 2-chlorobutane, the chlorine atom is attached to the second carbon atom, so the double bond can form between the first and second carbon atoms or between the second and third carbon atoms.
Hence, the correct answer is option (b) 2.
Direction (Q, Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.Q.Statement I : 2-butyne on reduction with Pd/CaCO3 gives c/s-2-butene.
Statement II : Hydrogenation proceed through adsorption mechanism.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Direction (Q, Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : 2-butyne on reduction with Pd/CaCO3 gives c/s-2-butene.
Statement II : Hydrogenation proceed through adsorption mechanism.
Statement II : Hydrogenation proceed through adsorption mechanism.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Raghav Bansal answered |
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulphate which is then poisoned with various forms of lead or sulphur. It is used for the hydrogenation of alkynes to cis alkenes (i.e. without further reduction into alkanes) When the triple bond is (2-butyne) hydrogenated over the Lindlar’s catalyst i.e.Pd/CaCO3
it gives predominantly cis alkene(2-butene).
it gives predominantly cis alkene(2-butene).
Ethylene reacts with HBr to give:- a)Acetylene
- b)Ethyl alcohol
- c)Acetaldehyde
- d)Ethyl bromide
Correct answer is option 'D'. Can you explain this answer?
Ethylene reacts with HBr to give:
a)
Acetylene
b)
Ethyl alcohol
c)
Acetaldehyde
d)
Ethyl bromide
|
|
Preeti Iyer answered |
Ethylene reacts with HBr to form Ethyl bromide. The reaction propagates as follow:-
H2C=CH2 + HBr → H2C+-CH3 →H2BrC-CH3
Since π cloud is electron rich, so HBr dissociates into H+ and Br-. H+ attacks on alkene to give a carbocation and then Br- attacks to get ethyl bromide.
H2C=CH2 + HBr → H2C+-CH3 →H2BrC-CH3
Since π cloud is electron rich, so HBr dissociates into H+ and Br-. H+ attacks on alkene to give a carbocation and then Br- attacks to get ethyl bromide.
Ethene and ethyne can be distinguished by:- a)Bromine water
- b)KMnO4 solution
- c)Ammoniacal Cuprous chloride solution
- d)Any of the above
Correct answer is option 'C'. Can you explain this answer?
Ethene and ethyne can be distinguished by:
a)
Bromine water
b)
KMnO4 solution
c)
Ammoniacal Cuprous chloride solution
d)
Any of the above
|
|
Nandini Patel answered |
The two hydrocarbons can be easily distinguished by simple chemical tests, as ethyne molecule is supposed to have acidic hydrogen.
1. When ethyne is bubbled through ammoniacal silver nitrate solution , a yellow-white precipitate of silver acetylide would be formed.
C2H2 + 2AgNO3 = Ag2C2 + 2HNO3
2. Similarly, ethyne forms a red precipitate of copper acetylide (Cu2C2) when it is passed through ammoniacal cuprous chloride solution.
Ethene does not react with AgNO3 or Cu2Cl2 solution.
Direction (Q. Nos. 17 - 20) This section contains 4 questions. When worked o u t wili result in an integer from 0 to 9 (both inclusive).Q. If 3-bromo-4-methyl hexane is treated with ethanolic KOH solution, how many different alkenes would be formed?
Correct answer is '6'. Can you explain this answer?
Direction (Q. Nos. 17 - 20) This section contains 4 questions. When worked o u t wili result in an integer from 0 to 9 (both inclusive).
Q. If 3-bromo-4-methyl hexane is treated with ethanolic KOH solution, how many different alkenes would be formed?
|
|
Raghav Bansal answered |
2 is the right answer.
Alcoholic KOH causes dehydrohalogenation reaction i.e. elimination reaction.
Cis isomer have:- a)High boiling point than trans isomer
- b)Lower boiling point than trans isomer
- c)Same boiling point
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Cis isomer have:
a)
High boiling point than trans isomer
b)
Lower boiling point than trans isomer
c)
Same boiling point
d)
None of the above
|
|
Rajat Kapoor answered |
The cis isomer in this case has a boiling point of 60.3 degC, while the trans isomer has a boiling point of 47.5 degC. In the cis isomer the two polar C-Cl bond dipole moments combine to give an overall molecular dipole, so that there are intermolecular dipole–dipole forces (or Keesom forces), which add to the London.
Which among these is not a structural isomer of the compound C4H8?- a)But-1-ene
- b)But-2-ene
- c)But-3-ene
- d)2-methylpropene
Correct answer is option 'C'. Can you explain this answer?
Which among these is not a structural isomer of the compound C4H8?
a)
But-1-ene
b)
But-2-ene
c)
But-3-ene
d)
2-methylpropene

|
Saqib Shabir answered |
The answer is c. But-3-ene. But-3-ene is not a structural isomer of C4H8 because it is the same molecule as But-1-ene, just numbered differently. The structural isomers of C4H8 are But-1-ene, But-2-ene, and 2-methylpropene. But-1-ene and But-2-ene are position isomers, differing in the position of the double bond. 2-methylpropene is a branched isomer. But-3-ene is not a distinct isomer because the numbering of the carbon chain starts from the end closest to the double bond, making it identical to But-1-ene.
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. How many distinct alkynes exist for C6H10 which gives effervescence on heating with Na?- a)5
- b)2
- c)3
- d)4
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many distinct alkynes exist for C6H10 which gives effervescence on heating with Na?
a)
5
b)
2
c)
3
d)
4
|
|
Upasana Bose answered |
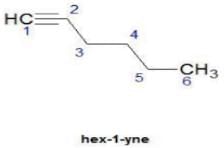
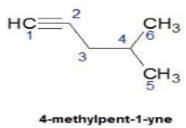
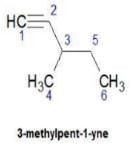
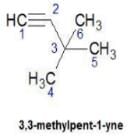
Hence D
Addition of halogens to alkenes is an example of:- a)Nucleophilic addition reaction
- b)Electrophilic addition reaction
- c)Electrophilic substitution reaction
- d)Nucleophilic substitution reaction
Correct answer is option 'B'. Can you explain this answer?
Addition of halogens to alkenes is an example of:
a)
Nucleophilic addition reaction
b)
Electrophilic addition reaction
c)
Electrophilic substitution reaction
d)
Nucleophilic substitution reaction

|
Avantika Chakraborty answered |
Halogens can act as electrophiles to attack a double bond in alkene. Double bond represents a region of electron density and therefore functions as a nucleophile.
What is the major dehydration product in the following reaction ?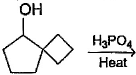
- a)

- b)

- c)
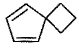
- d)

Correct answer is option 'D'. Can you explain this answer?
What is the major dehydration product in the following reaction ?
a)
b)
c)
d)
|
|
Pooja Shah answered |
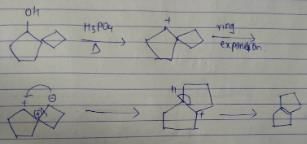
Note that H2SO4, H3PO4 and KHSO4 are dehydrating agents with rearrangement possible. While P2O5, P4O10, ThO2, POCl3 and Al2O3 are dehydrating agents without any possibility of rearrangement.
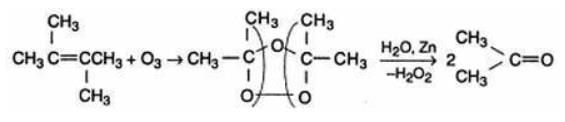
The alkene which on ozonolysis gives only acetone is:- a)(CH3)2C = CH2
- b)CH3CH = CHCH3
- c)CH3CH = C(CH3)2
- d)(CH3)2C = C(CH3)2
Correct answer is option 'D'. Can you explain this answer?
The alkene which on ozonolysis gives only acetone is:
a)
(CH3)2C = CH2
b)
CH3CH = CHCH3
c)
CH3CH = C(CH3)2
d)
(CH3)2C = C(CH3)2

|
Pioneer Academy answered |
The reductive ozaonalysis of 2,3 -Dimethyl-2-butene yields acetone.The reaction is as follows: 2,3 -Dimethyl-2-butene acetone
An optically active hydrocarbon X has molecular formula C6H12. X on catalytic hydrogenation gives optically inactive C6H14. X could be- a)3-methyl-1-pentene
- b)3-methyl-2-pentene
- c)4-methyl-2-pentene
- d)2-ethyl-1 -butene
Correct answer is option 'A'. Can you explain this answer?
An optically active hydrocarbon X has molecular formula C6H12. X on catalytic hydrogenation gives optically inactive C6H14. X could be
a)
3-methyl-1-pentene
b)
3-methyl-2-pentene
c)
4-methyl-2-pentene
d)
2-ethyl-1 -butene
|
|
Pritam Kapoor answered |
The optically active C6H1 2 hydrocarbon is 3-Methylpent-1-ene, having one chiral carbon shown in fig A.
On catalytic hydrogenation , the compound obtained is shown in fig B, and the molecule does not have chiral carbon.
The reaction is C6H1 2 → C6H1 4 and this reaction takes place inthe presence of H2 and Pd.
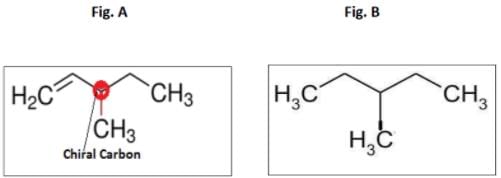
On catalytic hydrogenation , the compound obtained is shown in fig B, and the molecule does not have chiral carbon.
The reaction is C6H1 2 → C6H1 4 and this reaction takes place inthe presence of H2 and Pd.

In which of the following reactions , only single is omer of alkene is formed ?- a)
- b)
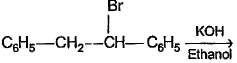
- c)
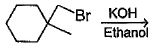
- d)

Correct answer is option 'A'. Can you explain this answer?
In which of the following reactions , only single is omer of alkene is formed ?
a)
b)
c)
d)
|
|
Pooja Shah answered |
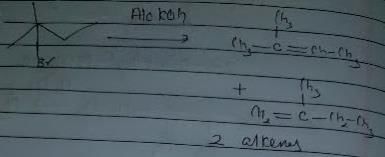
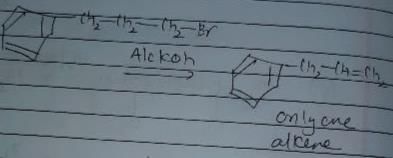
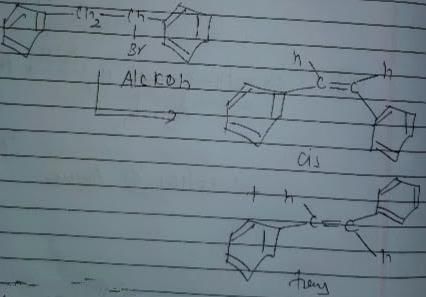
As there is no hydrogen at adjacent position for E2 elimination.
When H+ attacks CH3 – CH = CH2 , carbonation which is more stable is- a)CH3 – CH2 – CH2
- b)CH2+ – CH2 – CH3
- c)CH3 – CH+ – CH2
- d)CH3 – CH2 – CH2+
Correct answer is option 'C'. Can you explain this answer?
When H+ attacks CH3 – CH = CH2 , carbonation which is more stable is
a)
CH3 – CH2 – CH2
b)
CH2+ – CH2 – CH3
c)
CH3 – CH+ – CH2
d)
CH3 – CH2 – CH2+
|
|
Nitin Khanna answered |
CH3 – CH = CH2 → CH3 – CH+ – CH2
The reason for this is only that carbocation is formed which has maximum stability. In this case, we have 6 α-H while for option a, b and d; we have 0, 2 and 2 α-H respectively. So only carbocation in option c forms.
The reason for this is only that carbocation is formed which has maximum stability. In this case, we have 6 α-H while for option a, b and d; we have 0, 2 and 2 α-H respectively. So only carbocation in option c forms.
Which of the following compounds react most readily with Br2(g)?
- a) C2H2
- b) C3H6
- c)C2H4
- d)C4H10
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds react most readily with Br2(g)?
a)
C2H2
b)
C3H6
c)
C2H4
d)
C4H10
|
|
Siddharth Iyer answered |
The compound that reacts most readily with Br(g) is C3H6. Here's why:
Explanation:
When a compound reacts with Br(g), it undergoes a substitution reaction called bromination. In this reaction, a Br atom replaces a hydrogen atom in the compound. The reactivity of a compound towards bromination depends on its structure and the stability of the resulting product.
Comparing the compounds:
Let's compare the given compounds and analyze their structures to determine which one is most reactive towards bromination.
a) C2H2:
C2H2 is an alkyne with a triple bond between two carbon atoms. This triple bond is very strong and stable, making it difficult for Br(g) to break it and substitute a hydrogen atom. Therefore, C2H2 is less reactive towards bromination.
b) C3H6:
C3H6 is an alkene with a double bond between two carbon atoms. The double bond is weaker and less stable than a triple bond. Therefore, it is easier for Br(g) to break the double bond and substitute a hydrogen atom. This makes C3H6 more reactive towards bromination compared to C2H2.
c) C2H4:
C2H4 is also an alkene with a double bond between two carbon atoms, similar to C3H6. It has the same structure as C3H6, but it has fewer carbon atoms. Since the number of carbon atoms does not significantly affect the reactivity towards bromination, C2H4 is also reactive towards bromination, but less reactive compared to C3H6.
d) C4H10:
C4H10 is an alkane with only single bonds between carbon atoms. Alkanes are generally less reactive towards bromination because the single bonds are strong and stable. Breaking a single bond to substitute a hydrogen atom is more difficult for Br(g) compared to breaking a double or triple bond. Therefore, C4H10 is the least reactive towards bromination among the given compounds.
Conclusion:
Based on the structural analysis and the stability of the bonds, C3H6 is the most reactive compound towards bromination among the given options.
Explanation:
When a compound reacts with Br(g), it undergoes a substitution reaction called bromination. In this reaction, a Br atom replaces a hydrogen atom in the compound. The reactivity of a compound towards bromination depends on its structure and the stability of the resulting product.
Comparing the compounds:
Let's compare the given compounds and analyze their structures to determine which one is most reactive towards bromination.
a) C2H2:
C2H2 is an alkyne with a triple bond between two carbon atoms. This triple bond is very strong and stable, making it difficult for Br(g) to break it and substitute a hydrogen atom. Therefore, C2H2 is less reactive towards bromination.
b) C3H6:
C3H6 is an alkene with a double bond between two carbon atoms. The double bond is weaker and less stable than a triple bond. Therefore, it is easier for Br(g) to break the double bond and substitute a hydrogen atom. This makes C3H6 more reactive towards bromination compared to C2H2.
c) C2H4:
C2H4 is also an alkene with a double bond between two carbon atoms, similar to C3H6. It has the same structure as C3H6, but it has fewer carbon atoms. Since the number of carbon atoms does not significantly affect the reactivity towards bromination, C2H4 is also reactive towards bromination, but less reactive compared to C3H6.
d) C4H10:
C4H10 is an alkane with only single bonds between carbon atoms. Alkanes are generally less reactive towards bromination because the single bonds are strong and stable. Breaking a single bond to substitute a hydrogen atom is more difficult for Br(g) compared to breaking a double or triple bond. Therefore, C4H10 is the least reactive towards bromination among the given compounds.
Conclusion:
Based on the structural analysis and the stability of the bonds, C3H6 is the most reactive compound towards bromination among the given options.
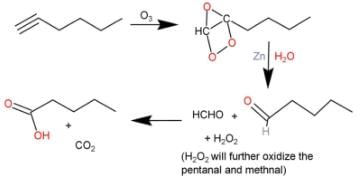
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has fo ur choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. What is/are true about ozonolysis of 1-hexyne followed by aqueous work-up?- a)Pentanoic acid and CO2 are formed
- b)Hydrolysis of ozonide of alkyne form H2O2 by product that oxidises the intermediate product into acids
- c)The ozonide has a C— C bond
- d)Pentanal and methanal are formed
Correct answer is option 'A,B,C'. Can you explain this answer?
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has fo ur choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. What is/are true about ozonolysis of 1-hexyne followed by aqueous work-up?
a)
Pentanoic acid and CO2 are formed
b)
Hydrolysis of ozonide of alkyne form H2O2 by product that oxidises the intermediate product into acids
c)
The ozonide has a C— C bond
d)
Pentanal and methanal are formed
|
|
Pooja Shah answered |
The correct answers are Options A, B and C.

A hydrocarbon X (C14H22)o n treatm ent with H2/Pt gives C14H26. Also X on treatm ent with alkaline KMnO4 followed by hydrolysis of products yields C7H12O2 which on further heating with soda lime gives cyclohexane. Hence, X is- a)
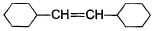
- b)
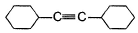
- c)
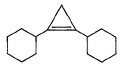
- d)
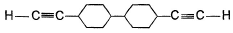
Correct answer is option 'B'. Can you explain this answer?
A hydrocarbon X (C14H22)o n treatm ent with H2/Pt gives C14H26. Also X on treatm ent with alkaline KMnO4 followed by hydrolysis of products yields C7H12O2 which on further heating with soda lime gives cyclohexane. Hence, X is
a)
b)
c)
d)
|
|
Neha Joshi answered |
The correct answers are option B.
As only 4 hydrogen atoms are increased after hydrogenation, there should be only 1 3× bond. So option B is correct.
As only 4 hydrogen atoms are increased after hydrogenation, there should be only 1 3× bond. So option B is correct.
Photochemical chlorination of alkane is initiated by process called:- a)Homolysis
- b)Pyrolysis
- c)Substitution
- d)Peroxidation
Correct answer is option 'A'. Can you explain this answer?
Photochemical chlorination of alkane is initiated by process called:
a)
Homolysis
b)
Pyrolysis
c)
Substitution
d)
Peroxidation
|
|
Rahul Bansal answered |
Photochemical chlorination of alkane take place by free radical mechanism which are possible by Homolysis of C - C bond
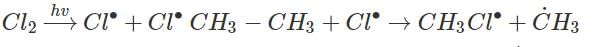
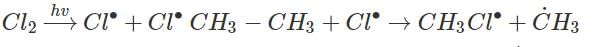
Which of the following reagent(s) can be used to distinguish between 1-hexyne and 2-hexyne?- a)Ammoniacal AgNO3 solution
- b)Ammoniacal solution of Cu(11) tartarate
- c)Cold, dilute and alkaline KMnO4
- d)H2SO4/HgSO4
Correct answer is option 'A,B'. Can you explain this answer?
Which of the following reagent(s) can be used to distinguish between 1-hexyne and 2-hexyne?
a)
Ammoniacal AgNO3 solution
b)
Ammoniacal solution of Cu(11) tartarate
c)
Cold, dilute and alkaline KMnO4
d)
H2SO4/HgSO4
|
|
Raghav Bansal answered |
The correct answers are Options A and B.
AgNO3 is called Tollen's reagent.
It reacts with terminal alkynes (1-hexyne) but not with alkenes (1-hexene).
2CH≡CCH2CH2CH3 + 2AgNO3 + 2NH4OH——›
1-hexyne
2CH≡CCH2CH2CH3Ag + 2NH4NO3 + 2H2O
AgNO3 is called Tollen's reagent.
It reacts with terminal alkynes (1-hexyne) but not with alkenes (1-hexene).
2CH≡CCH2CH2CH3 + 2AgNO3 + 2NH4OH——›
1-hexyne
2CH≡CCH2CH2CH3Ag + 2NH4NO3 + 2H2O
Terminal alkynes (hex-1-yne) react with ammoniacal
Cu2Cl2 solution. Tollens reagent, etc.
Cu2Cl2 solution. Tollens reagent, etc.
Consider the following addition reaction on a pure enantiomer of the shown bromoalkene.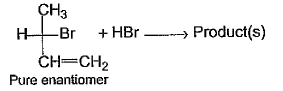 Q. What is/are true regarding products of the above reaction ?
Q. What is/are true regarding products of the above reaction ?- a)Four stereoisomers of products are formed
- b)A pair of enantiomers and a meso-dibromide are formed
- c)Only a pair of diastereomers are formed
- d)One of the product is meso-dibromide
Correct answer is option 'C,D'. Can you explain this answer?
Consider the following addition reaction on a pure enantiomer of the shown bromoalkene.
Q. What is/are true regarding products of the above reaction ?
a)
Four stereoisomers of products are formed
b)
A pair of enantiomers and a meso-dibromide are formed
c)
Only a pair of diastereomers are formed
d)
One of the product is meso-dibromide

|
Sushil Kumar answered |
The correct answer is Option C and D.
One of the product is a meso dibromide
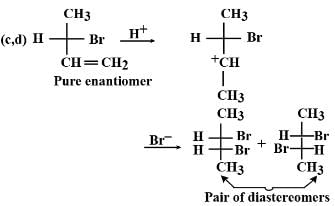
One of the product is a meso dibromide

Which reagent(s) below results in a visible change withl-butyne and can be used to differentiate it from 2-butyne?- a)

- b)

- c)
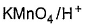
- d)
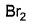
Correct answer is option 'A,B'. Can you explain this answer?
Which reagent(s) below results in a visible change withl-butyne and can be used to differentiate it from 2-butyne?
a)
b)
c)
d)
|
|
Priyanka Patil answered |
Both of it contain unpaired electron in its oxidation
Which of the following reactions produces an alkene?- a)
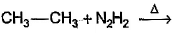
- b)
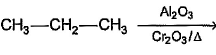
- c)
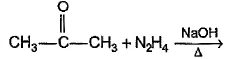
- d)
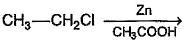
Correct answer is option 'B'. Can you explain this answer?
Which of the following reactions produces an alkene?
a)
b)
c)
d)
|
|
Hansa Sharma answered |
Option a) Diazene(N2H2) is a hydrogenating agent. So, there will be no reaction.
Option b) Al2O3+CrO3 acts as a dehydrogenation catalyst and so an alkene is formed. (Here 1-propene is formed)
Option c) This reaction is Wolff Kishner reaction. Here, acetone would be converted to alkane.
Option d) Zn/CH3COOH substitutes Cl with H and an alkane is formed.
Option b) Al2O3+CrO3 acts as a dehydrogenation catalyst and so an alkene is formed. (Here 1-propene is formed)
Option c) This reaction is Wolff Kishner reaction. Here, acetone would be converted to alkane.
Option d) Zn/CH3COOH substitutes Cl with H and an alkane is formed.
Consider the following reaction.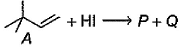 Molecular formula of both P and Q are C6H13l
Molecular formula of both P and Q are C6H13l
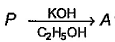 tetra substituted alkene isom er of A. Hence, P is most likely
tetra substituted alkene isom er of A. Hence, P is most likely- a)2, 2-dimethyl-3-iodobutane
- b)2-iodo-2, 3-dimethyl butane
- c)1-iodo-3, 3-dimethyl butane
- d)2, 3-diiodo-2, 3-dimethyl butan
Correct answer is option 'B'. Can you explain this answer?
Consider the following reaction.
Molecular formula of both P and Q are C6H13l
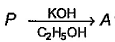 tetra substituted alkene isom er of A. Hence, P is most likely
tetra substituted alkene isom er of A. Hence, P is most likely
a)
2, 2-dimethyl-3-iodobutane
b)
2-iodo-2, 3-dimethyl butane
c)
1-iodo-3, 3-dimethyl butane
d)
2, 3-diiodo-2, 3-dimethyl butan
|
|
Geetika Shah answered |
Molecular formula of P and Q is C6H13I and this 3 degree reaction mechanism is SN1 mechanism so by that I molecules from HI attack as nucleophile to compound and when the reaction with KOH and C2H5OH rearrangement of I take place and the molecule formed is 2 iodo-2, 3- dimethyl butane.
Statement I : Boiling point of an alkene is slightly greater than that of its hydrogenated alkane.
Statement II : Alkenes have stronger van der Waals’ force of attraction than alkanes.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'D'. Can you explain this answer?
Statement I : Boiling point of an alkene is slightly greater than that of its hydrogenated alkane.
Statement II : Alkenes have stronger van der Waals’ force of attraction than alkanes.
Statement II : Alkenes have stronger van der Waals’ force of attraction than alkanes.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Ishani Pillai answered |
The correct answer is Option D.
The more intermolecular mass is added, the higher the boiling point. Intermolecular forces of alkenes get stronger with increase in the size of the molecules. In each case, the alkene has a boiling point which is a small number of degrees lower than the corresponding alkane.
The more intermolecular mass is added, the higher the boiling point. Intermolecular forces of alkenes get stronger with increase in the size of the molecules. In each case, the alkene has a boiling point which is a small number of degrees lower than the corresponding alkane.
When propene reacts with HBr in the presence of peroxide, it gives rise to
- a)Allyl bromide
- b)Isopropyl bromide
- c) n-propyl bromide
- d)3-bromopropane
Correct answer is option 'C'. Can you explain this answer?
When propene reacts with HBr in the presence of peroxide, it gives rise to
a)
Allyl bromide
b)
Isopropyl bromide
c)
n-propyl bromide
d)
3-bromopropane
|
|
Saanvi Nair answered |
Propene, also known as propylene, is an unsaturated hydrocarbon with the chemical formula C3H6. When propene reacts with HBr (hydrogen bromide) in the presence of peroxide, it undergoes a radical addition reaction to form n-propyl bromide (n-C3H7Br).
Here is a detailed explanation of the reaction:
1. Radical Initiation:
The presence of peroxide (often represented as ROOR, where R is an alkyl group) initiates the reaction by undergoing homolytic cleavage to produce two alkyl radicals, represented as R•. In this case, the peroxide could be tert-butyl peroxide (C(CH3)3OO•).
ROOR → 2R•
2. Radical Propagation:
The alkyl radical (R•) reacts with propene (C3H6) to form a more stable secondary radical intermediate.
R• + C3H6 → RCH2CH2•
The secondary radical intermediate then reacts with HBr to produce the alkyl bromide.
RCH2CH2• + HBr → RCH2CH2Br
This reaction step can occur at any position on the propene molecule, resulting in different possible alkyl bromide products.
3. Radical Termination:
The reaction can also undergo radical termination steps where two alkyl radicals combine to form a non-radical species. These termination steps play a role in consuming any excess alkyl radicals and stopping the chain reaction.
R• + R• → R-R
In the case of propene reacting with HBr in the presence of peroxide, the major product formed is n-propyl bromide (n-C3H7Br). This is because the secondary radical intermediate reacts preferentially with HBr at the terminal carbon of propene, resulting in the formation of n-propyl bromide. The other possible products, such as allyl bromide (C3H5Br), isopropyl bromide (C3H7Br), and 3-bromopropane (C3H7Br), are less favored due to the stability of the radical intermediate and the reactivity of HBr at the terminal carbon.
In summary, when propene reacts with HBr in the presence of peroxide, the major product obtained is n-propyl bromide (n-C3H7Br). This reaction follows a radical mechanism, initiated by the homolytic cleavage of peroxide and proceeds through radical propagation steps to form the alkyl bromide product.
Here is a detailed explanation of the reaction:
1. Radical Initiation:
The presence of peroxide (often represented as ROOR, where R is an alkyl group) initiates the reaction by undergoing homolytic cleavage to produce two alkyl radicals, represented as R•. In this case, the peroxide could be tert-butyl peroxide (C(CH3)3OO•).
ROOR → 2R•
2. Radical Propagation:
The alkyl radical (R•) reacts with propene (C3H6) to form a more stable secondary radical intermediate.
R• + C3H6 → RCH2CH2•
The secondary radical intermediate then reacts with HBr to produce the alkyl bromide.
RCH2CH2• + HBr → RCH2CH2Br
This reaction step can occur at any position on the propene molecule, resulting in different possible alkyl bromide products.
3. Radical Termination:
The reaction can also undergo radical termination steps where two alkyl radicals combine to form a non-radical species. These termination steps play a role in consuming any excess alkyl radicals and stopping the chain reaction.
R• + R• → R-R
In the case of propene reacting with HBr in the presence of peroxide, the major product formed is n-propyl bromide (n-C3H7Br). This is because the secondary radical intermediate reacts preferentially with HBr at the terminal carbon of propene, resulting in the formation of n-propyl bromide. The other possible products, such as allyl bromide (C3H5Br), isopropyl bromide (C3H7Br), and 3-bromopropane (C3H7Br), are less favored due to the stability of the radical intermediate and the reactivity of HBr at the terminal carbon.
In summary, when propene reacts with HBr in the presence of peroxide, the major product obtained is n-propyl bromide (n-C3H7Br). This reaction follows a radical mechanism, initiated by the homolytic cleavage of peroxide and proceeds through radical propagation steps to form the alkyl bromide product.
Direction (Q. Nos. 21 - 24) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. How many different isomeric alkynes on catalytic hydrogenation can give 2, 3, 4 -trimethyl heptane?
Correct answer is '8'. Can you explain this answer?
Direction (Q. Nos. 21 - 24) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. How many different isomeric alkynes on catalytic hydrogenation can give 2, 3, 4 -trimethyl heptane?

|
Ashish Mishra answered |
8 is correct.
Which of the following improperly describes the physical property of an alkyne?- a)Relatively non-polar
- b)Insoluble in alcohols and ethers
- c)Less dense than water
- d)Nearly insoluble in water
Correct answer is option 'B'. Can you explain this answer?
Which of the following improperly describes the physical property of an alkyne?
a)
Relatively non-polar
b)
Insoluble in alcohols and ethers
c)
Less dense than water
d)
Nearly insoluble in water
|
|
Mayank Chatterjee answered |
Alkynes are generally nonpolar molecules with little solubility in polar solvents, such as water. Solubility in nonpolar solvents, such as ether and acetone, is extensive. Like the alkanes and alkenes, alkynes of four or fewer carbon atoms tend to be gases.
Statement I : 2-bromobutane with (CH3)3COK in tertiary butanol gives 1 -butene as major product.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement I : 2-bromobutane with (CH3)3COK in tertiary butanol gives 1 -butene as major product.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Shivani Baliyan Baliyan answered |
B
If trans-2-pentene is treated with Br2 in CCI4 in presence of Lewis acid FeBr3- a)a pair of enantiomers of 2, 3-dibromopentane, but in unequal amounts are formed
- b)a racemic mixture of 2, 3-dibromopentane is formed
- c)a pure enantiomer of 2, 3-dibromopentane is formed
- d)a pair of diastereomers of 2, 3 dibromopentane is formed
Correct answer is option 'B'. Can you explain this answer?
If trans-2-pentene is treated with Br2 in CCI4 in presence of Lewis acid FeBr3
a)
a pair of enantiomers of 2, 3-dibromopentane, but in unequal amounts are formed
b)
a racemic mixture of 2, 3-dibromopentane is formed
c)
a pure enantiomer of 2, 3-dibromopentane is formed
d)
a pair of diastereomers of 2, 3 dibromopentane is formed
|
|
Sparsh Datta answered |
Trans-2-pentene on reaction with Br2 in CCl4 produces mainly (±) 2,3dibromopentene. A molecule of bromine is added across C=C double bond. A racemic mixture is obtained as the product.
Direction (Q. No. 17 - 20) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. If a pure enantiomer of 5 -bromo -2 -hexene is treated with Br2— CCI4 , how many different tribromides would be formed ?
Correct answer is '2'. Can you explain this answer?
Direction (Q. No. 17 - 20) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. If a pure enantiomer of 5 -bromo -2 -hexene is treated with Br2— CCI4 , how many different tribromides would be formed ?
|
|
Chirag Aggarwal answered |
No
Select the incorrect statement regarding alkenes.- a)In alkenes, the carbons are connected by pi bonds
- b)Alkenes have almost same physical properties as that of the alkanes
- c)Alkenes are less reactive than alkanes
- d)Alkenes undergo polymerization reaction
Correct answer is option 'C'. Can you explain this answer?
Select the incorrect statement regarding alkenes.
a)
In alkenes, the carbons are connected by pi bonds
b)
Alkenes have almost same physical properties as that of the alkanes
c)
Alkenes are less reactive than alkanes
d)
Alkenes undergo polymerization reaction
|
|
Srishti Chakraborty answered |
Understanding Alkenes and Their Reactivity
Alkenes are hydrocarbons characterized by the presence of at least one carbon-carbon double bond (C=C). This unique structure significantly influences their chemical properties, especially their reactivity compared to alkanes.
Incorrect Statement: Alkenes are less reactive than alkanes
- Alkenes are generally more reactive than alkanes. This increased reactivity is due to the presence of the double bond, which is more susceptible to reactions than the single bonds found in alkanes.
- The double bond consists of one sigma bond and one pi bond. The pi bond is a region of high electron density that can engage in electrophilic addition reactions, making alkenes quite reactive.
Comparison of Alkenes and Alkanes
- Physical Properties: Alkenes have similar physical properties to alkanes, such as boiling and melting points, primarily due to their similar structures and molecular weights.
- Reactivity: Alkenes react readily with electrophiles, undergoing reactions like hydrogenation, halogenation, and polymerization, enhancing their chemical activity.
Key Reactions of Alkenes
- Polymerization: Alkenes can undergo polymerization, a process where small alkene molecules (monomers) combine to form large polymer chains. This reaction is a fundamental aspect of synthetic materials.
- Electrophilic Addition: Alkenes are also involved in electrophilic addition reactions, where an electrophile reacts with the double bond, leading to the formation of more complex molecules.
In summary, the assertion that alkenes are less reactive than alkanes is incorrect. Alkenes are indeed more reactive due to their double bonds, allowing them to participate in a variety of chemical reactions.
Alkenes are hydrocarbons characterized by the presence of at least one carbon-carbon double bond (C=C). This unique structure significantly influences their chemical properties, especially their reactivity compared to alkanes.
Incorrect Statement: Alkenes are less reactive than alkanes
- Alkenes are generally more reactive than alkanes. This increased reactivity is due to the presence of the double bond, which is more susceptible to reactions than the single bonds found in alkanes.
- The double bond consists of one sigma bond and one pi bond. The pi bond is a region of high electron density that can engage in electrophilic addition reactions, making alkenes quite reactive.
Comparison of Alkenes and Alkanes
- Physical Properties: Alkenes have similar physical properties to alkanes, such as boiling and melting points, primarily due to their similar structures and molecular weights.
- Reactivity: Alkenes react readily with electrophiles, undergoing reactions like hydrogenation, halogenation, and polymerization, enhancing their chemical activity.
Key Reactions of Alkenes
- Polymerization: Alkenes can undergo polymerization, a process where small alkene molecules (monomers) combine to form large polymer chains. This reaction is a fundamental aspect of synthetic materials.
- Electrophilic Addition: Alkenes are also involved in electrophilic addition reactions, where an electrophile reacts with the double bond, leading to the formation of more complex molecules.
In summary, the assertion that alkenes are less reactive than alkanes is incorrect. Alkenes are indeed more reactive due to their double bonds, allowing them to participate in a variety of chemical reactions.
Ethylene on reaction with bromine forms which among the following product?- a)BrH2C=CH2Br
- b)BrH2C-CH2Br
- c)Br2HC=CHBr2
- d)Br2HC-CHBr2
Correct answer is option 'B'. Can you explain this answer?
Ethylene on reaction with bromine forms which among the following product?
a)
BrH2C=CH2Br
b)
BrH2C-CH2Br
c)
Br2HC=CHBr2
d)
Br2HC-CHBr2

|
Lead Academy answered |
The above reaction between Ethene and bromine is known as electrophilic halogenation reaction and the products usually formed are ethylene dihalides.
Statement I : 2, 3-dibromo butane with Zn-dust gives frans-2-butene as major product.Statement II : frans-2-butene is more stable than c/s-2-butene.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement I : 2, 3-dibromo butane with Zn-dust gives frans-2-butene as major product.
Statement II : frans-2-butene is more stable than c/s-2-butene.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Amrutha Das answered |
Zn reacts with 2,3 - dibromobutane to give alkene. As trans 2-butene is more stable than cis 2-butene(owing tosterric hinderancce), former is major product.
How many different alkyne isomers upon hydrogenation in the presence of CaCO3 / Pd gives 4-methyl hexene which is simultaneously c/s at double bond?
Correct answer is '2'. Can you explain this answer?
How many different alkyne isomers upon hydrogenation in the presence of CaCO3 / Pd gives 4-methyl hexene which is simultaneously c/s at double bond?
|
|
Ananya Datta answered |
Solution:
Given: Hydrogenation of alkyne in the presence of CaCO3 / Pd gives 4-methyl hexene which is simultaneously cis/trans at double bond.
To find: Number of different alkyne isomers.
Step 1: Write the formula of 4-methyl hexene.
4-methyl hexene formula: CH3-CH2-CH=CH-CH2-CH3
Step 2: Draw the structural formula of 4-methyl hexene.
H H
│ │
H-C-C-C=C-C-H
│ │
H CH3
Step 3: Identify the position of the double bond.
Double bond is present between 3rd and 4th carbon atoms.
Step 4: Identify the alkynes which can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd.
There are 2 possible alkyne isomers which can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd.
- 3-methyl-1-pentene - This alkyne can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd. The double bond is present between 2nd and 3rd carbon atoms. On hydrogenation, the double bond is converted into a single bond and a methyl group is added to the 3rd carbon atom.
- 2-methyl-2-pentene - This alkyne can also give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd. The double bond is present between 2nd and 3rd carbon atoms. On hydrogenation, the double bond is converted into a single bond and a methyl group is added to the 4th carbon atom.
Both of these alkyne isomers give 4-methyl hexene which is simultaneously cis/trans at double bond.
Therefore, the correct answer is '2'.
Given: Hydrogenation of alkyne in the presence of CaCO3 / Pd gives 4-methyl hexene which is simultaneously cis/trans at double bond.
To find: Number of different alkyne isomers.
Step 1: Write the formula of 4-methyl hexene.
4-methyl hexene formula: CH3-CH2-CH=CH-CH2-CH3
Step 2: Draw the structural formula of 4-methyl hexene.
H H
│ │
H-C-C-C=C-C-H
│ │
H CH3
Step 3: Identify the position of the double bond.
Double bond is present between 3rd and 4th carbon atoms.
Step 4: Identify the alkynes which can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd.
There are 2 possible alkyne isomers which can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd.
- 3-methyl-1-pentene - This alkyne can give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd. The double bond is present between 2nd and 3rd carbon atoms. On hydrogenation, the double bond is converted into a single bond and a methyl group is added to the 3rd carbon atom.
- 2-methyl-2-pentene - This alkyne can also give 4-methyl hexene upon hydrogenation in the presence of CaCO3 / Pd. The double bond is present between 2nd and 3rd carbon atoms. On hydrogenation, the double bond is converted into a single bond and a methyl group is added to the 4th carbon atom.
Both of these alkyne isomers give 4-methyl hexene which is simultaneously cis/trans at double bond.
Therefore, the correct answer is '2'.
When 2,3-dibromobutane is treated with KOH in ethanol, 2-bromo-2-butene is formed which does not undergo further dehydrobromination to form 2-butyne under similar condition because- a)H and Br are not trans
- b)there is no further B— H available
- c)vinyl bromide are very less reactive in E2 elimination reaction
- d)reactant is more stable than product
Correct answer is option 'C'. Can you explain this answer?
When 2,3-dibromobutane is treated with KOH in ethanol, 2-bromo-2-butene is formed which does not undergo further dehydrobromination to form 2-butyne under similar condition because
a)
H and Br are not trans
b)
there is no further B— H available
c)
vinyl bromide are very less reactive in E2 elimination reaction
d)
reactant is more stable than product
|
|
Rahul Kumar answered |
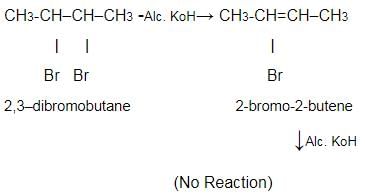
Because vinyl bromide is less reactive and Alc. KoH is not that strong a base; we need a strong base like NaNH2 .
Passage IITwo isomeric alkynes A and B have molecular formula C8H14 A on treatment with ammoniacal AgNO3 solution forms a white precipitate while B on similar treatment formed no precipitate. Also both A and B are chiral and hydrogenation of either A or B with H2/Pt gives the same achiral hydrocarbon C(C8H18). Treatment of A with HgSO4 /H2SO4 (aq) gives D(C8H16O) a s major product while sim ilar treatment of B, mixture of D and E in comparable amounts are formed. Q. What is true regarding A and B?- a)Both will evolve H2(g) on heating with Na metal
- b)A will give same product with either HgSO4 /H2SO4 or B2H6/H2O2/NaOH
- c)B will give same product with either HgSO4/H2SO4 or B2H6/H2O2/NaOH
- d)Both will produce a stereoisomers with Lindlar’s catalyst
Correct answer is option 'C,D'. Can you explain this answer?
Passage II
Two isomeric alkynes A and B have molecular formula C8H14 A on treatment with ammoniacal AgNO3 solution forms a white precipitate while B on similar treatment formed no precipitate. Also both A and B are chiral and hydrogenation of either A or B with H2/Pt gives the same achiral hydrocarbon C(C8H18). Treatment of A with HgSO4 /H2SO4 (aq) gives D(C8H16O) a s major product while sim ilar treatment of B, mixture of D and E in comparable amounts are formed.
Q. What is true regarding A and B?
a)
Both will evolve H2(g) on heating with Na metal
b)
A will give same product with either HgSO4 /H2SO4 or B2H6/H2O2/NaOH
c)
B will give same product with either HgSO4/H2SO4 or B2H6/H2O2/NaOH
d)
Both will produce a stereoisomers with Lindlar’s catalyst

|
Ashish Mishra answered |
C and D is correct.
The correct statement(s) regarding relative reactivity of an alkyne and an aklene is/are- a)Alkynes are more reactive than an alkene in electrophilic addition reaction
- b)In catalytic hydrogenation, alkynes are more reactive than alkene
- c)Alkynes give acids while alkenes give aldehydes and ketones
- d)Alkynes are less reactive than alkenes in addition reactions of halogens
Correct answer is option 'B,C,D'. Can you explain this answer?
The correct statement(s) regarding relative reactivity of an alkyne and an aklene is/are
a)
Alkynes are more reactive than an alkene in electrophilic addition reaction
b)
In catalytic hydrogenation, alkynes are more reactive than alkene
c)
Alkynes give acids while alkenes give aldehydes and ketones
d)
Alkynes are less reactive than alkenes in addition reactions of halogens
|
|
Yash Ghoshal answered |
b) Alkynes suffer less steric hindrance during adsorption on the surface of metal catalyst.
Hence, alkynes are more reactive than alkene towards catalytic hydrogenation.
c) Oxidation of alkenes with the help of ozone can give alcohols, aldehydes, ketones, or carboxylic acids.
Alkynes undergo ozonolysis to give acid anhydrides or diketones. If water is present in the reaction, the acid anhydride undergoes hydrolysis to yield two carboxylic acids.
d) The triple bonds of alkynes, because of its high electron density, are easily attacked by electrophiles, but less reactive than alkenes due to the compact C-C electron cloud.
Hence, alkynes are more reactive than alkene towards catalytic hydrogenation.
c) Oxidation of alkenes with the help of ozone can give alcohols, aldehydes, ketones, or carboxylic acids.
Alkynes undergo ozonolysis to give acid anhydrides or diketones. If water is present in the reaction, the acid anhydride undergoes hydrolysis to yield two carboxylic acids.
d) The triple bonds of alkynes, because of its high electron density, are easily attacked by electrophiles, but less reactive than alkenes due to the compact C-C electron cloud.
A chiral hydrocarbon has molar mass 82 and it gives effervescence when heated with Na metal. What is true about the original hydrocarbon?- a)Treatment with Pd/CaCO3/H2 would make it achiral
- b)Treatment with Na/NH3 (l) would make it achiral
- c)Treatment with Raney nickel-H2 would make it achiral
- d)It’s reaction with HgSO4/ H2SO4(aq) gives a mixture of ketones
Correct answer is option 'C'. Can you explain this answer?
A chiral hydrocarbon has molar mass 82 and it gives effervescence when heated with Na metal. What is true about the original hydrocarbon?
a)
Treatment with Pd/CaCO3/H2 would make it achiral
b)
Treatment with Na/NH3 (l) would make it achiral
c)
Treatment with Raney nickel-H2 would make it achiral
d)
It’s reaction with HgSO4/ H2SO4(aq) gives a mixture of ketones
|
|
Bhargavi Yadav answered |
A chiral hydrocarbon -Na → H2
There is terminal triple bond in the reactant hydrocarbon because,
R - C ≡ C - H -NaR - C ≡ O Na++H2
Molecular mass = 82i.e., It is C6H10
(a) CH3 - CH2 - CH - C ≡ CH
|
CH3
H2 | Pd | CaCO3
CH3 - CH2 - *CH - CH = CH2
|
CH3
Option A is wrong as it is chiral
CH3 - CH2 - CH - C ≡ CH
|
CH3
Na| NH3
CH3 - CH2 - *CH - CH = CH2
|
CH3
Option B is wrong as it is chiral
CH3 - CH2 - CH - C ≡ CH
|
CH3
H2 | Ni
CH3 - CH2 - CH - CH2 = CH2
|
CH3
Option C is correct as it is achiral
There is terminal triple bond in the reactant hydrocarbon because,
R - C ≡ C - H -NaR - C ≡ O Na++H2
Molecular mass = 82i.e., It is C6H10
(a) CH3 - CH2 - CH - C ≡ CH
|
CH3
H2 | Pd | CaCO3
CH3 - CH2 - *CH - CH = CH2
|
CH3
Option A is wrong as it is chiral
CH3 - CH2 - CH - C ≡ CH
|
CH3
Na| NH3
CH3 - CH2 - *CH - CH = CH2
|
CH3
Option B is wrong as it is chiral
CH3 - CH2 - CH - C ≡ CH
|
CH3
H2 | Ni
CH3 - CH2 - CH - CH2 = CH2
|
CH3
Option C is correct as it is achiral
Chapter doubts & questions for Aliphatic Hydrocarbons - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aliphatic Hydrocarbons - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily