All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Chemical Bonding and Shapes of Compounds for Chemistry Exam
The hybridization state on BH3 is:
- a)sp3d
- b)sp
- c)sp3
- d)sp2
Correct answer is option 'D'. Can you explain this answer?
The hybridization state on BH3 is:
a)
sp3d
b)
sp
c)
sp3
d)
sp2
|
|
Vikram Kapoor answered |
In BH3
We know that,
Boron has three valence electron, so it is supposed to make 3 bond in a molecules with hybridization sp2 as only S and two p are used in hybridization because last p orbital vacant.
Which one shows maximum hydrogen bonding?
- a)H2Se
- b)H2S
- c)H2O
- d)All have same
Correct answer is option 'C'. Can you explain this answer?
Which one shows maximum hydrogen bonding?
a)
H2Se
b)
H2S
c)
H2O
d)
All have same
|
|
Pooja Choudhury answered |
The correct option is : a H2O Explanation:H2O shows maximum H-bonding because each H2O molecule is linked to four H2O molecules through H-bonds.
The hybridization state in triangle bipyramidal CH5+ is:
- a)sp
- b)sp3d
- c)sp3
- d)sp2
Correct answer is option 'D'. Can you explain this answer?
The hybridization state in triangle bipyramidal CH5+ is:
a)
sp
b)
sp3d
c)
sp3
d)
sp2

|
Sahana Sharma answered |
Carbonium ion methonium, CH5+ is produced by the addition of H+ to CH4. In CH5+ three hydrogen atoms are in a plane and one above and one below the plane.
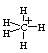
So its sp3d and the shape is trigonal bipyramid.
But this compound is considered a CH3+ carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond. As the two hydrogen atoms in H2 are in continuously exchange positions with the three hydrogen atoms in the CH3+ methonium CH5+ is considered as a fluxional molecule. Due to the existence of CH3+ this is sp2 hybridised.
Which type of bond if formed by overlapping of dxz and dxz orbitals if the molecular axis is x-axis:- a)σ bond
- b)π bond
- c)
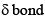
- d)μ bond
Correct answer is option 'B'. Can you explain this answer?
Which type of bond if formed by overlapping of dxz and dxz orbitals if the molecular axis is x-axis:
a)
σ bond
b)
π bond
c)
d)
μ bond
|
|
Pooja Choudhury answered |
Lateral overlap along the X-axis between two dzx orbitals leads to the formation of a Pi-bond.
The percentage of p-character in the orbitals forming P−P bonds in P4 is- a)25
- b)33
- c)50
- d)75
Correct answer is option 'D'. Can you explain this answer?
The percentage of p-character in the orbitals forming P−P bonds in P4 is
a)
25
b)
33
c)
50
d)
75
|
|
Pooja Choudhury answered |
The percentage of p-character in forming P-P bonds in P4 molecule is: 0. In forming
P4 molecule, P atom uses sp3 hybrid orbitals. Thus, the p-character in hybrid orbitals is 75%.
P4 molecule, P atom uses sp3 hybrid orbitals. Thus, the p-character in hybrid orbitals is 75%.
Examples of refractory materials include- a)MgO
- b)LiCl
- c)KF
- d)CaCl2
Correct answer is option 'A'. Can you explain this answer?
Examples of refractory materials include
a)
MgO
b)
LiCl
c)
KF
d)
CaCl2

|
Bijoy Kapoor answered |
They are namely based on oxides and their compounds, the most important oxides are Al2O3, CaO, MgO, SiO2, Cr2O3, and ZrO2. The second type is non-oxide refractories. Example of non-oxide refractories are carbon-based refractory materials, and carbides, nitrides, borides, and silicides.
According to VSEPR theory, the molecule/ ion having ideal tetrahedral shape is:- a)SF4
- b)SO42–
- c)S2Cl2
- d)SO2Cl2
Correct answer is option 'B'. Can you explain this answer?
According to VSEPR theory, the molecule/ ion having ideal tetrahedral shape is:
a)
SF4
b)
SO42–
c)
S2Cl2
d)
SO2Cl2

|
Jay Nambiar answered |
B)SO42- (sulfate ion) has ideal tetrahedral shape with four surrounding oxygen atoms arranged symmetrically around the central sulfur atom.
Arrange the following molecules in order of increasing bond polarit :(I) H2O
(II) NH3
(III) PH3
(IV) H2S- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < II < I
- d)III < IV < I < II
Correct answer is option 'C'. Can you explain this answer?
Arrange the following molecules in order of increasing bond polarit :
(I) H2O
(II) NH3
(III) PH3
(IV) H2S
(II) NH3
(III) PH3
(IV) H2S
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < II < I
d)
III < IV < I < II

|
Asf Institute answered |
Correct Answer :- c
Explanation : The bond polarity is directly proportional to the electronegativity of the atom.
As we move down the group, radius of elements increases and electronegativity decreases so bond angle decreases.
So, bond polarity will be : H2O < NH3< H2S < PH3
Correct order of bond angles is:- a)PF3 < PH3
- b)
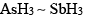
- c)OH2 < OF2
- d)OF2 > OCl2
Correct answer is option 'D'. Can you explain this answer?
Correct order of bond angles is:
a)
PF3 < PH3
b)
c)
OH2 < OF2
d)
OF2 > OCl2

|
Ravi Maurya answered |
Wrong ans bcs order is OCl2>H2O>OF2 due to large size of Cl so ans a is ryt
In the cyanide ion the negative charge is on
- a)N
- b)C
- c)Both C and N
- d)Resonate between C and N
Correct answer is option 'A'. Can you explain this answer?
In the cyanide ion the negative charge is on
a)
N
b)
C
c)
Both C and N
d)
Resonate between C and N
|
|
Sakib Mahamud answered |
From formal charge calculation c got the negative charge
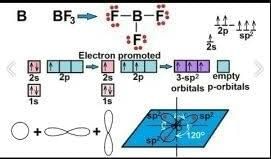
The hybridization state in ‘B’ when BF3 form adduct with ether is:
- a)sp
- b)sp3
- c) sp2
- d)sp4
Correct answer is option 'C'. Can you explain this answer?
The hybridization state in ‘B’ when BF3 form adduct with ether is:
a)
sp
b)
sp3
c)
sp2
d)
sp4

|
Soumya Sengupta answered |
For boron to bond with three fluoride atoms in boron trifluoride (BF3), the atomic s- and p-orbitals in boron's outer shell mix to form three equivalent sp2 hybrid orbitals.
The direction of dipole moment is correct in:- a)
- b)
- c)
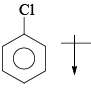
- d)All are correct.
Correct answer is option 'B'. Can you explain this answer?
The direction of dipole moment is correct in:
a)
b)
c)
d)
All are correct.
|
|
Vedika Singh answered |
In pyrolle there is greater resonance effect n due to more electronegativity in furan more inductive effect pronounced so the direction of dipole moment in furan is towards oxygen.
The structure of XeF4 is:
- a)Tetrahedral
- b)Square Pyramidal
- c)Square Planar
- d)Octahedral
Correct answer is option 'C'. Can you explain this answer?
The structure of XeF4 is:
a)
Tetrahedral
b)
Square Pyramidal
c)
Square Planar
d)
Octahedral

|
Gopal Sawai answered |
Question is wrong it should ask shape of this molecule
Hybridization in N2 is:- a)sp4
- b)sp2
- c)sp3
- d)None
Correct answer is option 'D'. Can you explain this answer?
Hybridization in N2 is:
a)
sp4
b)
sp2
c)
sp3
d)
None

|
Sagarika Patel answered |
The hybridization of O2 ,N2 and F2 is Sp2 , Sp, Sp3 . Steric factor = number of bonded atoms + number of lone pair . The hybridization of O2, N2, and F2are sp2, sp, sp3 respectively. As we know between O-O , there are two bonds one is sigma and other is pi bond.
Which has an exact angle of 120° among:- a)C2H4
- b)BF3
- c)HCHO
- d)All
Correct answer is option 'B'. Can you explain this answer?
Which has an exact angle of 120° among:
a)
C2H4
b)
BF3
c)
HCHO
d)
All
|
|
Chirag Verma answered |
Boron trifluoride is planar and the bond angle is 120 degrees. This can be predicted from VSEPR theory. There are three bonding pairs of electrons and no lone pairs on the central atom
Hybridization and shape of N(SiH3)3 is:- a)Sp3, tetrahedral
- b)sp2, planar
- c)sp3, planar
- d)sp2, angular
Correct answer is option 'B'. Can you explain this answer?
Hybridization and shape of N(SiH3)3 is:
a)
Sp3, tetrahedral
b)
sp2, planar
c)
sp3, planar
d)
sp2, angular

|
Ishani Dasgupta answered |
Hybridization and shape of N(SiH3)3:
Hybridization is the process of combining atomic orbitals to form hybrid orbitals that have different properties from the original atomic orbitals. The shape of a molecule is determined by the arrangement of its atoms and the hybridization of its orbitals.
The molecule N(SiH3)3 contains one nitrogen atom and three silicon atoms bonded to it. The nitrogen atom has one unpaired electron in its 2p orbital, which can be used to form three sigma bonds with the silicon atoms. The hybridization of the nitrogen atom is therefore determined by the number of sigma bonds it forms.
Option B is the correct answer because the hybridization of the nitrogen atom in N(SiH3)3 is sp2, and the shape of the molecule is planar.
Explanation:
1. Hybridization of the Nitrogen atom:
The nitrogen atom in N(SiH3)3 forms three sigma bonds with the silicon atoms, which requires three orbitals. Therefore, the nitrogen atom undergoes sp2 hybridization, in which one 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals.
2. Shape of the Molecule:
The shape of the molecule is determined by the arrangement of its atoms and the hybridization of its orbitals. In N(SiH3)3, the three sp2 hybrid orbitals on the nitrogen atom are arranged in a trigonal planar geometry, with bond angles of 120 degrees. The three Si-H bonds are also arranged in a trigonal planar geometry around each silicon atom. Therefore, the shape of the molecule is planar.
Conclusion:
In summary, the correct answer for the hybridization and shape of N(SiH3)3 is sp2 and planar, respectively. The nitrogen atom undergoes sp2 hybridization to form three sp2 hybrid orbitals, which are arranged in a trigonal planar geometry around the nitrogen atom. The three silicon atoms are also arranged in a trigonal planar geometry around the nitrogen atom, resulting in a planar shape for the molecule.
Hybridization is the process of combining atomic orbitals to form hybrid orbitals that have different properties from the original atomic orbitals. The shape of a molecule is determined by the arrangement of its atoms and the hybridization of its orbitals.
The molecule N(SiH3)3 contains one nitrogen atom and three silicon atoms bonded to it. The nitrogen atom has one unpaired electron in its 2p orbital, which can be used to form three sigma bonds with the silicon atoms. The hybridization of the nitrogen atom is therefore determined by the number of sigma bonds it forms.
Option B is the correct answer because the hybridization of the nitrogen atom in N(SiH3)3 is sp2, and the shape of the molecule is planar.
Explanation:
1. Hybridization of the Nitrogen atom:
The nitrogen atom in N(SiH3)3 forms three sigma bonds with the silicon atoms, which requires three orbitals. Therefore, the nitrogen atom undergoes sp2 hybridization, in which one 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals.
2. Shape of the Molecule:
The shape of the molecule is determined by the arrangement of its atoms and the hybridization of its orbitals. In N(SiH3)3, the three sp2 hybrid orbitals on the nitrogen atom are arranged in a trigonal planar geometry, with bond angles of 120 degrees. The three Si-H bonds are also arranged in a trigonal planar geometry around each silicon atom. Therefore, the shape of the molecule is planar.
Conclusion:
In summary, the correct answer for the hybridization and shape of N(SiH3)3 is sp2 and planar, respectively. The nitrogen atom undergoes sp2 hybridization to form three sp2 hybrid orbitals, which are arranged in a trigonal planar geometry around the nitrogen atom. The three silicon atoms are also arranged in a trigonal planar geometry around the nitrogen atom, resulting in a planar shape for the molecule.
Which of the following species has two non bonded electron pairs on the central atom:- a)TeCl4
- b)ClF3
- c)ICl2
- d)PCl3
Correct answer is option 'D'. Can you explain this answer?
Which of the following species has two non bonded electron pairs on the central atom:
a)
TeCl4
b)
ClF3
c)
ICl2
d)
PCl3

|
Garima Chavan answered |
In TeCl4 out of 6 electrons:bonded electrons are 4,non-bonded electron pair is 1.
In ClF3 out of 7 electrons:bonded electrons are 3,non-bonded electron pairs are 2.
In PCl3 out of 5 electrons:bonded electrons are 3,non-bonded electron pair is 1.
In ICl2− out of (7+1=8) electrons:bonded electrons are 2,non-bonded electron pairs are 3.
In ClF3 out of 7 electrons:bonded electrons are 3,non-bonded electron pairs are 2.
In PCl3 out of 5 electrons:bonded electrons are 3,non-bonded electron pair is 1.
In ICl2− out of (7+1=8) electrons:bonded electrons are 2,non-bonded electron pairs are 3.
In which of the following molecule are all the bonds not equal ?- a)NF3
- b)CIF3
- c)BF3
- d)AlF3
Correct answer is option 'B'. Can you explain this answer?
In which of the following molecule are all the bonds not equal ?
a)
NF3
b)
CIF3
c)
BF3
d)
AlF3
|
|
Biswa Nath answered |
In case of ClF3, there have two axial bond and one equatorial bond.
With respect to hyper valence theory, which will have multicenter bonding:
- a)B2H6
- b)CH6++
- c) SF4
- d)All are correct
Correct answer is option 'A'. Can you explain this answer?
With respect to hyper valence theory, which will have multicenter bonding:
a)
B2H6
b)
CH6++
c)
SF4
d)
All are correct

|
Yashvi Roy answered |
Multicenter bonding is typically associated with compounds that exhibit bonding that cannot be explained by conventional two-center two-electron bonds. This often involves compounds with electron-deficient atoms or those that form hypervalent bonds.
Let's evaluate the options:
1. B2H6: Diborane (B2H6) is a classic example of a molecule with multicenter bonding. It has two bridging hydrogen atoms that are involved in three-center two-electron bonds, connecting two boron atoms. This is a well-known case of multicenter bonding.
2. CH6++: This is not a standard compound and seems hypothetical. Such a molecule would be highly unstable and could theoretically involve multicenter bonding, but it's not a commonly recognized species.
3. SF4: Sulfur tetrafluoride (SF4) is hypervalent and has 10 electrons around sulfur, but it does not involve multicenter bonding. The bonding in SF4 can be explained using standard two-center bonds.
2. CH6++: This is not a standard compound and seems hypothetical. Such a molecule would be highly unstable and could theoretically involve multicenter bonding, but it's not a commonly recognized species.
3. SF4: Sulfur tetrafluoride (SF4) is hypervalent and has 10 electrons around sulfur, but it does not involve multicenter bonding. The bonding in SF4 can be explained using standard two-center bonds.
Given that B2H6 clearly has multicenter bonding and the other options are either incorrect or ambiguous:
Correct Answer: 1. B2H6
Which will have zero dipole moment:- a)O2F2
- b)H2O2
- c)MnO4–
- d)All
Correct answer is option 'C'. Can you explain this answer?
Which will have zero dipole moment:
a)
O2F2
b)
H2O2
c)
MnO4–
d)
All

|
Baishali Bajaj answered |
Zero dipole moment is the dipole moment between two atoms being zero. It depends on the polarities of individual bonds and the geometry of the atoms.
For example CO2 , two opposing oxygen atom with carbon in the center as it’s geometry . The dipole bonds are equal in magnitude but opposite in nature. So, it has zero dipole moment.
But on the other hand water (H2O) has a oxygen atom at the top of a triangular structure while two hydrogen atoms take the place of the base sides. Here also the dipole bonds are equal in magnitude but not opposing each other. So, it has non-zero dipole moment.
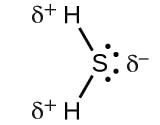
The geometry of H2S and its dipole moment are :- a)angular and non-zero
- b)angular and zero
- c)linear and non zero
- d)linear and zero
Correct answer is option 'A'. Can you explain this answer?
The geometry of H2S and its dipole moment are :
a)
angular and non-zero
b)
angular and zero
c)
linear and non zero
d)
linear and zero

|
Asf Institute answered |
- H2S molecule has an angular geometry because it has two lone pairs of electrons that make the molecule bend. The dipole moment is not zero.
- This is because sulfur is more electronegative than hydrogen, making the molecule slightly polar.
In which, lone pair is present in almost pure orbital:- a)

- b)
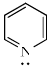
- c)SbH3
- d)NH3
Correct answer is option 'C'. Can you explain this answer?
In which, lone pair is present in almost pure orbital:
a)
b)
c)
SbH3
d)
NH3
|
|
Pooja Choudhury answered |
According to Drago's rule when the following conditions are satisfied, then the energy difference between the participating atomic orbitals will be very high and thus no mixing of orbitals or hybridization takes place.
- At least one lone pair must be present on the central atom.
- The central atom must be of 2nd period.
- The electronegativity of the surrounding atom must be less than or equal to 2.1.
- No hybridisation means bonding will take place through pure atomic p orbitals as in SbH3 and thus bond angle will be approximately equal to 900.
Which of the set of isomers of C6H4Cl2 is having equal dipole moment with C6H5Cl and C6H6 respectively
- a)ortho and meta
- b)meta and para
- c)ortho and para
- d)para and ortho
Correct answer is option 'B'. Can you explain this answer?
Which of the set of isomers of C6H4Cl2 is having equal dipole moment with C6H5Cl and C6H6 respectively
a)
ortho and meta
b)
meta and para
c)
ortho and para
d)
para and ortho
|
|
Pooja Choudhury answered |
The dipole moment of a molecule depends on the magnitude and direction of the bond dipole moments and the molecular geometry. C6H5Cl (chlorobenzene) has a dipole moment of 1.7 D, while C6H6 (benzene) has zero dipole moment.
Out of the isomers of C6H4Cl2 (1,2-dichlorobenzene), the ortho (1,2-) and meta (1,3-) isomers have non-zero dipole moments due to the asymmetrical distribution of the chlorine atoms around the benzene ring.
The para (1,4-) isomer has a zero dipole moment because the two bond dipole moments of the chlorine atoms are oriented in opposite directions, canceling each other out.
Therefore, the isomers that have equal dipole moment with C6H5Cl (1.7 D) are the ortho and meta isomers.
Which of the following set of ions/ molecules is isoelectric and structural?- a)SF4, SiF4, XeF4, PF4+
- b)CO2, CN22-, C34-, SO2
- c)BeF42-, BF4-, CF4, NF4+
- d)CO32-, NO3-, SO32-, PO32-
Correct answer is option 'C'. Can you explain this answer?
Which of the following set of ions/ molecules is isoelectric and structural?
a)
SF4, SiF4, XeF4, PF4+
b)
CO2, CN22-, C34-, SO2
c)
BeF42-, BF4-, CF4, NF4+
d)
CO32-, NO3-, SO32-, PO32-

|
Asf Institute answered |
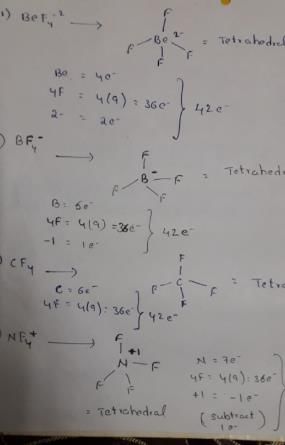 Isoelectronic species are those have same number of electrons.
Isoelectronic species are those have same number of electrons.Hybridization on central atom in Be2C is:- a)sp
- b)sp2
- c)sp3
- d)None
Correct answer is option 'A'. Can you explain this answer?
Hybridization on central atom in Be2C is:
a)
sp
b)
sp2
c)
sp3
d)
None

|
Vaibhav Ghosh answered |
Hybridization of central atom in Be2C
Explanation:
Be2C is a diatomic molecule in which two Be atoms are covalently bonded to one C atom. In Be2C, the central atom is C and the valence shell electronic configuration of C is 2s22p2.
The hybridization of an atom is defined as the mixing of atomic orbitals to form hybrid orbitals that have different shapes and energies than the original atomic orbitals. The hybrid orbitals are used to explain the geometry of molecules and the bonding between atoms.
To determine the hybridization of the central atom in Be2C, we need to first determine the number of hybrid orbitals needed to accommodate the electron pairs around the central atom.
In Be2C, the C atom is surrounded by two electron pairs (one from each Be atom) and therefore needs two hybrid orbitals. The type of hybridization that occurs depends on the number and type of atomic orbitals that are mixed.
In C, the 2s and three 2p orbitals can hybridize to form four sp3 hybrid orbitals, three sp2 hybrid orbitals, or two sp hybrid orbitals.
However, in Be2C, only two hybrid orbitals are needed, and therefore only two atomic orbitals can hybridize. The most likely hybridization is sp hybridization, which involves mixing the 2s and one 2p orbital.
Therefore, the correct answer is option "A" (sp).
Conclusion:
The hybridization of the central atom in Be2C is sp.
Explanation:
Be2C is a diatomic molecule in which two Be atoms are covalently bonded to one C atom. In Be2C, the central atom is C and the valence shell electronic configuration of C is 2s22p2.
The hybridization of an atom is defined as the mixing of atomic orbitals to form hybrid orbitals that have different shapes and energies than the original atomic orbitals. The hybrid orbitals are used to explain the geometry of molecules and the bonding between atoms.
To determine the hybridization of the central atom in Be2C, we need to first determine the number of hybrid orbitals needed to accommodate the electron pairs around the central atom.
In Be2C, the C atom is surrounded by two electron pairs (one from each Be atom) and therefore needs two hybrid orbitals. The type of hybridization that occurs depends on the number and type of atomic orbitals that are mixed.
In C, the 2s and three 2p orbitals can hybridize to form four sp3 hybrid orbitals, three sp2 hybrid orbitals, or two sp hybrid orbitals.
However, in Be2C, only two hybrid orbitals are needed, and therefore only two atomic orbitals can hybridize. The most likely hybridization is sp hybridization, which involves mixing the 2s and one 2p orbital.
Therefore, the correct answer is option "A" (sp).
Conclusion:
The hybridization of the central atom in Be2C is sp.
The bond energies of H2, F2 and Cl2 decreases in the following order:- a)H2 > F2 > Cl2
- b)Cl2 > F2 > H2
- c)F2 > Cl2 > H2
- d)H2 > Cl2 > F2
Correct answer is option 'D'. Can you explain this answer?
The bond energies of H2, F2 and Cl2 decreases in the following order:
a)
H2 > F2 > Cl2
b)
Cl2 > F2 > H2
c)
F2 > Cl2 > H2
d)
H2 > Cl2 > F2

|
Garima Chavan answered |
Bond Energies of H2, F2, and Cl2
Bond energy is the energy required to break a chemical bond in a molecule. It is also known as bond dissociation energy. The bond energies of H2, F2, and Cl2 are determined by the strength of the bond between the atoms. The bond energy decreases in the order H2 > F2 > Cl2 because of the difference in the electronegativity of the atoms.
Explanation of the Correct Answer
The correct answer is option 'D', which means the bond energies of H2, Cl2, and F2 decrease in the order H2 > Cl2 > F2. This order can be explained as follows:
H2: Hydrogen molecule consists of two hydrogen atoms that are held together by a covalent bond. The bond energy of H2 is 436 kJ/mol. The bond energy is relatively high because the two hydrogen atoms have the same electronegativity, which means the electrons are shared equally between the two atoms.
Cl2: Chlorine molecule consists of two chlorine atoms that are also held together by a covalent bond. The bond energy of Cl2 is 242 kJ/mol. The bond energy is lower than H2 because the two chlorine atoms have a larger difference in electronegativity, which means the electrons are not shared equally between the two atoms.
F2: Fluorine molecule also consists of two fluorine atoms that are held together by a covalent bond. The bond energy of F2 is 158 kJ/mol. The bond energy is the lowest among the three molecules because the two fluorine atoms have the largest difference in electronegativity, which means the electrons are strongly attracted to one of the atoms.
Conclusion
In summary, the bond energies of H2, F2, and Cl2 decrease in the order H2 > Cl2 > F2. This is because of the difference in electronegativity of the atoms. When the electronegativity difference is small, the bond energy is higher, and when the electronegativity difference is large, the bond energy is lower.
Bond energy is the energy required to break a chemical bond in a molecule. It is also known as bond dissociation energy. The bond energies of H2, F2, and Cl2 are determined by the strength of the bond between the atoms. The bond energy decreases in the order H2 > F2 > Cl2 because of the difference in the electronegativity of the atoms.
Explanation of the Correct Answer
The correct answer is option 'D', which means the bond energies of H2, Cl2, and F2 decrease in the order H2 > Cl2 > F2. This order can be explained as follows:
H2: Hydrogen molecule consists of two hydrogen atoms that are held together by a covalent bond. The bond energy of H2 is 436 kJ/mol. The bond energy is relatively high because the two hydrogen atoms have the same electronegativity, which means the electrons are shared equally between the two atoms.
Cl2: Chlorine molecule consists of two chlorine atoms that are also held together by a covalent bond. The bond energy of Cl2 is 242 kJ/mol. The bond energy is lower than H2 because the two chlorine atoms have a larger difference in electronegativity, which means the electrons are not shared equally between the two atoms.
F2: Fluorine molecule also consists of two fluorine atoms that are held together by a covalent bond. The bond energy of F2 is 158 kJ/mol. The bond energy is the lowest among the three molecules because the two fluorine atoms have the largest difference in electronegativity, which means the electrons are strongly attracted to one of the atoms.
Conclusion
In summary, the bond energies of H2, F2, and Cl2 decrease in the order H2 > Cl2 > F2. This is because of the difference in electronegativity of the atoms. When the electronegativity difference is small, the bond energy is higher, and when the electronegativity difference is large, the bond energy is lower.
A simplified application of MO theory to the hypothetical ‘molecule’ OF would give its bond order as :- a)2
- b)1.5
- c)1.0
- d)0.5
Correct answer is option 'B'. Can you explain this answer?
A simplified application of MO theory to the hypothetical ‘molecule’ OF would give its bond order as :
a)
2
b)
1.5
c)
1.0
d)
0.5
|
|
Vivek Khatri answered |
In MO theory, bond order is used to indicate the stability of a bond. A higher bond order corresponds to a stronger, more stable bond. Bond order can be calculated as the difference between the number of electrons in bonding and antibonding orbitals, divided by 2.
For the hypothetical molecule OF, we can consider the atomic orbitals of oxygen (O) and fluorine (F). Oxygen has the electron configuration 1s2 2s2 2p4, while fluorine has the configuration 1s2 2s2 2p5. The valence electrons, which are the electrons in the outermost shell, are involved in bonding. Oxygen has 6 valence electrons, and fluorine has 7 valence electrons.
When these two atoms come together to form a bond, their atomic orbitals will combine to form molecular orbitals. In this case, the 2p orbitals of oxygen and fluorine will overlap. The 2p orbitals can combine to form two bonding orbitals (σ and π) and two antibonding orbitals (σ* and π*).
The 7 valence electrons from fluorine and the 6 valence electrons from oxygen will fill these molecular orbitals, starting with the lowest energy bonding orbitals and following Hund's rule and the Pauli Exclusion Principle. There will be a total of 13 electrons to distribute:
σ : 2 electrons
π : 4 electrons (2 electrons in each of the two degenerate π orbitals)
σ* : 2 electrons
π* : 5 electrons (3 electrons in one π* orbital, 2 electrons in the other)
Now we can calculate the bond order:
Bond order = (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) / 2
Bond order = (6 - 7) / 2
Bond order = -1 / 2
Bond order = 1.5
Thus, the bond order of the hypothetical molecule OF is 1.5 (option B).
For the hypothetical molecule OF, we can consider the atomic orbitals of oxygen (O) and fluorine (F). Oxygen has the electron configuration 1s2 2s2 2p4, while fluorine has the configuration 1s2 2s2 2p5. The valence electrons, which are the electrons in the outermost shell, are involved in bonding. Oxygen has 6 valence electrons, and fluorine has 7 valence electrons.
When these two atoms come together to form a bond, their atomic orbitals will combine to form molecular orbitals. In this case, the 2p orbitals of oxygen and fluorine will overlap. The 2p orbitals can combine to form two bonding orbitals (σ and π) and two antibonding orbitals (σ* and π*).
The 7 valence electrons from fluorine and the 6 valence electrons from oxygen will fill these molecular orbitals, starting with the lowest energy bonding orbitals and following Hund's rule and the Pauli Exclusion Principle. There will be a total of 13 electrons to distribute:
σ : 2 electrons
π : 4 electrons (2 electrons in each of the two degenerate π orbitals)
σ* : 2 electrons
π* : 5 electrons (3 electrons in one π* orbital, 2 electrons in the other)
Now we can calculate the bond order:
Bond order = (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) / 2
Bond order = (6 - 7) / 2
Bond order = -1 / 2
Bond order = 1.5
Thus, the bond order of the hypothetical molecule OF is 1.5 (option B).
Which of the following contains a covalent bond?- a)Mg3N2
- b)Li2O
- c)NaCl
- d)NO3
Correct answer is option 'D'. Can you explain this answer?
Which of the following contains a covalent bond?
a)
Mg3N2
b)
Li2O
c)
NaCl
d)
NO3
|
|
Pooja Choudhury answered |
- As we know that ionic bond is formed between an electropositive and an electronegative atom and a covalent bond is formed between two electronegative or electropositive elements.
- In all the above options NO3 the bonds are ionic in nature but NO3 is covalent in nature as bond is formed between two electronegative atoms i.e. N and O.
Hybridization in carbon atoms of 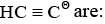
- a)sp and sp
- b)sp and sp3
- c)sp and sp2
- d)None
Correct answer is option 'A'. Can you explain this answer?
Hybridization in carbon atoms of 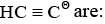
a)
sp and sp
b)
sp and sp3
c)
sp and sp2
d)
None

|
Rising Star answered |
In hybridization We calculate Sigma bond+no.of loan pair electron+no.of negative charge. in this question 1st carbon has two Sigma bond so hybridization is sp and 2nd carbon has one Sigma and one negative charge so hybridization is sp .
Hybridization state of boron and oxygen in boric acid is:- a)sp3, sp2
- b)sp2, sp3
- c)sp2, sp2
- d)sp3, sp3
Correct answer is option 'C'. Can you explain this answer?
Hybridization state of boron and oxygen in boric acid is:
a)
sp3, sp2
b)
sp2, sp3
c)
sp2, sp2
d)
sp3, sp3

|
Rahul Chatterjee answered |
The answer is b.
The central boron atom is binded to three hydroxyl groups. As boron has three electrons in the outermost shell and has to form three sigma bonds, it is sp2 hybridised. This means all the three bonds are in the same plane. Each oxygen atom is sp3 hybridised.
Which is correct order of bond angle:- a)CCl4 > BF3 > NO2+
- b)NH3 > NCl3 > NBr3
- c)Br2O > Cl2O > OF2
- d)PCl3 > PBr3 > PI3
Correct answer is option 'C'. Can you explain this answer?
Which is correct order of bond angle:
a)
CCl4 > BF3 > NO2+
b)
NH3 > NCl3 > NBr3
c)
Br2O > Cl2O > OF2
d)
PCl3 > PBr3 > PI3

|
Stuti Patel answered |
The correct order of bond angle is determined by the repulsion between the bonding pairs and lone pairs of electrons present in the molecule. The greater the repulsion, the larger the bond angle. Let's analyze the given options to determine the correct order of bond angle.
a) CCl4, BF3, NO2
- CCl4 has a tetrahedral geometry with bond angle of 109.5°.
- BF3 has a trigonal planar geometry with bond angle of 120°.
- NO2 has a bent geometry with bond angle of 115°.
Therefore, the correct order of bond angle for option a) is: BF3 > NO2 > CCl4.
b) NH3, NCl3, NBr3
- NH3 has a trigonal pyramidal geometry with bond angle of 107°.
- NCl3 has a trigonal pyramidal geometry with bond angle of 107°.
- NBr3 has a trigonal pyramidal geometry with bond angle of 102°.
Therefore, the correct order of bond angle for option b) is: NH3 = NCl3 > NBr3.
c) Br2O, Cl2O, OF2
- Br2O has a bent geometry with bond angle of 110°.
- Cl2O has a bent geometry with bond angle of 111°.
- OF2 has a bent geometry with bond angle of 103°.
Therefore, the correct order of bond angle for option c) is: Cl2O > Br2O > OF2.
d) PCl3, PBr3, PI3
- PCl3 has a trigonal pyramidal geometry with bond angle of 107°.
- PBr3 has a trigonal pyramidal geometry with bond angle of 107°.
- PI3 has a trigonal pyramidal geometry with bond angle of 102°.
Therefore, the correct order of bond angle for option d) is: PCl3 = PBr3 > PI3.
Hence, the correct order of bond angle among the given options is c) Cl2O > Br2O > OF2.
a) CCl4, BF3, NO2
- CCl4 has a tetrahedral geometry with bond angle of 109.5°.
- BF3 has a trigonal planar geometry with bond angle of 120°.
- NO2 has a bent geometry with bond angle of 115°.
Therefore, the correct order of bond angle for option a) is: BF3 > NO2 > CCl4.
b) NH3, NCl3, NBr3
- NH3 has a trigonal pyramidal geometry with bond angle of 107°.
- NCl3 has a trigonal pyramidal geometry with bond angle of 107°.
- NBr3 has a trigonal pyramidal geometry with bond angle of 102°.
Therefore, the correct order of bond angle for option b) is: NH3 = NCl3 > NBr3.
c) Br2O, Cl2O, OF2
- Br2O has a bent geometry with bond angle of 110°.
- Cl2O has a bent geometry with bond angle of 111°.
- OF2 has a bent geometry with bond angle of 103°.
Therefore, the correct order of bond angle for option c) is: Cl2O > Br2O > OF2.
d) PCl3, PBr3, PI3
- PCl3 has a trigonal pyramidal geometry with bond angle of 107°.
- PBr3 has a trigonal pyramidal geometry with bond angle of 107°.
- PI3 has a trigonal pyramidal geometry with bond angle of 102°.
Therefore, the correct order of bond angle for option d) is: PCl3 = PBr3 > PI3.
Hence, the correct order of bond angle among the given options is c) Cl2O > Br2O > OF2.
I3- ion is linear having the hybridization:- a)sp
- b)sp2
- c)sp3d
- d)sp3
Correct answer is option 'C'. Can you explain this answer?
I3- ion is linear having the hybridization:
a)
sp
b)
sp2
c)
sp3d
d)
sp3
|
|
Avinash Mehta answered |
I3^- has sp3d hybridisation as it has 3 lone pairs and 2 bond pairs. It is sp3d and three lone pairs of electrons occupy equatorial positions in TBP structure. The shape is almost linear.
If six lobes of one orbital and six lobes of another orbital are overlapped then the resultant bond is:- a)σ
- b)π
- c)
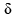
- d)
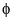
Correct answer is option 'D'. Can you explain this answer?
If six lobes of one orbital and six lobes of another orbital are overlapped then the resultant bond is:
a)
σ
b)
π
c)
d)
|
|
Pooja Choudhury answered |
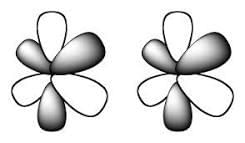
In chemistry, phi bonds (φ bonds) are covalent chemical bonds, where six lobes of one involved atomic orbital overlap six lobes of the other involved atomic orbital.
The experimental value of the dipole moment of HCI is 1.03 D. The length of the H-CI bond is 1.275 A. The percentage of ionic character in HCl is- a)43
- b)21
- c)17
- d)7
Correct answer is option 'C'. Can you explain this answer?
The experimental value of the dipole moment of HCI is 1.03 D. The length of the H-CI bond is 1.275 A. The percentage of ionic character in HCl is
a)
43
b)
21
c)
17
d)
7

|
Ishani Dasgupta answered |
Calculation of Percentage of Ionic Character in HCl
Experimental Value of Dipole Moment of HCl
The experimental value of the dipole moment of HCl is given as 1.03 D.
Length of H-Cl Bond
The length of the H-Cl bond is given as 1.275 A.
Calculation of Percentage of Ionic Character
The percentage of ionic character in HCl can be calculated using the following formula:
% Ionic Character = (1 - exp(-μ/(4.8 × d^2))) × 100
Where,
μ = dipole moment in Debye units
d = bond length in Angstroms
Plugging in the values, we get:
% Ionic Character = (1 - exp(-1.03/(4.8 × 1.275^2))) × 100
% Ionic Character = (1 - 0.335) × 100
% Ionic Character = 0.665 × 100
% Ionic Character = 66.5%
Therefore, the percentage of ionic character in HCl is 66.5%, which is closest to option C (17%). The correct answer is option C.
Experimental Value of Dipole Moment of HCl
The experimental value of the dipole moment of HCl is given as 1.03 D.
Length of H-Cl Bond
The length of the H-Cl bond is given as 1.275 A.
Calculation of Percentage of Ionic Character
The percentage of ionic character in HCl can be calculated using the following formula:
% Ionic Character = (1 - exp(-μ/(4.8 × d^2))) × 100
Where,
μ = dipole moment in Debye units
d = bond length in Angstroms
Plugging in the values, we get:
% Ionic Character = (1 - exp(-1.03/(4.8 × 1.275^2))) × 100
% Ionic Character = (1 - 0.335) × 100
% Ionic Character = 0.665 × 100
% Ionic Character = 66.5%
Therefore, the percentage of ionic character in HCl is 66.5%, which is closest to option C (17%). The correct answer is option C.
Which is not paramagnetic among the following?- a)B2
- b)C2
- c)O2
- d)None
Correct answer is option 'B'. Can you explain this answer?
Which is not paramagnetic among the following?
a)
B2
b)
C2
c)
O2
d)
None

|
Akash Kulkarni answered |
Explanation:
Paramagnetism is the property of the substance to get attracted towards the magnetic field. The substances which have unpaired electrons are attracted to the magnetic field. The substances which do not have unpaired electrons are diamagnetic, i.e., they are not attracted to the magnetic field.
Let's check the electronic configuration of the given molecules to identify which one is paramagnetic and which one is diamagnetic.
a) B2: The atomic number of boron is 5. The electronic configuration of B2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)1. Here, there is an unpaired electron in the π2px orbital. So, B2 is paramagnetic.
b) C2: The atomic number of carbon is 6. The electronic configuration of C2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)2. Here, all the electrons are paired. So, C2 is diamagnetic.
c) O2: The atomic number of oxygen is 8. The electronic configuration of O2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)2(π*2py)2(π*2px)1. Here, there are two unpaired electrons in the π*2py and π*2px orbitals. So, O2 is paramagnetic.
d) None: Since both B2 and O2 are paramagnetic, the answer is None.
Therefore, the correct answer is option 'B' which is C2 as it is diamagnetic and not paramagnetic.
Paramagnetism is the property of the substance to get attracted towards the magnetic field. The substances which have unpaired electrons are attracted to the magnetic field. The substances which do not have unpaired electrons are diamagnetic, i.e., they are not attracted to the magnetic field.
Let's check the electronic configuration of the given molecules to identify which one is paramagnetic and which one is diamagnetic.
a) B2: The atomic number of boron is 5. The electronic configuration of B2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)1. Here, there is an unpaired electron in the π2px orbital. So, B2 is paramagnetic.
b) C2: The atomic number of carbon is 6. The electronic configuration of C2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)2. Here, all the electrons are paired. So, C2 is diamagnetic.
c) O2: The atomic number of oxygen is 8. The electronic configuration of O2 is (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)2(π2px)2(π*2py)2(π*2px)1. Here, there are two unpaired electrons in the π*2py and π*2px orbitals. So, O2 is paramagnetic.
d) None: Since both B2 and O2 are paramagnetic, the answer is None.
Therefore, the correct answer is option 'B' which is C2 as it is diamagnetic and not paramagnetic.
Hybridization in Boron in BF3-ether mixture is:- a)sp2
- b)sp3
- c)sp
- d)sp3d
Correct answer is option 'A'. Can you explain this answer?
Hybridization in Boron in BF3-ether mixture is:
a)
sp2
b)
sp3
c)
sp
d)
sp3d

|
Dipanjan Sharma answered |
- BF3 molecule is formed by bonding between three sp2 orbitals of B and p of 3 F atoms.
The structure of XeF6 is :- a)pentagonal bipyramidal
- b)octahedral
- c)capped octahedral
- d)square pyramidal
Correct answer is option 'C'. Can you explain this answer?
The structure of XeF6 is :
a)
pentagonal bipyramidal
b)
octahedral
c)
capped octahedral
d)
square pyramidal

|
Asf Institute answered |
The structure of XeF6 (Xenon hexafluoride) is distorted octahedral (option C) because of its molecular geometry and the number of electron pairs surrounding the central atom.
XeF6 is formed when xenon (Xe) reacts with fluorine (F) to form a compound with six covalent bonds between the central xenon atom and six fluorine atoms. Xenon has 8 valence electrons, and it uses 6 of these electrons to form bonds with the 6 fluorine atoms. The remaining 2 valence electrons form a lone pair.
In the case of XeF6, the central xenon atom is surrounded by six bonding pairs of electrons and one lone pair of electrons. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs will arrange themselves to minimize repulsion, which results in a distorted octahedral molecular geometry.
In a capped octahedral structure, there are six fluorine atoms bonded to the central xenon atom. Five of these fluorine atoms are arranged in a square pyramid around the xenon atom, while the sixth fluorine atom is positioned above the central atom. The lone pair of electrons occupies the position opposite to the sixth fluorine atom, "distorting" the octahedral structure.
This unique molecular geometry of XeF6 distinguishes it from other geometries like pentagonal bipyramidal, octahedral, or square pyramidal.
XeF6 is formed when xenon (Xe) reacts with fluorine (F) to form a compound with six covalent bonds between the central xenon atom and six fluorine atoms. Xenon has 8 valence electrons, and it uses 6 of these electrons to form bonds with the 6 fluorine atoms. The remaining 2 valence electrons form a lone pair.
In the case of XeF6, the central xenon atom is surrounded by six bonding pairs of electrons and one lone pair of electrons. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs will arrange themselves to minimize repulsion, which results in a distorted octahedral molecular geometry.
In a capped octahedral structure, there are six fluorine atoms bonded to the central xenon atom. Five of these fluorine atoms are arranged in a square pyramid around the xenon atom, while the sixth fluorine atom is positioned above the central atom. The lone pair of electrons occupies the position opposite to the sixth fluorine atom, "distorting" the octahedral structure.
This unique molecular geometry of XeF6 distinguishes it from other geometries like pentagonal bipyramidal, octahedral, or square pyramidal.
In allene (C3H4), the type(s) of hybridisation of the carbon atoms is (are)- a)sp and sp3
- b)sp and sp2
- c)Only sp2
- d)sp2 and sp3
Correct answer is option 'B'. Can you explain this answer?
In allene (C3H4), the type(s) of hybridisation of the carbon atoms is (are)
a)
sp and sp3
b)
sp and sp2
c)
Only sp2
d)
sp2 and sp3
|
|
Rajesh Khatri answered |
S - P Hybridisation -
When one S - and one P - orbital belonging to the same main shell of an atom are mixed together to form two new SP - orbitals.
-wherein
shapes
Diagonal or Linear
sp2 Hybridisation -
When one s - and two p - orbitals of the same shell of an atom mix to form three new equivalent orbitals.The hybridised orbital is called sp2 orbital.
- wherein
Shape is Trigonal planar
Which one of the following molecules is planar?
- a)NF3
- b)NCl3
- c)PH3
- d)BH3
Correct answer is option 'D'. Can you explain this answer?
Which one of the following molecules is planar?
a)
NF3
b)
NCl3
c)
PH3
d)
BH3

|
Preethi Joshi answered |
Explanation:
Planarity in molecules:
In chemistry, a molecule is considered planar if all of its atoms lie in a single plane.
Analysis of the molecules:
Let's analyze each of the given molecules to determine their planarity.
NF3:
NF3 is a trigonal pyramidal molecule, where the N atom is bonded to three F atoms. Due to the lone pair on the N atom, the molecule is not planar.
NCl3:
NCl3 is also a trigonal pyramidal molecule similar to NF3, where the N atom is bonded to three Cl atoms. Just like NF3, the lone pair on the N atom causes it to be non-planar.
PH3:
PH3 is a trigonal pyramidal molecule where the P atom is bonded to three H atoms. The lone pair on the P atom makes it non-planar.
BH3:
BH3 is a trigonal planar molecule, where the B atom is bonded to three H atoms. The absence of a lone pair on the B atom allows all the atoms to lie in a single plane, making it planar.
Therefore, among the given molecules, only BH3 is planar, while NF3, NCl3, and PH3 are non-planar due to the presence of a lone pair on the central atom.
Planarity in molecules:
In chemistry, a molecule is considered planar if all of its atoms lie in a single plane.
Analysis of the molecules:
Let's analyze each of the given molecules to determine their planarity.
NF3:
NF3 is a trigonal pyramidal molecule, where the N atom is bonded to three F atoms. Due to the lone pair on the N atom, the molecule is not planar.
NCl3:
NCl3 is also a trigonal pyramidal molecule similar to NF3, where the N atom is bonded to three Cl atoms. Just like NF3, the lone pair on the N atom causes it to be non-planar.
PH3:
PH3 is a trigonal pyramidal molecule where the P atom is bonded to three H atoms. The lone pair on the P atom makes it non-planar.
BH3:
BH3 is a trigonal planar molecule, where the B atom is bonded to three H atoms. The absence of a lone pair on the B atom allows all the atoms to lie in a single plane, making it planar.
Therefore, among the given molecules, only BH3 is planar, while NF3, NCl3, and PH3 are non-planar due to the presence of a lone pair on the central atom.
Compound with maximum ionic character is formed fiom- a)Na and C
- b)Cs and F
- c)Cs and I
- d)Na and F
Correct answer is option 'B'. Can you explain this answer?
Compound with maximum ionic character is formed fiom
a)
Na and C
b)
Cs and F
c)
Cs and I
d)
Na and F
|
|
Sakib Mahamud answered |
According to fajan's rule smaller the cation and anion and greater the charge ..the compound will be more ionic. so it should be NaF.
The cationic part of solid Cl2O6 is having the “_______ ” shape- a)linear
- b)angular
- c)Tetrahedron
- d)undefined
Correct answer is option 'D'. Can you explain this answer?
The cationic part of solid Cl2O6 is having the “_______ ” shape
a)
linear
b)
angular
c)
Tetrahedron
d)
undefined

|
Anshika Chavan answered |
Oxidation state of +6. The cationic part of Cl2O6 is [ClO4]+.
What is the electronic configuration of carbon in it’s excited state?
- a)1s22s22p4
- b)1s22s12p3
- c)1s22s22p5
- d)1s22s12p4
Correct answer is option 'B'. Can you explain this answer?
What is the electronic configuration of carbon in it’s excited state?
a)
1s22s22p4
b)
1s22s12p3
c)
1s22s22p5
d)
1s22s12p4

|
Asf Institute answered |
Electronic configuration: Electronic configuration is the distribution of the electrons in orbitals of atoms using some basic principles like the Pauli exclusion principle and the Aufbau principle.
Ground state:
- The ground state is the state of lower energy occupied by an electron in an atomic orbital.
- The ground state is a highly stable state.
For Carbon:
- The atomic number of carbon is 6.
- The ground state electronic configuration of neutral carbon atom is 1s22s22p2
Excited state:
- The excited state is the state of higher energy occupied by an electron in an atomic orbital.
- The excited state is a less stable state.
- The excited-state electronic configuration of a carbon atom is 1s22s12p3 i.e, the one electron from the 2s orbital gets excited to the 2p orbital by absorbing some energy.
Stability increases, as the energy ___________- a)increases
- b)doesn’t change
- c)decreases
- d)increases and then decreases
Correct answer is option 'C'. Can you explain this answer?
Stability increases, as the energy ___________
a)
increases
b)
doesn’t change
c)
decreases
d)
increases and then decreases
|
|
Hema answered |
Stability is inversely proportional to energy.
A sigma bond may be formed by the overlap of 2 atomic orbitals of atoms A and B. If the bond is formed along the x-axis, which of the following overlaps is acceptable ?- a)s orbital of A and pz orbital of B
- b)px orbital of A and py orbital of B
- c)pz orbital of A and px orbital of B
- d)px orbital of A and s orbital of B
Correct answer is option 'D'. Can you explain this answer?
A sigma bond may be formed by the overlap of 2 atomic orbitals of atoms A and B. If the bond is formed along the x-axis, which of the following overlaps is acceptable ?
a)
s orbital of A and pz orbital of B
b)
px orbital of A and py orbital of B
c)
pz orbital of A and px orbital of B
d)
px orbital of A and s orbital of B

|
Partho Gupta answered |
Explanation:
A sigma bond is formed by the end-to-end overlap of two atomic orbitals. The overlap can occur along any axis, but in this case, it is along the x-axis.
Acceptable Overlap:
The only acceptable overlap for a sigma bond along the x-axis is between a p orbital of one atom and an s orbital of the other atom. Therefore, the correct option is D, which states the overlap between the px orbital of A and the s orbital of B.
Unacceptable Overlaps:
The other options are not acceptable because:
a) s orbital of A and pz orbital of B - overlap is not along x-axis
b) px orbital of A and py orbital of B - overlap is not between an s and a p orbital
c) pz orbital of A and px orbital of B - overlap is not between an s and a p orbital
Conclusion:
In conclusion, to form a sigma bond along the x-axis, there must be an overlap between an s orbital and a p orbital. Therefore, option D is the correct answer.
A sigma bond is formed by the end-to-end overlap of two atomic orbitals. The overlap can occur along any axis, but in this case, it is along the x-axis.
Acceptable Overlap:
The only acceptable overlap for a sigma bond along the x-axis is between a p orbital of one atom and an s orbital of the other atom. Therefore, the correct option is D, which states the overlap between the px orbital of A and the s orbital of B.
Unacceptable Overlaps:
The other options are not acceptable because:
a) s orbital of A and pz orbital of B - overlap is not along x-axis
b) px orbital of A and py orbital of B - overlap is not between an s and a p orbital
c) pz orbital of A and px orbital of B - overlap is not between an s and a p orbital
Conclusion:
In conclusion, to form a sigma bond along the x-axis, there must be an overlap between an s orbital and a p orbital. Therefore, option D is the correct answer.
In which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic? - a)N2 → N2+
- b) O2 → O2+
- c)O2 → O22-
- d)NO → NO+
Correct answer is option 'D'. Can you explain this answer?
In which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic?
a)
N2 → N2+
b)
O2 → O2+
c)
O2 → O22-
d)
NO → NO+

|
Ipsita Chopra answered |
Both Ionic and Covalent bond arise from the tendency of atoms to attain stable configuration of electrons.
Chapter doubts & questions for Chemical Bonding and Shapes of Compounds - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Bonding and Shapes of Compounds - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup