All questions of Thermodynamics for Chemistry Exam
Which of the following is true for the reaction: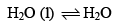 at 1000C at one atmosphere:
at 1000C at one atmosphere:- a)ΔE = 0
- b)ΔH = 0
- c)ΔH = ΔE
- d)ΔH = TΔS
Correct answer is option 'D'. Can you explain this answer?

|
Veda Institute answered |
For a system of constant composition, the pressure P is given by:- a)
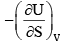
- b)
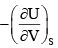
- c)
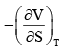
- d)
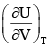
Correct answer is option 'D'. Can you explain this answer?
|
|
Dronacharya Institute answered |
Can you explain the answer of this question below:Heat product in calories by the combustion of one gram of carbon is called:
- A:
Heat of combustion of carbon.
- B:
Heat of formation of carbon.
- C:
Calorific Value of carbon.
- D:
Heat of product of carbon.
The answer is c.
Heat product in calories by the combustion of one gram of carbon is called:
Heat of combustion of carbon.
Heat of formation of carbon.
Calorific Value of carbon.
Heat of product of carbon.

|
Shivam Sharma answered |
Identify the correct statement regarding entropy:- a)At 00C, the entropy of a perfect ly crystalline substance is taken to be zero
- b)At absolute zero of the temperature, the entropy of all perfectly crystalline substance is positive
- c)At absolute zero of the temperature the entropy of all perfectly crystalline substance is taken to be zero
- d)At absolute zero temperature, the entropy of a perfectly crystalline substance is taken to be zero
Correct answer is option 'D'. Can you explain this answer?

|
Pranavi Mishra answered |
The values of ΔH for the combustion of ethene and ethyne are -341.1 and -310.0 K cal respectively. Which of the following is a better fuel:- a)C2H2
- b)C2H4
- c)Both a and b
- d)None of these
Correct answer is option 'A'. Can you explain this answer?

|
Anagha Bajaj answered |
Standard molar enthalpy of formation (∆Hf°) is the energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states under standard conditions of temperature and pressure (298 K and 1 atm).
The species which has a zero standard molar enthalpy of formation means that the energy change that occurs when one mole of the compound is formed from its constituent elements is zero. This can happen only if the compound is an element itself.
Answer Explanation
Among the given options, Cl2(g) is the species which has zero standard molar enthalpy of formation at 298 K.
When one mole of Cl2(g) is formed from its constituent elements Cl(g), the energy change involved is zero at 298 K. This is because Cl2(g) is an element itself and its formation from Cl(g) requires no energy input or release.
On the other hand, Br2(g), H2O(g), and CH4(g) are compounds and their formation from their constituent elements requires energy input or release. Hence, their standard molar enthalpy of formation is not zero.
Therefore, the correct answer is option 'B' (Cl2(g)).
dU for Isothermal Process for Vander Waals Gas and Ideal Gas respectively are:
- a)0 ,0
- b)
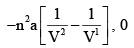
- c)
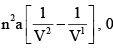
- d)
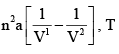
Correct answer is option 'B'. Can you explain this answer?

|
Pie Academy answered |
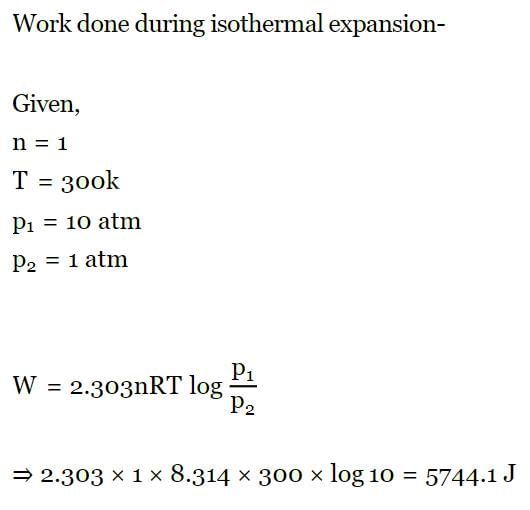
A piston-cylinder contains 0.5 kg of air at 500 kPa and 500 K. The air expands in a process so the pressure is linearly decreasing with volume to a final state of 100 kPa and 300 K. Find the work in the process.- a)56.1 kJ
- b)66.1 kJ
- c)76.1 kJ
- d)86.1 kJ
Correct answer is option 'D'. Can you explain this answer?

|
Edurev.iitjam answered |
For the isothermal expansion of an ideal gas:- a)E and H increases.
- b)E increases but H decreases.
- c)H increases and E decreases.
- d)E and H are unaltered.
Correct answer is 'D'. Can you explain this answer?
|
|
Aditya Deshmukh answered |
Can you explain the answer of this question below:At 270C one mole of an ideal gas is compressed isothermally and reversibly from a pressure of 2 atm to 10 atm. The value of ΔE and q are (R = 2):
- A:
0, -965.84 cal
- B:
-965.84 cal, -865.58 cal
- C:
865.58 cal, -865.58 cal
- D:
-865.58 cal, -865.58 cal
The answer is a.
At 270C one mole of an ideal gas is compressed isothermally and reversibly from a pressure of 2 atm to 10 atm. The value of ΔE and q are (R = 2):
0, -965.84 cal
-965.84 cal, -865.58 cal
865.58 cal, -865.58 cal
-865.58 cal, -865.58 cal

|
Pie Academy answered |
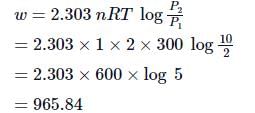
ΔE = Q + W,
The temperature of the system decreases in an:- a)Adiabatic Compression.
- b)Isothermal Compression.
- c)Isothermal Expansion.
- d)Adiabatic Expansion.
Correct answer is option 'D'. Can you explain this answer?
|
|
Aditya Deshmukh answered |
Heat of neutralization of strong acid and weak base is:- a)57.1 KJ mol–1
- b)13.7 KJ mol–1
- c)Less than 13.7 Kcal mol–1
- d)More than 13.7 Kcal mol–1
Correct answer is option 'C'. Can you explain this answer?

|
Jaya Sen answered |
When a strong acid and a weak base react together in a neutralization reaction, the heat of neutralization is less than 13.7 kcal/mol. This is because the weak base cannot completely neutralize the strong acid, resulting in the formation of a weakly acidic solution.
Factors Affecting Heat of Neutralization
The heat of neutralization depends on several factors, including:
1. Strength of Acid and Base: Strong acids and bases produce more heat of neutralization compared to weak acids and bases.
2. Concentration of Acid and Base: Higher concentrations of acid and base produce more heat of neutralization.
3. Temperature: The heat of neutralization increases with temperature.
4. Nature of Reaction: The heat of neutralization depends on the nature of the reaction, whether it is an exothermic or endothermic reaction.
Application of Heat of Neutralization
The heat of neutralization is an important concept in various fields, including:
1. Chemical Engineering: Heat of neutralization is used in the design of chemical reactors, which involve neutralization reactions.
2. Pharmaceutical Industry: Heat of neutralization is used in the design of drug formulations, which involve neutralization reactions.
3. Environmental Science: Heat of neutralization is used in the study of acid rain and its effects on the environment.
Conclusion
In conclusion, the heat of neutralization of a strong acid and weak base is less than 13.7 kcal/mol. This is due to the incomplete neutralization of the strong acid by the weak base, resulting in the formation of a weakly acidic solution. The heat of neutralization is an important concept in various fields and is affected by factors such as the strength and concentration of acid and base, temperature, and nature of the reaction.
Choose the correct criterion of spontaneity in terms of the properties of the system alone:- a)(dS)U, V > 0
- b)(dS)T. P > 0
- c)(dS)H, P < 0
- d)(dG)T, V = 0
Correct answer is option 'A'. Can you explain this answer?

|
Aditi Basak answered |
The spontaneity of a process is determined by the properties of the system. The correct criterion of spontaneity in terms of the properties of the system alone is given by:
(dS)U, V 0
Explanation:
Entropy (S) is a measure of the disorder or randomness of a system. The change in entropy (dS) is related to the heat (q) that flows into or out of the system during a process, as well as the temperature (T) of the system. The criterion of spontaneity can be expressed in terms of the change in entropy:
(dS) q/T
For a process to be spontaneous, the change in entropy (dS) must be positive. However, the sign of q depends on the conditions of the process, such as the temperature and pressure. Therefore, the criterion of spontaneity must be expressed in terms of system properties that do not depend on the conditions of the process.
One such property is the internal energy (U) of the system, which is the sum of the kinetic and potential energies of the particles in the system. The change in internal energy (dU) is related to the heat (q) that flows into or out of the system, as well as the work (w) done on or by the system:
dU q + w
For a closed system (i.e., one that does not exchange matter with its surroundings), the work done on or by the system can be expressed in terms of the volume (V) of the system:
w -PdV
where P is the pressure.
Using these equations, the change in entropy can be expressed in terms of the internal energy and volume of the system:
(dS)U, V (dU/T) (P/T)(dV/T) 0
This criterion of spontaneity states that a process is spontaneous if the change in entropy is positive for a given internal energy and volume of the system. It does not depend on the conditions of the process, such as the temperature and pressure.
Conclusion:
The correct criterion of spontaneity in terms of the properties of the system alone is option 'A': (dS)U, V 0. This criterion states that a process is spontaneous if the change in entropy is positive for a given internal energy and volume of the system. It does not depend on the temperature and pressure of the system.
Which of the following statement is correct: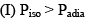
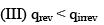
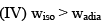
- a)I, II, & IV
- b)I, II, III, & IV
- c)II, & III
- d)I, III, & IV
Correct answer is option 'A'. Can you explain this answer?
|
|
Chirag Verma answered |
For which of the following reactions ΔH = ΔU?- a)N2(g) + 3H2(g) → 2NH3(g)
- b)PCl3(g) + Cl2(g) → PCl5(g)
- c)H2(g) + I2(g) → 2HI(g)
- d)2KClO3(s) → 2KCl(s) + 3O2(g)
Correct answer is option 'C'. Can you explain this answer?
|
|
Lavanya Menon answered |
Where Δng denotes the stoichiometric difference between the gaseous products and the gaseous reactants.
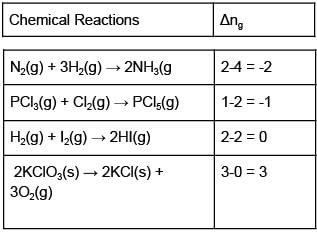
Hence for reaction c) ΔH = ΔU as Δng = 0.
ΔU is zero in
- a)Isothermal process
- b)Isochoric process
- c)Cyclic process
- d)All of these
Correct answer is option 'D'. Can you explain this answer?

|
Aditi Basak answered |
Among the following, the system require higher amount of thermal energy to giving the temperature 800C:- a)200 gm at 400C
- b)100 gm at 200C
- c)150 gm at 500C
- d)300 gm at 300C
Correct answer is option 'D'. Can you explain this answer?

|
Mrinalini Singh answered |
Q = m × c × ΔT
where Q is the amount of thermal energy in joules, m is the mass of the substance in grams, c is the specific heat capacity of the substance in joules per gram per degree Celsius, and ΔT is the temperature difference in degrees Celsius.
To determine which system requires the highest amount of thermal energy to reach 800C, we need to calculate the amount of thermal energy required for each option and compare them.
Calculations:
a) 200 gm at 400C
Q = 200 × 0.5 × (800 - 400) = 40,000 J
b) 100 gm at 200C
Q = 100 × 0.5 × (800 - 200) = 30,000 J
c) 150 gm at 500C
Q = 150 × 0.5 × (800 - 500) = 22,500 J
d) 300 gm at 300C
Q = 300 × 0.5 × (800 - 300) = 90,000 J
Comparing the results, we can see that option D requires the highest amount of thermal energy to reach 800C.
Conclusion:
Therefore, the correct answer is option D, 300 gm at 300C, requires the highest amount of thermal energy to reach 800C because it has the highest mass and the greatest temperature difference.
Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb. ΔU = Q + W c. ΔU = Q - W d. ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer?

|
Shivam Sharma answered |
Among the following, intensive property is:- a)Mass
- b)Volume
- c)Surface Tension
- d)Enthalpy
Correct answer is option 'C'. Can you explain this answer?

|
Yashvi Roy answered |
The heat required to raise the temperature of a body by 1K is called:- a)Specific Heat
- b)Thermal Capacity
- c)Water Equivalent
- d)None of these
Correct answer is option 'B'. Can you explain this answer?

|
Baishali Bajaj answered |
Which of the following is true for an adiabatic process:- a)ΔH = 0
- b)ΔW = 0
- c)ΔQ = 0
- d)ΔV = 0
Correct answer is option 'C'. Can you explain this answer?

|
Pranavi Mishra answered |
All the enthalpies of fusion are positive.- a)true
- b)false
- c)can not be determined
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?

|
Niharika Kulkarni answered |
The enthalpy of fusion, also known as the heat of fusion, is the amount of heat energy required to change a substance from a solid to a liquid state at its melting point. It is a thermodynamic property that is specific to each substance and is typically measured in joules per gram (J/g).
Positive enthalpies of fusion
The enthalpy of fusion is positive for all substances. This means that it requires an input of heat energy to break the intermolecular forces holding the solid together and convert it into a liquid. The positive sign indicates that the process is endothermic, meaning it absorbs heat from its surroundings.
Explanation
When a solid substance is heated, its temperature gradually increases until it reaches its melting point. At this point, the substance starts to change from a solid to a liquid, but its temperature remains constant until the entire solid has melted. This constant temperature is known as the melting point plateau.
During the melting process, the heat energy being supplied is used to overcome the attractive forces between the particles in the solid. These forces, such as ionic bonds, metallic bonds, or intermolecular forces, hold the particles together and give the solid its rigid structure.
To break these forces and convert the solid into a liquid, energy must be supplied. This energy is absorbed as heat, causing the temperature of the substance to remain constant during the melting process. The amount of heat energy required to completely melt a given amount of substance is the enthalpy of fusion.
Since energy is being absorbed from the surroundings, the enthalpy of fusion is positive. The positive sign indicates that the process is endothermic, meaning it requires an input of heat energy. The magnitude of the enthalpy of fusion depends on the strength of the intermolecular forces in the substance. Substances with stronger intermolecular forces generally have higher enthalpies of fusion.
Conclusion
In conclusion, all enthalpies of fusion are positive because the process of melting a solid requires an input of heat energy. The positive sign indicates that the process is endothermic and that energy is being absorbed from the surroundings.
The change in entropy when three moles of argon gas are heated at constant volume from 200 K to 300 K is:- a)15.16 JK–1mol–1
- b)– 15.16 JK–1mol–1
- c)– 5.05 JK–1mol–1
- d)5.05 JK–1mol–1
Correct answer is option 'D'. Can you explain this answer?

|
Niharika Kulkarni answered |
To calculate the change in entropy, we can use the formula:
ΔS = nCv ln(T2/T1)
where ΔS is the change in entropy, n is the number of moles, Cv is the molar heat capacity at constant volume, T1 is the initial temperature, and T2 is the final temperature.
Given, n = 3 moles, Cv = 12.5 J K-1 mol-1 for monoatomic gases, T1 = 200 K, and T2 = 300 K.
ΔS = 3 × 12.5 J K-1 mol-1 × ln(300 K/200 K)
ΔS = 5.05 J K-1 mol-1
Therefore, the correct answer is option 'D' (5.05 JK-1mol-1).
Heat product in calories by the combustion of one gram of carbon is called:
- a)Heat of combustion of carbon.
- b)Heat of formation of carbon.
- c)Calorific Value of carbon.
- d)Heat of product of carbon.
Correct answer is 'C'. Can you explain this answer?
Heat product in calories by the combustion of one gram of carbon is called:
|
|
Aditya Deshmukh answered |
Standard entropy of crystalline carbon monoxide (in KJ/mol) at 00K is around:- a)0.03
- b)2.50
- c)Zero
- d)5.76
Correct answer is option 'D'. Can you explain this answer?

|
Vandana Gupta answered |
Explanation:
- Entropy is a measure of randomness or disorder of a system. It is denoted by the letter "S" and has units of J/K or KJ/mol.
- Standard entropy (S°) is the entropy of a substance under standard conditions of temperature (298 K) and pressure (1 atm).
- Crystalline carbon monoxide is a solid form of CO where the molecules are arranged in a regular pattern or crystal lattice.
- The standard entropy of crystalline CO at 0K is 5.76 KJ/mol, which means that at absolute zero temperature, the disorder or randomness of the CO molecules in the crystal lattice is 5.76 KJ/mol.
- This value is determined experimentally and is based on the heat capacity and vibrational frequencies of the CO molecules in the crystal lattice.
- It is important to note that entropy increases with temperature, so the entropy of CO at higher temperatures would be greater than 5.76 KJ/mol.
In summary, the standard entropy of crystalline carbon monoxide at 0K is 5.76 KJ/mol, which represents the disorder or randomness of the CO molecules in the crystal lattice at absolute zero temperature.
The relationship between volume change in an isothermal process (Δ Vi) and an adiabatic process (Δ Va) for a pressure change from P1 to P2 is:- a)Δ Vi > Δ Va
- b)Δ Vi < Δ Va
- c)Δ Vi = Δ Va
- d)Δ Vi = Δ Va = 0
Correct answer is option 'A'. Can you explain this answer?

|
Siddharth Majumdar answered |
Isothermal Process (Vi)
- Isothermal process is a thermodynamic process that occurs at a constant temperature.
- In an isothermal process, the volume change is directly proportional to the pressure change.
- This relationship is described by the following equation: Vi = nRT/Pi, where n is the number of moles of gas, R is the gas constant, T is the temperature, and Pi is the initial pressure.
Adiabatic Process (Va)
- Adiabatic process is a thermodynamic process that occurs without any heat exchange with the surroundings.
- In an adiabatic process, the volume change is related to the pressure change by the following equation: Va = Vi(Pi/Pf)^(1/γ), where Pf is the final pressure, and γ is the ratio of specific heats.
Relationship Between Vi and Va for Pressure Change from P1 to P2
- When there is a pressure change from P1 to P2 in both isothermal and adiabatic processes, the initial volume Vi in the isothermal process is equal to the volume Va in the adiabatic process.
- This is because the pressure change is the same in both processes, and the initial temperature is also the same.
- Therefore, the relationship between volume change in an isothermal process (Vi) and an adiabatic process (Va) for a pressure change from P1 to P2 is Vi = Va.
The heat capacity of 10 mol of an ideal gas at a certain temperature is 300 JK–1 at constant pressure. The heat capacity of the same gas at the same temperature and at constant volume would be:- a)383 JK–1
- b)217 JK–1
- c)134 JK–1
- d)466 JK–1
Correct answer is option 'B'. Can you explain this answer?

|
Asf Institute answered |
For a spontaneous process, the correct statement is:- a)Entropy of the system always increases.
- b)Free energy of the system always increases.
- c)Total entropy change is always negative.
- d)Total entropy change is always positive.
Correct answer is option 'D'. Can you explain this answer?

|
Shivani Mehta answered |
Spontaneous processes are those processes which occur without any external intervention. These processes occur naturally and are irreversible.
The correct statement for a spontaneous process is:
Total entropy change is always positive.
Entropy:
Entropy is a measure of the degree of randomness or disorder in a system. It is denoted by the symbol "S".
The second law of thermodynamics states that the entropy of an isolated system always increases over time. This means that the degree of disorder of the system always increases with time.
Free energy:
Free energy is the energy available to do work in a system. It is denoted by the symbol "G".
For a spontaneous process, the free energy of the system always decreases. This means that the energy available to do work in the system decreases during a spontaneous process.
Total entropy change:
The total entropy change is the sum of the entropy changes of the system and its surroundings.
For a spontaneous process, the total entropy change is always positive. This means that the degree of disorder of the system and its surroundings always increases during a spontaneous process.
Conclusion:
The correct statement for a spontaneous process is that the total entropy change is always positive. This means that the degree of disorder of the system and its surroundings always increases during a spontaneous process.
In an isochoric process, the increased internal energy is:- a)Equal to the heat absorbed.
- b)Equal to heat evolved.
- c)Equal to the work done.
- d)Equal to sum of heat absorbed and work done.
Correct answer is option 'A'. Can you explain this answer?

|
Swara Dasgupta answered |
Heat of formation of H2O is –286 KJ per mole and H2O2 is –188 KJ mol–1. The enthalpy change for the reaction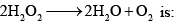
- a)- 196 KJ
- b)196 KJ
- c)984 KJ
- d)–984 KJ
Correct answer is option 'A'. Can you explain this answer?

|
Edurev.iitjam answered |
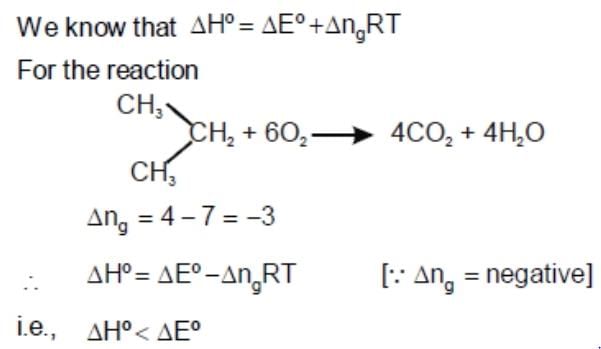
Consider the reaction: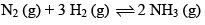 Carried out at constant temperature and pressure. If ΔH and ΔU are enthalpy and internal energy changes for the reaction, which of the following expressions is true?
Carried out at constant temperature and pressure. If ΔH and ΔU are enthalpy and internal energy changes for the reaction, which of the following expressions is true?- a)ΔH = 0
- b)ΔH = ΔU
- c)ΔH < ΔU
- d)ΔH > ΔU
Correct answer is option 'B'. Can you explain this answer?

|
Bijoy Kapoor answered |
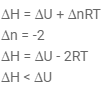
At 270C one mole of an ideal gas is compressed isothermally and reversibly from a pressure of 2 atm to 10 atm. The value of ΔE and q are (R = 2):- a)0, -965.84 cal
- b)-965.84 cal, -865.58 cal
- c)865.58 cal, -865.58 cal
- d)-865.58 cal, -865.58 cal
Correct answer is 'A'. Can you explain this answer?

|
Bhavana Dasgupta answered |
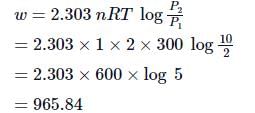
ΔE = Q + W,
The work done in ergs for the reversible expansion of one mole of an ideal gas from a volume of 10L to 20L is:
- a)
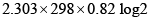 ergs
ergs
- b)
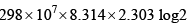 ergs
ergs
- c)
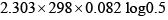 ergs
ergs
- d)
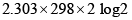 ergs
ergs
Correct answer is option 'B'. Can you explain this answer?
|
|
Dronacharya Institute answered |
Can you explain the answer of this question below:If enthalpies of formation C2H4 (g), CO2 (g) and H2O (l) at 2500C and 1 atm. pressure be 52, –394 and –286 KJ mol–1 respectively. The enthalpy of combustion of C2H4 (g) will be:
- A:
+ 1412 KJ mol–1
- B:
– 1412 KJ mol–1
- C:
+ 141.2 KJ mol–1
- D:
– 141.2 KJ mol–1
The answer is b.
If enthalpies of formation C2H4 (g), CO2 (g) and H2O (l) at 2500C and 1 atm. pressure be 52, –394 and –286 KJ mol–1 respectively. The enthalpy of combustion of C2H4 (g) will be:
+ 1412 KJ mol–1
– 1412 KJ mol–1
+ 141.2 KJ mol–1
– 141.2 KJ mol–1

|
Baishali Bajaj answered |
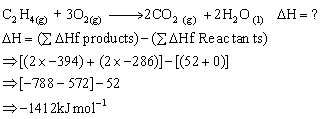
What is the internal pressure for 4 mole of Vander Waals gas:- a)Zero
- b)a/V2
- c)4a/V2
- d)16a/V2
Correct answer is option 'D'. Can you explain this answer?

|
Preethi Joshi answered |
Internal pressure of Vander Waals gas can be given by the following formula:
Pint = (nRT)/(V-nb) - a(n/V)^2
where,
Pint = Internal pressure
n = number of moles
R = Gas constant
T = Temperature
V = Volume
a = Vander Waals constant
b = Volume correction constant
Given,
n = 4 moles
We need to find the value of Pint.
To find the value of Pint, we need to know the values of Vander Waals constants 'a' and 'b'.
Vander Waals constant 'a' is a measure of the attractive forces between the gas molecules.
Vander Waals constant 'b' is a measure of the volume occupied by the gas molecules.
For a Vander Waals gas, the values of 'a' and 'b' can be calculated using the following equations:
a = (27/64) (R^2) (Tc^2) / Pc
b = (1/8) (RTc) / Pc
where,
Tc = Critical temperature
Pc = Critical pressure
The values of Tc and Pc for a gas can be found in tables or given in the problem.
Let's assume that the values of Tc and Pc are known and we have calculated the values of 'a' and 'b' for the gas.
Now, we can substitute the values of n, R, T, V, 'a', and 'b' in the formula for Pint and simplify to get the final answer.
Substituting the values, we get:
Pint = (4RT)/(V-4b) - 16a/V^2
We can see that the second term in the equation is proportional to 'a/V^2'. Therefore, the internal pressure of the Vander Waals gas is directly proportional to 'a/V^2'.
As the value of 'a' is a measure of the attractive forces between the gas molecules and 'V' is the volume occupied by the gas molecules, a larger value of 'a/V^2' indicates stronger attractive forces and hence a higher internal pressure.
Therefore, the correct option is D) 16a/V^2.
One mole of an ideal gas (CP = 29.234 JK–1mol–1) is expanded reversibly and adiabatically from 1dm3 to 10 dm3. The initial temperature is 750 K, the final temperature will be:- a)1000 K
- b)750 K
- c)300 K
- d)100 K
Correct answer is option 'C'. Can you explain this answer?
|
|
Biman Kaushik answered |
taking log and putting the values, i got 305K
and the ans is 300K.
The change in the entropy for the fusion of 1 mole of ice is:(Melting point of ice = 273 K, molar enthalpy of fusion of ice = 6.0 KJ mol–1):- a)11.73 JK–1mol–1
- b)18.84 JK–1mol–1
- c)21.97 JK–1mol–1
- d)24.47 JK–1mol–1
Correct answer is option 'C'. Can you explain this answer?

|
Priya Saha answered |
 =
= 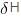 (Enthaply of fusion) /T (Temperature)
(Enthaply of fusion) /T (Temperature)Two moles of a monoatomic perfect gas initially 4.0 bars and 470C undergoes reversible expansion in an insulated container. The temperature at which the pressure reduces to 3.0 bar is:- a)200 K
- b)285 K
- c)310 K
- d)320 K
Correct answer is option 'B'. Can you explain this answer?

|
Bijoy Patel answered |
- Two moles of a monoatomic perfect gas
- Initial pressure (P1) = 4.0 bar
- Initial temperature (T1) = 470C
- Final pressure (P2) = 3.0 bar
- Process is reversible and adiabatic (insulated container)
To find: Final temperature (T2)
Formula used:
- For reversible adiabatic process, PV^γ = constant, where γ = Cp/Cv for a monoatomic gas is 5/3
- Also, PV = nRT (Ideal gas equation)
Solution:
1. Using the ideal gas equation, we can find the initial volume (V1) of the gas:
PV = nRT
V1 = nRT1/P1 = 2 x 8.314 x (470+273)/4.0 = 0.078 m3
2. Using the initial pressure and volume, we can find the initial value of PV^γ:
PV^γ = (4.0 bar x 0.078 m3)^5/3 = 1.10 x 10^6 bar3/K5/3
3. Since the process is reversible adiabatic, PV^γ remains constant:
P1V1^γ = P2V2^γ
4. Using the final pressure (P2) and the constant value of PV^γ, we can find the final volume (V2):
V2 = (P1V1^γ/P2)^(1/γ) = (1.10 x 10^6 bar3/K5/3 / 3.0 bar)^(1/5/3) = 0.105 m3
5. Using the final volume and the ideal gas equation, we can find the final temperature (T2):
PV = nRT
T2 = P2V2/nR = 3.0 bar x 0.105 m3 / 2 x 8.314 = 285 K
Therefore, the final temperature at which the pressure reduces to 3.0 bar is 285 K, which is option B.
Internal energy(ΔE) is equal to the heat supplied(q) in- a)Adiabatic change
- b)Isochoric change
- c)Isothermal reversible change
- d)Isothermal irreversible change
Correct answer is option 'B'. Can you explain this answer?

|
Asf Institute answered |
ince, ΔE = q + w
This implies, ΔE = q
The process in which no heat enters or leaves the system is termed as:- a)Isochoric
- b)Isobaric
- c)Isothermal
- d)Adiabatic
Correct answer is option 'D'. Can you explain this answer?

|
Hrishikesh Verma answered |
An adiabatic process is a thermodynamic process in which no heat enters or leaves the system. In other words, it is a process where there is no transfer of energy in the form of heat between the system and its surroundings. The term "adiabatic" comes from the Greek words "a" (meaning without) and "diabaino" (meaning to pass through).
Explanation:
During an adiabatic process, the system is insulated or isolated from its surroundings, preventing any heat exchange. This can be achieved by using a well-insulated container or by carrying out the process quickly enough that there is no time for heat transfer to occur.
Key Points:
- Adiabatic processes are characterized by a change in the internal energy of the system without any heat exchange.
- The first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system, can be simplified for adiabatic processes as: ΔU = -W, where ΔU is the change in internal energy and W is the work done.
- In an adiabatic expansion, the system does work on its surroundings, resulting in a decrease in internal energy.
- In an adiabatic compression, work is done on the system, increasing its internal energy.
- Adiabatic processes can occur in various systems, such as gases, liquids, and solids.
- Adiabatic processes are commonly encountered in areas such as thermodynamics, atmospheric science, and engineering.
Example:
One example of an adiabatic process is the compression or expansion of a gas in a cylinder with a piston. If the piston is moved quickly, there is no time for heat transfer to occur between the gas and its surroundings, and the process can be considered adiabatic.
In conclusion, an adiabatic process is one in which no heat enters or leaves the system. It is characterized by a change in internal energy without any heat exchange. Adiabatic processes are important in understanding the behavior of systems and are commonly encountered in various fields of science and engineering.
An open system allows the transfer of ________- a)only mass
- b)only energy
- c)both mass and energy
- d)neither mass nor energy
Correct answer is option 'C'. Can you explain this answer?

|
Anushka Basak answered |
- Open System: An open system allows the exchange of both matter and energy with its surroundings. This means that both mass and energy can flow in and out of the system freely. For example, an open beaker containing a solution that can be heated or cooled is an open system. The solution can evaporate, and new substances can be added or removed.
- Closed System: A closed system only allows the transfer of energy with its surroundings. In this case, matter cannot enter or leave the system. However, energy can still be exchanged in the form of heat or work. For instance, a closed flask containing a gas is a closed system. The gas molecules can move and collide, but no new substance can be added or removed.
- Isolated System: An isolated system does not allow the transfer of matter or energy with its surroundings. It is completely isolated and self-contained. In an isolated system, the total energy and mass remain constant. An example of an isolated system is a perfectly insulated thermos containing a hot liquid. No heat or matter can enter or leave the thermos.
- Open System Characteristics:
- Allows the transfer of both mass and energy.
- Mass and energy can freely flow in and out of the system.
- External substances can enter or leave the system.
- The system can exchange heat, work, and undergo chemical reactions with the surroundings.
- Examples include open beakers, living organisms, and natural ecosystems.
- Importance of Open Systems in Chemistry:
- Open systems allow for the study of chemical reactions in real-world scenarios where matter and energy exchange occurs.
- They provide a more realistic representation of chemical processes that occur in nature.
- Open systems allow scientists to understand the behavior of substances under various conditions and influences.
- The ability to exchange matter and energy with the surroundings makes open systems more versatile and applicable to real-world situations.
In an isochoric process, the increase in internal energy is- a)Equal to the heat absorbed.
- b)Equal to the heat evolved.
- c)Equal to the work done.
- d)Equal to the sum of the heat absorbed and work done.
Correct answer is option 'A'. Can you explain this answer?

|
Sarthak Chavan answered |
In an isochoric process, also known as an isovolumetric process, the volume of the system remains constant. This means that no work is done by or on the system due to a change in volume. In such a process, the increase in internal energy is equal to the heat absorbed by the system.
Explanation:
Definition of Internal Energy:
Internal energy is the sum of the kinetic and potential energies of all the particles in a system. It is a state function and depends only on the present state of the system, regardless of how the system reached that state.
Isochoric Process:
In an isochoric process, the volume of the system remains constant. This implies that the work done by or on the system (W) is zero, as work is defined as the product of force and displacement in the direction of the force. Since there is no change in volume, there is no displacement, and therefore, no work is done.
First Law of Thermodynamics:
According to the first law of thermodynamics, the change in internal energy (ΔU) of a system is equal to the heat (Q) absorbed by the system minus the work (W) done by the system.
ΔU = Q - W
In an isochoric process, as mentioned earlier, the work done (W) is zero. Therefore, the equation simplifies to:
ΔU = Q
Therefore, the change in internal energy (ΔU) of the system is equal to the heat (Q) absorbed by the system.
Answer:
In an isochoric process, the increase in internal energy is equal to the heat absorbed by the system. Therefore, option 'A' is the correct answer.
Summary:
In an isochoric process, the volume of the system remains constant, resulting in no work done. According to the first law of thermodynamics, the change in internal energy is equal to the heat absorbed by the system. Hence, in an isochoric process, the increase in internal energy is equal to the heat absorbed by the system.
The internal energy is the heat evolved or absorbed at- a)Constant pressure
- b)Constant volume
- c)Constant temperature
- d)Constant moles
Correct answer is option 'B'. Can you explain this answer?

|
Shubham Rane answered |
Internal energy is a fundamental concept in thermodynamics that refers to the total energy contained within a system. It includes the kinetic and potential energies of the particles that make up the system.
Heat
Heat is a form of energy transfer that occurs due to a temperature difference between two objects or systems. It flows from a region of higher temperature to a region of lower temperature until thermal equilibrium is reached.
Constant Volume
In a constant volume process, the volume of the system remains constant throughout. This means that no work is done by or on the system, and any change in energy is solely due to heat transfer.
Constant Pressure
In a constant pressure process, the pressure of the system remains constant throughout. This allows work to be done by or on the system, in addition to heat transfer.
Constant Temperature
In a constant temperature process, the temperature of the system remains constant throughout. This means that the average kinetic energy of the particles remains constant, and any change in energy is solely due to heat transfer.
Constant Moles
In a constant moles process, the number of moles of a substance in the system remains constant throughout. This means that the composition of the system does not change, and any change in energy is solely due to heat transfer.
Heat Evolved or Absorbed
The question asks whether the internal energy is the heat evolved or absorbed. In a constant volume process, the volume remains constant, so no work is done. Therefore, any change in energy is solely due to heat transfer. This means that the internal energy is equal to the heat evolved or absorbed.
Conclusion
The correct answer is option 'B', constant volume. In a constant volume process, the internal energy is equal to the heat evolved or absorbed.
The temperature of an object increases slowly, then the energy of that object _________- a)increases slowly
- b)decreases quickly
- c)increases quickly
- d)decreases slowly
Correct answer is option 'A'. Can you explain this answer?
|
|
Vivek Khatri answered |
Which of the following property cannot be used to describe the state of a system?- a)pressure
- b)volume
- c)temperature
- d)universal gas constant
Correct answer is option 'D'. Can you explain this answer?
|
|
Vivek Khatri answered |
Chapter doubts & questions for Thermodynamics - Physical Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Physical Chemistry
90 videos|144 docs|67 tests
|

Contact Support
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|