All Exams >
Grade 12 >
Biology for Grade 12 >
All Questions
All questions of Lipids and Cholesterol for Grade 12 Exam
Cellulose is made up of- a)Fructose
- b)Glucose
- c)Sucrose
- d)Ribose
Correct answer is option 'B'. Can you explain this answer?
Cellulose is made up of
a)
Fructose
b)
Glucose
c)
Sucrose
d)
Ribose
|
|
Ayush Chauhan answered |
Cellulose is a third polymer made from beta glucose molecules and the polymer molecules are straight cellulose serves a very different purpose in nature to starch and glycogen it make up the cell walls in plant cell.
The bacterial cell wall is formed of- a)Cellulose
- b)Hemicellulose
- c)Peptidoglycan
- d)Glycogen
Correct answer is option 'C'. Can you explain this answer?
The bacterial cell wall is formed of
a)
Cellulose
b)
Hemicellulose
c)
Peptidoglycan
d)
Glycogen
|
|
Pooja Shah answered |
Bacterial cell walls are made of peptidoglycan (also called murein), which is made from polysaccharide chains cross-linked by unusual peptides containing D-amino acids. Bacterial cell walls are different from the cell walls of plants and fungi which are made of cellulose and chitin, respectively.
The number of amino acids found in proteins are- a)20
- b)21
- c)18
- d)16
Correct answer is option 'A'. Can you explain this answer?
The number of amino acids found in proteins are
a)
20
b)
21
c)
18
d)
16
|
|
Rohan Singh answered |
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. ... Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
The most abundant mineral of the animal body is- a)Potassium
- b)Sodium
- c)Calcium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
The most abundant mineral of the animal body is
a)
Potassium
b)
Sodium
c)
Calcium
d)
Iron
|
|
Hansa Sharma answered |
Calcium is the most abundant mineral in our body. It is essential for the development and maintenance of strong bones and teeth, where about 99% of the body's calcium is found. Calcium also helps the heart, nerves, muscles, and other body systems work properly.
Therefore, the correct answer is option C.
A homopolymer has only one type of building block called a monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers made of amino acids. While a nucleic acid like DNA or RNA is made of only 4 types of nucleotide monomers, proteins are made of- a)20 types of monomers
- b)3 types of monomers
- c)40 types of monomers
- d)Only one type of monomer
Correct answer is option 'A'. Can you explain this answer?
A homopolymer has only one type of building block called a monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers made of amino acids. While a nucleic acid like DNA or RNA is made of only 4 types of nucleotide monomers, proteins are made of
a)
20 types of monomers
b)
3 types of monomers
c)
40 types of monomers
d)
Only one type of monomer

|
Syed Hussain answered |
In living systems, like our own bodies, these larger molecules include carbohydrates, lipids, nucleic acids and proteins. The monomers of these organic groups are: Carbohydrates - monosaccharides. Lipids - glycerol and fatty acids
The most abundant organic molecule present on earth is- a)Protein
- b)Lipid
- c)Steroids
- d)Cellulose
Correct answer is option 'D'. Can you explain this answer?
The most abundant organic molecule present on earth is
a)
Protein
b)
Lipid
c)
Steroids
d)
Cellulose
|
|
Vijay Bansal answered |
The most abundant organic compound on Earth is cellulose, which is made up of many, many glucose molecules all linked together. Cellulose is found in the cell walls of every plant, which is why it is the most abundant organic molecule.
When we homogenise any tissue in an acid, the acid-soluble pool represents- a)Mitochondria
- b)Nucleus
- c)Cell membrane
- d)Cytoplasm
Correct answer is option 'D'. Can you explain this answer?
When we homogenise any tissue in an acid, the acid-soluble pool represents
a)
Mitochondria
b)
Nucleus
c)
Cell membrane
d)
Cytoplasm
|
|
Rohit Shah answered |
When we homogenise any tissue in an acid, the acid soluble pool represents cytoplasm.
Ester linkages occur in- a)Proteins
- b)Lipids
- c)Nucleic acids
- d)Carbohydrates
Correct answer is option 'B'. Can you explain this answer?
Ester linkages occur in
a)
Proteins
b)
Lipids
c)
Nucleic acids
d)
Carbohydrates
|
|
Anjali Iyer answered |
Lipids are actually triglycerides. A triglyceride consists of glycerol and fatty acids which are held together by ester linkages.
An ester bond is present between- a)Amino acids
- b)Nucleoside
- c)Nucleotide
- d)Monosaccharides
Correct answer is option 'C'. Can you explain this answer?
An ester bond is present between
a)
Amino acids
b)
Nucleoside
c)
Nucleotide
d)
Monosaccharides

|
Syed Hussain answered |
Phosphodiester bond definition. A bondbetween a two sugar groups and a phosphate group; such bonds form the sugar-phosphate-sugar backbone of DNA and RNA. A diester bond (between phosphoric acid and two sugar molecules) linking two nucleotides together to form the nucleotide polymers DNA and RNA.
The oils have- a)High melting point
- b)Low melting point
- c)Optimum melting point
- d)No melting point
Correct answer is option 'B'. Can you explain this answer?
The oils have
a)
High melting point
b)
Low melting point
c)
Optimum melting point
d)
No melting point
|
|
Geetika Shah answered |
Oils have lower melting point (e.g., gingely oil) and hence remain as oil in winters.
A nucleoside differs from a nucleotide in not having a- a)Phosphate group
- b)Glucose
- c)Sugar
- d)Nitrogen base
Correct answer is option 'A'. Can you explain this answer?
A nucleoside differs from a nucleotide in not having a
a)
Phosphate group
b)
Glucose
c)
Sugar
d)
Nitrogen base

|
Aliya Agrawal answered |
Phosphate group...
because nucleoside = sugar + nitrogen base...
while nucleotides = sugar + nitrogen base + phosphate group.....
because nucleoside = sugar + nitrogen base...
while nucleotides = sugar + nitrogen base + phosphate group.....
Double hydrogen bond occurs in DNA between- a)Adenine and guanine
- b)Thymine and cytosine
- c)Adenine and thymine
- d)Uracil and thymine
Correct answer is option 'C'. Can you explain this answer?
Double hydrogen bond occurs in DNA between
a)
Adenine and guanine
b)
Thymine and cytosine
c)
Adenine and thymine
d)
Uracil and thymine
|
|
Gaurav Kumar answered |
The complementary base pairs of guanine with cytosine and adenine with thymine connect to one another using hydrogen bonds. In addition to holding the DNA strands together, the hydrogen bonding between the complementary bases also sequester the bases in the interior of the double helix. Since, the option of Guanine and Cytosine is not provided. Hence, the correct option is Option C.
A segment of DNA has 120 adenine and 120 cytosine bases. The total number of nucleotides present in the segment is- a)480
- b)240
- c)60
- d)120
Correct answer is option 'A'. Can you explain this answer?
A segment of DNA has 120 adenine and 120 cytosine bases. The total number of nucleotides present in the segment is
a)
480
b)
240
c)
60
d)
120
|
|
Pooja Shah answered |
According to Chargaff’s rule, the amount of adenine is always equal to that of thymine and the amount of guanine is always equal to that of cytosine.
A = T(120), G = C(120)
The total number of nucleotides would be 120 × 4 = 480.
Which is a reducing sugar?- a)Cellulose
- b)Maltose
- c)Starch
- d)Sucrose
Correct answer is option 'B'. Can you explain this answer?
Which is a reducing sugar?
a)
Cellulose
b)
Maltose
c)
Starch
d)
Sucrose
|
|
Prem Darade answered |
The sugar that is capable of acting as a reducing sugar because it has free aldehyde group or a free ketone group ...
DNA nucleotides are attached by
- a)Hydrogen bond
- b)Covalent bond
- c)Van der Waals bond
- d)Electrovalent bond
Correct answer is option 'A'. Can you explain this answer?
DNA nucleotides are attached by
a)
Hydrogen bond
b)
Covalent bond
c)
Van der Waals bond
d)
Electrovalent bond
|
|
Gopikas S answered |
Explanation: DNA nucleotides are attached by the Hydrogen bond. A nucleotide is the basic unit of polynucleotide chain of DNA (deoxyribonucleic acid) or RNA (Ribonucleic acid).
The nitrogenous bases are found in the strand's inward direction. The nitrogenous bases of the two antiparallel strands form hydrogen bonds, resulting in the formation of two helical strands.
The nitrogenous bases used in DNA (double-stranded helical structure) are adenine (A), cytosine (C), guanine (G), and thymine (T).
Adenine is joined to thymine with two hydrogen bonds, whereas guanine is joined to cytosine by three hydrogen bonds.
Thus, DNA nucleotides are attached by Hydrogen bond.
The plant cell wall are made up of- a)Cellulose
- b)Starch
- c)Glycogen Bacteria
- d)Inulin
Correct answer is option 'A'. Can you explain this answer?
The plant cell wall are made up of
a)
Cellulose
b)
Starch
c)
Glycogen Bacteria
d)
Inulin

|
Srishti Sen answered |
Plant cell walls are made of cellulose. Paper made from plant pulp is cellulose.
Enormous diversity of protein molecules is due to- a)R groups of amino acids
- b)Sequence of amino acids
- c)Peptide bonds
- d)Amino groups of amino acids
Correct answer is option 'B'. Can you explain this answer?
Enormous diversity of protein molecules is due to
a)
R groups of amino acids
b)
Sequence of amino acids
c)
Peptide bonds
d)
Amino groups of amino acids
|
|
Rajat Kapoor answered |
The third is tertiary; this is the folding of the secondary structures into the final 3D structure of a protein. Amino acids have properties that guide this; some interact easily with water (hydrophilic) and these orient themselves on the outside of a protein, while others don't interact well with water (hydrophobic) and will try to get themselves on the inside of the folded structure where they will be protected. Hydrophobicity/philicity is the major driving force in protein folding but other bonds will also be formed between amino acids like S-S linkages, other ionic bonds and HYDROGEN BONDS (tons of these are made). These smaller interactions generally stabilize the protein and keep it folded in the most ideal conformation possible.
The nucleotide chemical components are- a)Heterocyclic compounds, sugar and phosphate
- b)Sugar and Phosphate
- c)Heterocyclic compounds and sugar
- d)Phosphate and heterocyclic compounds
Correct answer is option 'A'. Can you explain this answer?
The nucleotide chemical components are
a)
Heterocyclic compounds, sugar and phosphate
b)
Sugar and Phosphate
c)
Heterocyclic compounds and sugar
d)
Phosphate and heterocyclic compounds

|
Akshat Chavan answered |
The nucleotide has three chemically distinct components. One is a heterocyclic compound, the second is a monosaccharide and the third a phosphoric acid or phosphate.
Which of the following carbohydrates is not a disaccharide?- a)Lactose
- b)Maltose
- c)Sucrose
- d)Galactose
Correct answer is option 'D'. Can you explain this answer?
Which of the following carbohydrates is not a disaccharide?
a)
Lactose
b)
Maltose
c)
Sucrose
d)
Galactose
|
|
Gaurav Kumar answered |
Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase.
Chemical formula: C6H12O6
Solubility in water: 650 g/L (20 °C)
Chemical formula: C6H12O6
Solubility in water: 650 g/L (20 °C)
Proteins perform many physiological functions. For example, some function as enzymes. Which one of the following represents an additional function which some proteins discharge?- a)Antibiotics
- b)Pigments making colours of flowers
- c)Hormones
- d)Pigments conferring colour to skin
Correct answer is option 'C'. Can you explain this answer?
Proteins perform many physiological functions. For example, some function as enzymes. Which one of the following represents an additional function which some proteins discharge?
a)
Antibiotics
b)
Pigments making colours of flowers
c)
Hormones
d)
Pigments conferring colour to skin
|
|
Anjali Iyer answered |
Proteins perform many physiological functions. For example, some proteins function as enzymes. Hormones represents an additional function that some proteins discharge (like insulin).
Lactose is made of
- a)Glucose + Fructose
- b)Fructose + Fructose
- c)Glucose + Glucose
- d)Glucose + Galactose
Correct answer is option 'D'. Can you explain this answer?
Lactose is made of
a)
Glucose + Fructose
b)
Fructose + Fructose
c)
Glucose + Glucose
d)
Glucose + Galactose

|
Ciel Knowledge answered |
Glucose+Galactose
- Lactose is a disaccharide that breaks down into two saccharides, glucose and galactose on hydrolysis.
- Both saccharides are joined by a beta glycosidic link.
- It is a natural sugar found in milk in amounts ranging from 2 to 8%.
- Lactose is a reducing sugar because it contains one free hemiacetal hydroxide.
- Hence, it is the correct option.
Which one of the following pairs of nitrogenous bases of nucleic acids is wrongly matched with the category mentioned against it?- a)Adenine, Thymine – Purines
- b)Uracil, Cytosine – Pyrimidines
- c)Guanine, Adenine – Purines
- d)Thymine, Uracil – Pyrimidines
Correct answer is option 'A'. Can you explain this answer?
Which one of the following pairs of nitrogenous bases of nucleic acids is wrongly matched with the category mentioned against it?
a)
Adenine, Thymine – Purines
b)
Uracil, Cytosine – Pyrimidines
c)
Guanine, Adenine – Purines
d)
Thymine, Uracil – Pyrimidines

|
Aastha Kulshreshtha answered |
Answer= a
As,
•purines= Adenine and Guanine.
•pyrimidine= Cytosine, Uracil and Thymine.
therefore the wrongly matched pair would be A.
As,
•purines= Adenine and Guanine.
•pyrimidine= Cytosine, Uracil and Thymine.
therefore the wrongly matched pair would be A.
Fehling’s solution is used for the detection of- a)Fats
- b)Starch
- c)Glucose
- d)All carbohydrates
Correct answer is option 'C'. Can you explain this answer?
Fehling’s solution is used for the detection of
a)
Fats
b)
Starch
c)
Glucose
d)
All carbohydrates
|
|
Athira Mukherjee answered |
Fehling is a chemical reagent used to test for the presence of aldehyde functional groups. It consists of two separate solutions, Fehling's A and Fehling's B, which are mixed together before use. When an aldehyde is added to Fehling's solution and heated, a red precipitate of copper(I) oxide is formed, indicating the presence of the aldehyde. Fehling's reagent is commonly used in organic chemistry laboratories to identify and distinguish aldehydes from other types of compounds.
Which of the following is not a conjugated protein?- a)Peptone
- b)Glycoprotein
- c)Chromoprotein
- d)Lipoprotein
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a conjugated protein?
a)
Peptone
b)
Glycoprotein
c)
Chromoprotein
d)
Lipoprotein
|
|
Nandini Patel answered |
A conjugated protein is a protein that functions in interaction with other (non-polypeptide) chemical groups attached by covalent bonding or weak interactions. Many proteins contain only amino acids and no other chemical groups, and they are called simple proteins.
In yeast, during fermentation the glycolysis pathway leads to- a)Production of glucose
- b)Production of oxygen
- c)Production of pyruvic acid
- d)Production of ethanol
Correct answer is option 'D'. Can you explain this answer?
In yeast, during fermentation the glycolysis pathway leads to
a)
Production of glucose
b)
Production of oxygen
c)
Production of pyruvic acid
d)
Production of ethanol

|
Jatin Chakraborty answered |
In yeast, during fermentation, the same pathway leads to the production of ethanol(alcohol).
DNA differs from RNA in having- a)Thymine but no uracil
- b)Uracil but no thymine
- c)Thymine but no cytosine
- d)Cytosine but no guanine
Correct answer is option 'A'. Can you explain this answer?
DNA differs from RNA in having
a)
Thymine but no uracil
b)
Uracil but no thymine
c)
Thymine but no cytosine
d)
Cytosine but no guanine
|
|
Om Desai answered |
Uracil is energetically less expensive to produce than thymine, which may account for its use in RNA. In DNA, however, uracil is readily produced by chemical degradation of cytosine, so having thymine as the normal base makes detection and repair of such incipient mutations more efficient.
Which is the most abundant chemical for the living organisms?- a)Lipids
- b)Proteins
- c)Ions
- d)Water
Correct answer is option 'D'. Can you explain this answer?
Which is the most abundant chemical for the living organisms?
a)
Lipids
b)
Proteins
c)
Ions
d)
Water

|
Anand Jain answered |
Water is the most abundant chemical for the living organisms.
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding. Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.- a)If both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)If Assertion is true but Reason is false
- d)If both Assertion and Reason are false
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding.
Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.
a)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion
b)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
c)
If Assertion is true but Reason is false
d)
If both Assertion and Reason are false

|
Ciel Knowledge answered |
- The Assertion is correct because competitive inhibitors indeed bind to the active site of an enzyme, which prevents the substrate from binding.
- The Reason is also correct as increasing the substrate concentration can outcompete the inhibitor for the active site, thereby restoring enzyme activity.
- Additionally, the Reason effectively explains the Assertion as it describes how competitive inhibition can be mitigated, confirming that both statements are true and that the Reason is the correct explanation for the Assertion.
The energy currency of cell is—- a)GDP
- b)ATP
- c)ADP
- d)NAD
Correct answer is option 'B'. Can you explain this answer?
The energy currency of cell is—
a)
GDP
b)
ATP
c)
ADP
d)
NAD
|
|
Rohan Singh answered |
When the ATP converts to ADP, the ATP is said to be spent. he molecule is used like a battery within cells and allows the consumption of one of its phosphorous molecules.The energy currency used by all cells from bacteria to man is adenosine triphosphate (ATP).
Which of the following is not obtained on hydrolysis of nucleic acid?- a)Purine
- b)Phosphoric acid
- c)Pyrimidine
- d)Pentose sugar
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not obtained on hydrolysis of nucleic acid?
a)
Purine
b)
Phosphoric acid
c)
Pyrimidine
d)
Pentose sugar

|
Ruchi Chopra answered |
Hydrolysis of nucleic acid (DNA and RNA) produces pentose sugar (ribose or deoxyribose) purine and pyrimidine. Phosphoric acid is not released on hydrolysis of DNA or RNA.
A peptide bond is formed by the process of- a)Amination
- b)Rehydration
- c)Deamination
- d)Dehydration
Correct answer is option 'D'. Can you explain this answer?
A peptide bond is formed by the process of
a)
Amination
b)
Rehydration
c)
Deamination
d)
Dehydration
|
|
Preeti Iyer answered |
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O). This is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids.
In a DNA molecule, two strands are held by- a)Nitrogen bonds
- b)Phosphate bonds
- c)Carbon bonds
- d)Hydrogen bonds
Correct answer is option 'D'. Can you explain this answer?
In a DNA molecule, two strands are held by
a)
Nitrogen bonds
b)
Phosphate bonds
c)
Carbon bonds
d)
Hydrogen bonds
|
|
Pooja Mehta answered |
By hydrogen bonds between the two bases. Basically, the bases are ‘polar’, meaning they have slight differences in electrical charge at certain points. This allows them to attract one another like a balloon sticking to your hair.
Hydrogen bonds are weaker than the covalent bonds that hold the rest of the molecule together, making them easier to break and re-form.
Which one is not a denaturing factor for protein?- a)High energy radiation
- b)High pressure
- c)Drastic change in pH
- d)High temperature
Correct answer is option 'B'. Can you explain this answer?
Which one is not a denaturing factor for protein?
a)
High energy radiation
b)
High pressure
c)
Drastic change in pH
d)
High temperature

|
Prasenjit Pillai answered |
Protein molecules get denatured due to high temperature, very high or low pH and high energy radiation but there is no effect due to high pressure.
A polysaccharide present as storehouse of energy of plant tissues- a)Chitin
- b)Starch
- c)Hemi cellulose
- d)Cellulose
Correct answer is option 'B'. Can you explain this answer?
A polysaccharide present as storehouse of energy of plant tissues
a)
Chitin
b)
Starch
c)
Hemi cellulose
d)
Cellulose
|
|
Jyoti Kapoor answered |
Polysaccharide
A polysaccharide is a large molecule made of many smaller monosaccharides. Monosaccharides are simple sugars, like glucose. Special enzymes bind these small monomers together creating large sugar polymers, or polysaccharides. A polysaccharide is also called a glycan. A polysaccharide can be a homopolysaccharide, in which all the monosaccharides are the same, or a heteropolysaccharide in which the monosaccharides vary. Depending on which monosaccharides are connected, and which carbons in the monosaccharides connects, polysaccharides take on a variety of forms. A molecule with a straight chain of monosaccharides is called a linear polysaccharide, while a chain that has arms and turns is known as a branched polysaccharide.
. __________ is a globular protein of 6 kDa consisting of 51 amino acids arranged in 2 polypeptide chains held together by a disulphide bridge- a)Fibrinogen
- b)Keratin
- c)Insulin
- d)Glucagon
Correct answer is option 'C'. Can you explain this answer?
. __________ is a globular protein of 6 kDa consisting of 51 amino acids arranged in 2 polypeptide chains held together by a disulphide bridge
a)
Fibrinogen
b)
Keratin
c)
Insulin
d)
Glucagon
|
|
Pooja Mehta answered |
Human insulin is a peptide hormone composed of 51 ammo acids and has a molecular weight of 5805 Da.(∼6 Kda). In this molecule, there are two polypeptide chains (A and B) held together by disulphide bridge.
One turn of the DNA double helix spans a distance of- a)3.4 nm
- b)4.26 nm
- c)4.56 nm
- d)2.46 nm
Correct answer is option 'A'. Can you explain this answer?
One turn of the DNA double helix spans a distance of
a)
3.4 nm
b)
4.26 nm
c)
4.56 nm
d)
2.46 nm
|
|
Suresh Kumar answered |
Refer biomolecules chapter in Ncert .Its mentioned in that chapter...
Which one of the following is fibrous protein?- a)Collagen
- b)Ribozymes
- c)Haemoglobin
- d)Hemicellulose
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is fibrous protein?
a)
Collagen
b)
Ribozymes
c)
Haemoglobin
d)
Hemicellulose

|
Shanaya Rane answered |
Collagen is a fibrous protein. It is the main structural protein in the extracellular space in thevarious connective tissues. It is the most abundant protein in mammals.
The part of enzyme bound to the protein part by a covalent bond is called- a)Holoenzyme
- b)Cofactor
- c)Prosthetic group
- d)Apoenzyme
Correct answer is option 'C'. Can you explain this answer?
The part of enzyme bound to the protein part by a covalent bond is called
a)
Holoenzyme
b)
Cofactor
c)
Prosthetic group
d)
Apoenzyme

|
Imk Pathsala answered |
A prosthetic group is a tightly covalently bound, specific non-polypeptide unit required for the biological function of some proteins. The prosthetic group may be organic (such as a vitamin, sugar, or lipid) or inorganic (such as a metal ion), but is not composed of amino acids.
An immediate source of cellular energy is- a)Pyruvic acid
- b)NADH
- c)Glucose
- d)ATP
Correct answer is option 'D'. Can you explain this answer?
An immediate source of cellular energy is
a)
Pyruvic acid
b)
NADH
c)
Glucose
d)
ATP
|
|
Supriya Senapati answered |
Because during cellular respiration, Glucose undergoes oxidation through various enzymatic machinery to produce energy which is stored as the ATP( energy currency of cell ) , ATP is further utilized by the different cell for their activities.
Select the right option regarding the given graph.

- a)X - axis = Rate of reaction
Y - axis = Enzymatic activity
- b)X - axis = Enzymatic activity
Y - axis = Rate of reaction
- c)X - axis = pH/Temperature
Y - axis = Enzymatic activity
- d)X - axis = Enzymatic activity
Y - axis = pH/Temperature
Correct answer is option 'C'. Can you explain this answer?
Select the right option regarding the given graph.

a)
X - axis = Rate of reaction
Y - axis = Enzymatic activity
Y - axis = Enzymatic activity
b)
X - axis = Enzymatic activity
Y - axis = Rate of reaction
Y - axis = Rate of reaction
c)
X - axis = pH/Temperature
Y - axis = Enzymatic activity
Y - axis = Enzymatic activity
d)
X - axis = Enzymatic activity
Y - axis = pH/Temperature
Y - axis = pH/Temperature
|
|
Jyoti Sengupta answered |
The variable with which we have to find the relation should always have to be on the y axis and the variable which does not show variation and will always increase will be on the x-axis.
Metal ions required for functioning of enzyme is- a)Holoenzyme
- b)Co-enzyme
- c)Prosthetic group
- d)Co-factor
Correct answer is option 'D'. Can you explain this answer?
Metal ions required for functioning of enzyme is
a)
Holoenzyme
b)
Co-enzyme
c)
Prosthetic group
d)
Co-factor

|
Mahi Shah answered |
A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site and at the same time form one or more cordination bonds with the substrate, e.g., zinc is a cofactor for the proteolytic enzyme carboxypeptidase.
How many carbon atoms are generally used in composition of monosaccharides?- a)3 to 7
- b)1 to 5
- c)5 to 10
- d)5 to 15
Correct answer is option 'A'. Can you explain this answer?
How many carbon atoms are generally used in composition of monosaccharides?
a)
3 to 7
b)
1 to 5
c)
5 to 10
d)
5 to 15

|
Ciel Knowledge answered |
Carbohydrates are also called saccharides because their basic components are sugars. Monosaccharides are those sugars or simple carbohydrate monomers which cannot be hydrolysed further into smaller components. They have a general formula of CnH2nOn. Depending upon the number of carbon atoms, monosaccharides are of five types : trioses (having 3 carbon atoms, glyceraldehyde), tetroses (having four carbon atoms, e.g., erythrose), pentoses (having five carbon atoms, e.g, ribose), hexoses (having six carbon atoms, e.g.; glucose), heptoses (having seven carbon atoms, e.g., sedoheptulose).
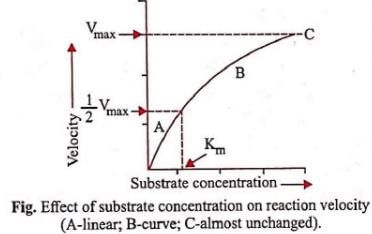
Michaelis Menten Constant (Km) is equal to- a)The rate of reaction
- b)The rate of enzymatic activity
- c)Substrate concentration at which the reaction attains half of its maximum velocity
- d)Substrate concentration at which the rate of reaction is maximum
Correct answer is option 'C'. Can you explain this answer?
Michaelis Menten Constant (Km) is equal to
a)
The rate of reaction
b)
The rate of enzymatic activity
c)
Substrate concentration at which the reaction attains half of its maximum velocity
d)
Substrate concentration at which the rate of reaction is maximum
|
|
Ajay Yadav answered |
Km or the Michaelis-Menten constant is defined as the substrate concentration (expressed in moles/l) at which half-maximum velocity in an enzyme catalysed reaction is achieved. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value. It was given by Leonor Michaelis and Maud Menten (1913). Km value is a characteristic feature of a given enzyme. It is a representative for measuring the strength of ES complex. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them. For majority of enzymes, the Km values are in the range of 10−5 to 10−2 moles.

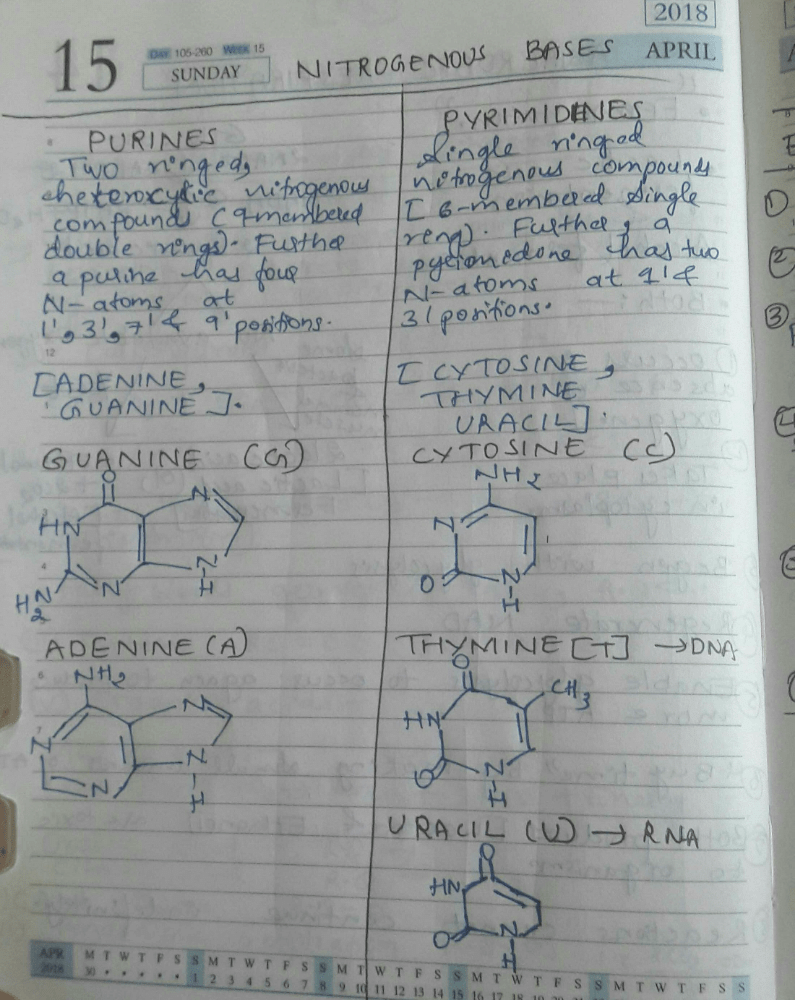
Read the following passage to answer the following questions:Proteins are polypeptide chains made up of amino acids. There are 20 types of amino acids joined together by peptide bond between amino and carboxylic group. There are two kinds of amino acids, Essential amino acids and Non-essential amino acids. The Primary structure of protein is the linear sequence of amino acids in a polypeptide chain. The first amino acid of sequence is called N-terminal amino acids and last amino acid of peptide chain is called C-terminal amino acids. The secondary structure proteins forms helix. There are three types of secondary structure: a helix, P pleated and collagen helix. In tertiary structure long protein chain is folded upon itself like a hollow woollen ball to give three-dimensional view of protein. In quaternary structure, each polypeptide develops its own tertiary structure and function as subunit of protein.Q. The smallest amino acid is- a)Phenol
- b)Formic acid
- c)Glycine
- d)Methane
Correct answer is option 'C'. Can you explain this answer?
Read the following passage to answer the following questions:
Proteins are polypeptide chains made up of amino acids. There are 20 types of amino acids joined together by peptide bond between amino and carboxylic group. There are two kinds of amino acids, Essential amino acids and Non-essential amino acids. The Primary structure of protein is the linear sequence of amino acids in a polypeptide chain. The first amino acid of sequence is called N-terminal amino acids and last amino acid of peptide chain is called C-terminal amino acids. The secondary structure proteins forms helix. There are three types of secondary structure: a helix, P pleated and collagen helix. In tertiary structure long protein chain is folded upon itself like a hollow woollen ball to give three-dimensional view of protein. In quaternary structure, each polypeptide develops its own tertiary structure and function as subunit of protein.
Q. The smallest amino acid is
a)
Phenol
b)
Formic acid
c)
Glycine
d)
Methane
|
|
Priya Menon answered |
The simplest, and smallest, amino acid is glycine for which the R-group is a hydrogen (H).
What is the term for the property of amino acids where the structure changes in solutions of different pH due to the ionizable nature of -NH2 and -COOH groups?- a)Isomerism
- b)Tautomeric shift
- c)Hydrolysis
- d)Acid dissociation
Correct answer is option 'D'. Can you explain this answer?
What is the term for the property of amino acids where the structure changes in solutions of different pH due to the ionizable nature of -NH2 and -COOH groups?
a)
Isomerism
b)
Tautomeric shift
c)
Hydrolysis
d)
Acid dissociation

|
EduRev NEET answered |
- Amino acids exhibit acid dissociation because their structure changes in solutions of different pH, primarily due to the ionizable nature of -NH2 and -COOH groups.
Chitin occurs in cell wall of- a)Bacteria
- b)Yeast
- c)Fungi
- d)Algae
Correct answer is option 'C'. Can you explain this answer?
Chitin occurs in cell wall of
a)
Bacteria
b)
Yeast
c)
Fungi
d)
Algae
|
|
Neha Sarkar answered |
Chitin in Fungi Cell Wall
Chitin is a long-chain polymer of a N-acetylglucosamine, a derivative of glucose. It is a structural polysaccharide found in the cell walls of fungi.
- **Fungi Cell Wall Composition**: The cell wall of fungi is primarily composed of chitin, along with other polysaccharides like glucans and proteins. Chitin provides strength and rigidity to the cell wall, protecting the fungal cell and providing structural support.
- **Function of Chitin in Fungi**: Chitin in the fungal cell wall plays a crucial role in maintaining cell shape, providing protection against environmental stresses, and serving as a barrier against harmful substances. It also helps in cell-cell communication and interaction with the external environment.
- **Unique Characteristics of Chitin**: Chitin is a unique polysaccharide that is not found in other organisms like bacteria, yeast, or algae. Its presence in the fungal cell wall sets fungi apart from other microorganisms in terms of structural composition.
- **Importance of Chitin**: Understanding the role of chitin in fungal cell walls is important for various applications, including the development of antifungal drugs, biocontrol agents, and biotechnological processes. Targeting chitin synthesis in fungi can lead to the development of novel strategies for controlling fungal infections and crop diseases.
In conclusion, chitin is a key component of the cell wall in fungi, providing structural integrity and protection to fungal cells. Its unique properties make it essential for the survival and growth of fungi, highlighting its significance in the field of microbiology and biotechnology.
Chitin is a long-chain polymer of a N-acetylglucosamine, a derivative of glucose. It is a structural polysaccharide found in the cell walls of fungi.
- **Fungi Cell Wall Composition**: The cell wall of fungi is primarily composed of chitin, along with other polysaccharides like glucans and proteins. Chitin provides strength and rigidity to the cell wall, protecting the fungal cell and providing structural support.
- **Function of Chitin in Fungi**: Chitin in the fungal cell wall plays a crucial role in maintaining cell shape, providing protection against environmental stresses, and serving as a barrier against harmful substances. It also helps in cell-cell communication and interaction with the external environment.
- **Unique Characteristics of Chitin**: Chitin is a unique polysaccharide that is not found in other organisms like bacteria, yeast, or algae. Its presence in the fungal cell wall sets fungi apart from other microorganisms in terms of structural composition.
- **Importance of Chitin**: Understanding the role of chitin in fungal cell walls is important for various applications, including the development of antifungal drugs, biocontrol agents, and biotechnological processes. Targeting chitin synthesis in fungi can lead to the development of novel strategies for controlling fungal infections and crop diseases.
In conclusion, chitin is a key component of the cell wall in fungi, providing structural integrity and protection to fungal cells. Its unique properties make it essential for the survival and growth of fungi, highlighting its significance in the field of microbiology and biotechnology.
Which of the following graphs shows the relationship between the rate of an enzymatic activity and substrate concentration (S)?- a)
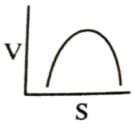
- b)
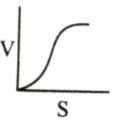
- c)
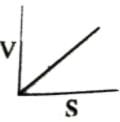
- d)
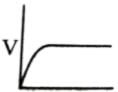
Correct answer is option 'D'. Can you explain this answer?
Which of the following graphs shows the relationship between the rate of an enzymatic activity and substrate concentration (S)?
a)

b)

c)

d)

|
|
Ajay Yadav answered |
Increase in substrate concentration increases the rate of reaction due to two factors: (i) occupation of more and more active sites by the substrate molecules, (ii) higher number of collisions between substrate molecules. The rise in velocity is quite high in the beginning but it decreases progressively with the increase in substate concentration. If a graph is plotted for substrate concentration versus reaction velocity, it appears as a hyperbolic curve. A stage is reached where velocity is maximum. It does not increase further by increasing the substrate concentration. At this stage the anzyme molecule becomes fully saturated and no active site is left free to bind additional substrate molecules.
Which of the following statements given above is/are correct?i. In the absence of any enzyme, the formation of H2CO3 is very slow, with about 200 molecules produced in an hour.ii. Carbonic anhydrase accelerates the reaction rate by approximately 10 million times, producing about 600,000 molecules of H2CO3 every second.iii. The metabolic pathway from glucose to pyruvic acid involves ten enzyme-catalyzed reactions.iv. Under anaerobic conditions in skeletal muscle, pyruvic acid is formed instead of lactic acid.- a)i and ii
- b) i, ii and iii
- c)i, iii and iv
- d)ii and iii
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements given above is/are correct?
i. In the absence of any enzyme, the formation of H2CO3 is very slow, with about 200 molecules produced in an hour.
ii. Carbonic anhydrase accelerates the reaction rate by approximately 10 million times, producing about 600,000 molecules of H2CO3 every second.
iii. The metabolic pathway from glucose to pyruvic acid involves ten enzyme-catalyzed reactions.
iv. Under anaerobic conditions in skeletal muscle, pyruvic acid is formed instead of lactic acid.
a)
i and ii
b)
i, ii and iii
c)
i, iii and iv
d)
ii and iii

|
Bs Academy answered |
To analyze the statements:
- Statement i is correct; it describes the slow reaction rate in the absence of an enzyme.
- Statement ii is also correct; it accurately describes the function of carbonic anhydrase and its dramatic acceleration of the reaction rate.
- Statement iii is accurate; it notes that the pathway from glucose to pyruvic acid consists of ten enzyme-catalyzed reactions.
- Statement iv is incorrect; under anaerobic conditions, lactic acid is produced in skeletal muscle, not pyruvic acid.
Therefore, the correct answer, which includes statements i, ii and iii, is Option B.
Which of the following nitrogenous base produces nucleoside only with ribose sugar?- a)Thymine
- b)Guanine
- c)Uracil
- d)Adenine
Correct answer is option 'C'. Can you explain this answer?
Which of the following nitrogenous base produces nucleoside only with ribose sugar?
a)
Thymine
b)
Guanine
c)
Uracil
d)
Adenine

|
Ruchi Chakraborty answered |
Uracil nitrogenous base is produces nucleoside only with ribose sugar. This nucleoside is present only inside the RNA and absent in DNA.
Chapter doubts & questions for Lipids and Cholesterol - Biology for Grade 12 2025 is part of Grade 12 exam preparation. The chapters have been prepared according to the Grade 12 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 12 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Lipids and Cholesterol - Biology for Grade 12 in English & Hindi are available as part of Grade 12 exam.
Download more important topics, notes, lectures and mock test series for Grade 12 Exam by signing up for free.
Biology for Grade 12
63 videos|111 docs|65 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup