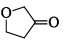All Exams >
Chemistry >
Organic Chemistry >
All Questions
All questions of Qualitative Organic Analysis and Spectroscopic Techniques for Chemistry Exam
Proton decoupled 13C NMR spectrum of a bicyclooctane (C8H14) exhibits only two signals. The structure of the compound is:- a)
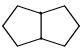
- b)
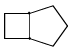
- c)
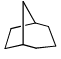
- d)
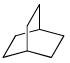
Correct answer is option 'A'. Can you explain this answer?
Proton decoupled 13C NMR spectrum of a bicyclooctane (C8H14) exhibits only two signals. The structure of the compound is:
a)
b)
c)
d)

|
Asf Institute answered |
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
- a)1:1
- b)1:2
- c)2:1
- d)6:1
Correct answer is option 'B'. Can you explain this answer?
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
a)
1:1
b)
1:2
c)
2:1
d)
6:1
|
|
Vikram Kapoor answered |
The signal intensity depends on number of equivalent hydrogen and acetonitrile have 3 hydrogen and benzene have six hydrogen.
Therefore the ratio is 1 : 2
N2 does not show pure vibrat ional spectral because:- a)Triple bond in N2 is very strong
- b)The dipole moment of N2 is zero
- c)Both a and b
- d)None of the above.
Correct answer is option 'B'. Can you explain this answer?
N2 does not show pure vibrat ional spectral because:
a)
Triple bond in N2 is very strong
b)
The dipole moment of N2 is zero
c)
Both a and b
d)
None of the above.

|
Akash Kulkarni answered |
Introduction:
The question asks why N2 does not show pure vibrational spectra. In order to answer this question, we need to understand the concept of vibrational spectra and the properties of N2.
Vibrational Spectra:
Vibrational spectra refers to the study of molecular vibrations, which involve the periodic motion of atoms within a molecule. When a molecule vibrates, it undergoes changes in bond lengths and bond angles, resulting in absorption or emission of infrared radiation. The vibrational spectra of a molecule can provide information about its structure and bonding.
Properties of N2:
N2 is a diatomic molecule consisting of two nitrogen atoms. It is held together by a triple bond, which is very strong due to the overlap of atomic orbitals. N2 is a symmetric molecule, meaning that the nitrogen atoms are identical and the molecule has a center of symmetry. As a result, N2 has a zero dipole moment.
Explanation:
The correct answer is option 'B' - the dipole moment of N2 is zero. This is because pure vibrational spectra are observed when a molecule has a dipole moment. A dipole moment occurs when there is an asymmetric distribution of charge in a molecule, resulting in a separation of positive and negative charges.
In the case of N2, the molecule is symmetric, and the nitrogen atoms are identical. As a result, the distribution of charge is symmetrical, and there is no separation of positive and negative charges. Therefore, N2 does not have a dipole moment.
Why is dipole moment important for vibrational spectra?
In vibrational spectroscopy, the electric field of the incident radiation interacts with the dipole moment of the molecule. This interaction leads to changes in the vibrational energy levels of the molecule, which can be observed as absorption or emission of infrared radiation.
If a molecule has a dipole moment, it can undergo a change in dipole moment during vibrational motion, resulting in a change in its electric field interaction with the incident radiation. This change in dipole moment leads to the absorption or emission of infrared radiation, which gives rise to vibrational spectra.
Conclusion:
In conclusion, N2 does not show pure vibrational spectra because it does not have a dipole moment. The symmetric nature of N2, along with the identical nitrogen atoms, leads to a symmetrical distribution of charge and a zero dipole moment. Therefore, N2 does not interact strongly with the electric field of incident radiation, resulting in the absence of pure vibrational spectra.
The question asks why N2 does not show pure vibrational spectra. In order to answer this question, we need to understand the concept of vibrational spectra and the properties of N2.
Vibrational Spectra:
Vibrational spectra refers to the study of molecular vibrations, which involve the periodic motion of atoms within a molecule. When a molecule vibrates, it undergoes changes in bond lengths and bond angles, resulting in absorption or emission of infrared radiation. The vibrational spectra of a molecule can provide information about its structure and bonding.
Properties of N2:
N2 is a diatomic molecule consisting of two nitrogen atoms. It is held together by a triple bond, which is very strong due to the overlap of atomic orbitals. N2 is a symmetric molecule, meaning that the nitrogen atoms are identical and the molecule has a center of symmetry. As a result, N2 has a zero dipole moment.
Explanation:
The correct answer is option 'B' - the dipole moment of N2 is zero. This is because pure vibrational spectra are observed when a molecule has a dipole moment. A dipole moment occurs when there is an asymmetric distribution of charge in a molecule, resulting in a separation of positive and negative charges.
In the case of N2, the molecule is symmetric, and the nitrogen atoms are identical. As a result, the distribution of charge is symmetrical, and there is no separation of positive and negative charges. Therefore, N2 does not have a dipole moment.
Why is dipole moment important for vibrational spectra?
In vibrational spectroscopy, the electric field of the incident radiation interacts with the dipole moment of the molecule. This interaction leads to changes in the vibrational energy levels of the molecule, which can be observed as absorption or emission of infrared radiation.
If a molecule has a dipole moment, it can undergo a change in dipole moment during vibrational motion, resulting in a change in its electric field interaction with the incident radiation. This change in dipole moment leads to the absorption or emission of infrared radiation, which gives rise to vibrational spectra.
Conclusion:
In conclusion, N2 does not show pure vibrational spectra because it does not have a dipole moment. The symmetric nature of N2, along with the identical nitrogen atoms, leads to a symmetrical distribution of charge and a zero dipole moment. Therefore, N2 does not interact strongly with the electric field of incident radiation, resulting in the absence of pure vibrational spectra.
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
- a)π-π* transition
- b)σ-σ* transition
- c)n-π* transition
- d)π-σ* transition
Correct answer is option 'C'. Can you explain this answer?
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
a)
π-π* transition
b)
σ-σ* transition
c)
n-π* transition
d)
π-σ* transition

|
Edurev.iitjam answered |
The type of transition is responsible for a band at λmax = 279 nm in absorption spectrum of acetone. In case of n → π* transitions, the polar solvents form hydrogen bonds with the ground state of polar molecules more readily than with their excited states. Therefore, in polar solvents the energies of electronic transitions are increased.
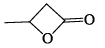
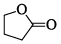
What will be the strength of coupling between geminal protons in the following molecules?
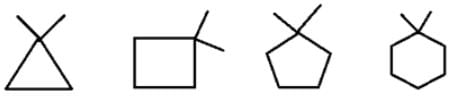
- a)Decrease as the size of ring increase
- b)Increase as the size of ring increase
- c)Remains same
- d)No relation between the size of the ring & coupling
Correct answer is option 'B'. Can you explain this answer?
What will be the strength of coupling between geminal protons in the following molecules?


a)
Decrease as the size of ring increase
b)
Increase as the size of ring increase
c)
Remains same
d)
No relation between the size of the ring & coupling

|
Asf Institute answered |
Geminal proton 2J coupling i.e. coupling of H & H on same carbon.
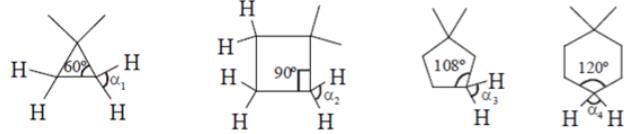
as ring size increases, bond angle outside decreases i.e. α1 > α2 > α3 > α4 (according % s character). If B.A. decreases, 2J coupling increases.

as ring size increases, bond angle outside decreases i.e. α1 > α2 > α3 > α4 (according % s character). If B.A. decreases, 2J coupling increases.
The compound which shows IR frequencies at both 3314 and 2126 cm–1 is:- a)CH3(CH2)4 CH2SH
- b)CH3(CH2)4 CH2C ≡ N
- c)CH3(CH2)4 CH2C ≡ C — H
- d)CH3(CH2)2 C ≡ C(CH2)2 CH3
Correct answer is option 'C'. Can you explain this answer?
The compound which shows IR frequencies at both 3314 and 2126 cm–1 is:
a)
CH3(CH2)4 CH2SH
b)
CH3(CH2)4 CH2C ≡ N
c)
CH3(CH2)4 CH2C ≡ C — H
d)
CH3(CH2)2 C ≡ C(CH2)2 CH3

|
Lekshmi Deshpande answered |
The compound which shows IR frequencies at both 3314 and 2126 cm-1 could be a nitrile compound, specifically benzonitrile (C6H5CN). The 3314 cm-1 peak corresponds to the stretching vibration of the C-H bond, while the 2126 cm-1 peak corresponds to the stretching vibration of the C≡N bond.
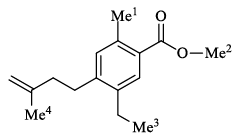
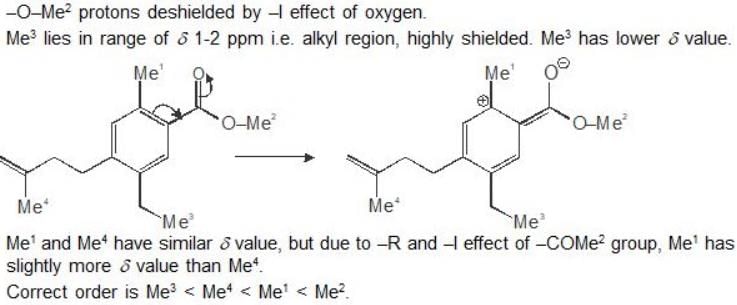
An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?- a)
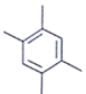
- b)
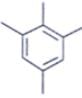
- c)
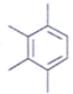
- d)
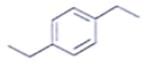
Correct answer is option 'A'. Can you explain this answer?
An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?
a)

b)

c)

d)


|
Asf Institute answered |
In compound a;
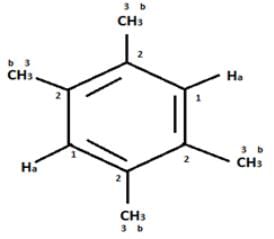
Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.

Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.
The number of IR active vibrational modes in ammonia is:- a)6
- b)4
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
The number of IR active vibrational modes in ammonia is:
a)
6
b)
4
c)
2
d)
3

|
Aryan Choudhary answered |
**Explanation:**
Ammonia (NH3) is a molecule composed of one nitrogen atom and three hydrogen atoms. In order to determine the number of IR active vibrational modes in ammonia, we need to consider its molecular symmetry and the number of atoms present.
**Molecular Symmetry:**
Ammonia belongs to the point group C3v, which has a threefold rotation axis (C3) passing through the nitrogen atom and a vertical mirror plane (σv) containing the nitrogen and hydrogen atoms.
**Vibrational Modes:**
Vibrational modes can be classified based on their symmetry properties. In general, there are three types of vibrational modes:
1. Stretching modes: These modes involve the stretching of bonds and can be further classified into symmetric stretching (ν1), asymmetric stretching (ν2), and degenerate stretching (ν3).
2. Bending modes: These modes involve the bending of bonds and can be further classified into in-plane bending (ν4) and out-of-plane bending (ν5).
3. Torsional modes: These modes involve the twisting or rotation around a bond.
**Application of Group Theory:**
Group theory can be used to determine the number of IR active vibrational modes in a molecule. In the case of ammonia, the C3v point group has three irreducible representations: A1, A2, and E.
**Application of Selection Rules:**
Selection rules help us determine which vibrational modes are IR active. In general, for IR active modes, the change in dipole moment (∆µ) should be non-zero during the vibration.
For ammonia, the selection rules are as follows:
- A1 modes: ∆µ = 0
- A2 modes: ∆µ = 0
- E modes: ∆µ ≠ 0
**Determination of IR Active Modes:**
By applying the selection rules, we find that the A1 and A2 modes are not IR active since they have ∆µ = 0. However, the E modes have ∆µ ≠ 0, indicating that they are IR active.
In ammonia, there are three E modes: ν2 (in-plane bending), ν3 (degenerate stretching), and ν4 (out-of-plane bending). Therefore, the number of IR active vibrational modes in ammonia is 3.
Hence, the correct answer is option A (6 is not the correct answer).
Ammonia (NH3) is a molecule composed of one nitrogen atom and three hydrogen atoms. In order to determine the number of IR active vibrational modes in ammonia, we need to consider its molecular symmetry and the number of atoms present.
**Molecular Symmetry:**
Ammonia belongs to the point group C3v, which has a threefold rotation axis (C3) passing through the nitrogen atom and a vertical mirror plane (σv) containing the nitrogen and hydrogen atoms.
**Vibrational Modes:**
Vibrational modes can be classified based on their symmetry properties. In general, there are three types of vibrational modes:
1. Stretching modes: These modes involve the stretching of bonds and can be further classified into symmetric stretching (ν1), asymmetric stretching (ν2), and degenerate stretching (ν3).
2. Bending modes: These modes involve the bending of bonds and can be further classified into in-plane bending (ν4) and out-of-plane bending (ν5).
3. Torsional modes: These modes involve the twisting or rotation around a bond.
**Application of Group Theory:**
Group theory can be used to determine the number of IR active vibrational modes in a molecule. In the case of ammonia, the C3v point group has three irreducible representations: A1, A2, and E.
**Application of Selection Rules:**
Selection rules help us determine which vibrational modes are IR active. In general, for IR active modes, the change in dipole moment (∆µ) should be non-zero during the vibration.
For ammonia, the selection rules are as follows:
- A1 modes: ∆µ = 0
- A2 modes: ∆µ = 0
- E modes: ∆µ ≠ 0
**Determination of IR Active Modes:**
By applying the selection rules, we find that the A1 and A2 modes are not IR active since they have ∆µ = 0. However, the E modes have ∆µ ≠ 0, indicating that they are IR active.
In ammonia, there are three E modes: ν2 (in-plane bending), ν3 (degenerate stretching), and ν4 (out-of-plane bending). Therefore, the number of IR active vibrational modes in ammonia is 3.
Hence, the correct answer is option A (6 is not the correct answer).
What are the number of signals in 1H NMR in the given molecules?
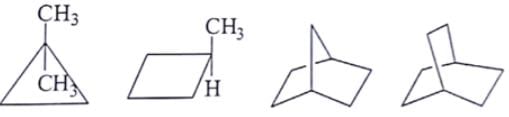
- a)3, 4, 4, 3 respectively
- b)2, 6, 4, 2 respectively
- c)2, 4, 6, 2 respectively
- d)2, 4, 2, 6 respectively
Correct answer is option 'B'. Can you explain this answer?
What are the number of signals in 1H NMR in the given molecules?


a)
3, 4, 4, 3 respectively
b)
2, 6, 4, 2 respectively
c)
2, 4, 6, 2 respectively
d)
2, 4, 2, 6 respectively

|
Pioneer Academy answered |
Different types of proton give the different type of signals, so the different hydrogen will give different numbers of signals.
As we can see first compound has two type of hydrogen Ha and Hb. Compound second has six type of hydrogen Ha, Hb, Hc, Hd, He and H. Compound third has four type of hydrogen Ha, Hb, Hc, Hd and l forth compound has two type of hydrogen Ha and Hb.
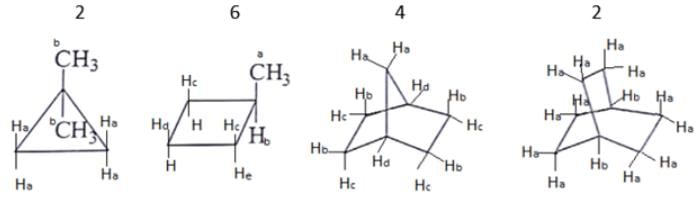
As we can see first compound has two type of hydrogen Ha and Hb. Compound second has six type of hydrogen Ha, Hb, Hc, Hd, He and H. Compound third has four type of hydrogen Ha, Hb, Hc, Hd and l forth compound has two type of hydrogen Ha and Hb.

Of the mo lecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiation are:- a)CH4, CO2, benzene
- b)CH4, benzene and H2
- c)CO2, benzene and H2
- d)CH4, CO2 and H2
Correct answer is option 'A'. Can you explain this answer?
Of the mo lecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiation are:
a)
CH4, CO2, benzene
b)
CH4, benzene and H2
c)
CO2, benzene and H2
d)
CH4, CO2 and H2
|
|
Anjali Bansal answered |
In H2 there is no mode of vibration in which change of dipole moment take place,while other consists change in dipole moment take place in mode of vibration. so answer is a
The bond that gives the most intense band in the infrared spectrum for its stretching vibrat ions is:- a)C—H
- b)N—H
- c)O—H
- d)S—H
Correct answer is option 'C'. Can you explain this answer?
The bond that gives the most intense band in the infrared spectrum for its stretching vibrat ions is:
a)
C—H
b)
N—H
c)
O—H
d)
S—H
|
|
Pooja Choudhury answered |
Approximate Infrared Stretching Frequencies:
- C–H - 3000 cm-1
- N–H - 3600 cm-1
- O–H - 3600 cm-1
- S–H - 2570 cm-1
The compound that exhibits sharp bands at 3300 and 2150 cm–1 in the IR spectrum is:- a)1-butyne
- b)2-butyne
- c)Butyronitrile
- d)Butylamine
Correct answer is option 'A'. Can you explain this answer?
The compound that exhibits sharp bands at 3300 and 2150 cm–1 in the IR spectrum is:
a)
1-butyne
b)
2-butyne
c)
Butyronitrile
d)
Butylamine

|
Mrinalini Singh answered |
Without additional information about the compound, it is impossible to determine its identity based solely on the given spectral data. However, the sharp bands at 3300 and 2150 cm^-1 suggest the presence of functional groups such as N-H or O-H (3300 cm^-1) and C≡C or C≡N (2150 cm^-1), respectively. Other spectral features and chemical tests would be needed to confirm the identity of the compound.
A molecule, AX, has a vibrational energy of 1000 cm–1 and rotational energy of 10 cm–1. Another molecule, BX, has a vibrational energy of 400 cm–1 and rotational energy of 40 cm–1. Which one of the following statements about the coupling of vibrational and rotational motion is true:- a)The coupling is stronger in BX.
- b)The coupling is stronger in AX.
- c)Magnitude of coupling is same in both AX and BX.
- d)There is no coupling in both AX and BX
Correct answer is option 'A'. Can you explain this answer?
A molecule, AX, has a vibrational energy of 1000 cm–1 and rotational energy of 10 cm–1. Another molecule, BX, has a vibrational energy of 400 cm–1 and rotational energy of 40 cm–1. Which one of the following statements about the coupling of vibrational and rotational motion is true:
a)
The coupling is stronger in BX.
b)
The coupling is stronger in AX.
c)
Magnitude of coupling is same in both AX and BX.
d)
There is no coupling in both AX and BX
|
|
Pooja Choudhury answered |
More the rotational energy, more will be the coupling.
Therefore, The coupling is stronger in BX.
Consider a 1H NMR spectrum in which a quartet and a doublet appeared at 9.72 and 2.40 ppm, respectively. Which of the following compounds is the most probable one:- a)CH3COCH3
- b)CH3CH2CHO
- c)CH3CHO
- d)CH3CH2CH2OH
Correct answer is option 'C'. Can you explain this answer?
Consider a 1H NMR spectrum in which a quartet and a doublet appeared at 9.72 and 2.40 ppm, respectively. Which of the following compounds is the most probable one:
a)
CH3COCH3
b)
CH3CH2CHO
c)
CH3CHO
d)
CH3CH2CH2OH

|
Ishaan Sengupta answered |
Explanation:
The quartet at 9.72 ppm indicates the presence of a group of 3 equivalent protons, which are coupled to a proton in a neighboring carbon atom. The coupling constant, J, can be determined using the formula:
J = Δν / (n + 1)
where Δν is the distance between the peaks in the quartet (in Hz) and n is the number of neighboring protons (in this case, n = 1).
J = (9.72 - 9.60) / (1 + 1) = 0.06 Hz
The doublet at 2.40 ppm indicates the presence of a group of 3 equivalent protons, which are not coupled to any neighboring protons.
Reasoning:
Based on the NMR data, the most probable compound is CH3CHO (acetaldehyde). This is because the quartet at 9.72 ppm corresponds to the protons on the aldehyde carbon, which is directly attached to the methyl group. The doublet at 2.40 ppm corresponds to the methyl group itself. The chemical shift of the aldehyde carbon is consistent with that of other aldehydes, and the absence of a coupling between the aldehyde protons and any neighboring protons indicates that they are not in close proximity to any other protons in the molecule. This is consistent with the structure of acetaldehyde. The other compounds listed (acetone, propanal, and 1-propanol) do not have the same NMR data as CH3CHO and can be eliminated as possibilities.
The quartet at 9.72 ppm indicates the presence of a group of 3 equivalent protons, which are coupled to a proton in a neighboring carbon atom. The coupling constant, J, can be determined using the formula:
J = Δν / (n + 1)
where Δν is the distance between the peaks in the quartet (in Hz) and n is the number of neighboring protons (in this case, n = 1).
J = (9.72 - 9.60) / (1 + 1) = 0.06 Hz
The doublet at 2.40 ppm indicates the presence of a group of 3 equivalent protons, which are not coupled to any neighboring protons.
Reasoning:
Based on the NMR data, the most probable compound is CH3CHO (acetaldehyde). This is because the quartet at 9.72 ppm corresponds to the protons on the aldehyde carbon, which is directly attached to the methyl group. The doublet at 2.40 ppm corresponds to the methyl group itself. The chemical shift of the aldehyde carbon is consistent with that of other aldehydes, and the absence of a coupling between the aldehyde protons and any neighboring protons indicates that they are not in close proximity to any other protons in the molecule. This is consistent with the structure of acetaldehyde. The other compounds listed (acetone, propanal, and 1-propanol) do not have the same NMR data as CH3CHO and can be eliminated as possibilities.
The number of normal modes of vibration in the benzene molecule is:- a)6
- b)30
- c)12
- d)36
Correct answer is option 'B'. Can you explain this answer?
The number of normal modes of vibration in the benzene molecule is:
a)
6
b)
30
c)
12
d)
36

|
Raksha Pillai answered |
Normal Modes of Vibration in Benzene Molecule:
Benzene is a planar molecule with six carbon atoms and six hydrogen atoms. The carbon atoms are arranged in a hexagonal ring with alternating double bonds. The normal modes of vibration in the benzene molecule can be determined using group theory and the concept of symmetry.
1. Symmetry Elements of Benzene Molecule:
The benzene molecule has several symmetry elements, including:
- C6 rotation axis: A six-fold rotation axis passing through the center of the ring
- C2 rotation axis: A two-fold rotation axis perpendicular to the plane of the ring
- Six C2 axes of symmetry: Each bisects the angle between two adjacent C-C bonds
- Mirror planes: Perpendicular to the C6 axis and bisecting the C-C bonds
2. Character Tables and Irreducible Representations:
Using group theory, we can construct a character table for the benzene molecule based on its symmetry elements. The character table lists the symmetry operations and their corresponding irreducible representations.
The irreducible representations are labeled with Greek letters, such as Γ, Σ, and Π. Each mode of vibration in the molecule corresponds to an irreducible representation.
3. Normal Modes of Vibration:
The benzene molecule has 30 normal modes of vibration, which can be classified into the following irreducible representations:
- 6 A1 modes: In-phase stretching of all C-C bonds
- 6 A2 modes: Out-of-phase stretching of adjacent C-C bonds
- 12 E modes: Combination of in-phase and out-of-phase stretching of C-C bonds and bending of C-H bonds
- 6 B2 modes: In-phase bending of the ring
Each normal mode of vibration has a characteristic frequency, which can be measured experimentally using techniques such as infrared spectroscopy or Raman spectroscopy.
Conclusion:
In summary, the benzene molecule has 30 normal modes of vibration, which can be determined using group theory and the concept of symmetry. These modes correspond to different combinations of in-phase and out-of-phase stretching and bending of the C-C and C-H bonds in the molecule. The normal modes of vibration can be used to study the structural and electronic properties of the molecule.
Benzene is a planar molecule with six carbon atoms and six hydrogen atoms. The carbon atoms are arranged in a hexagonal ring with alternating double bonds. The normal modes of vibration in the benzene molecule can be determined using group theory and the concept of symmetry.
1. Symmetry Elements of Benzene Molecule:
The benzene molecule has several symmetry elements, including:
- C6 rotation axis: A six-fold rotation axis passing through the center of the ring
- C2 rotation axis: A two-fold rotation axis perpendicular to the plane of the ring
- Six C2 axes of symmetry: Each bisects the angle between two adjacent C-C bonds
- Mirror planes: Perpendicular to the C6 axis and bisecting the C-C bonds
2. Character Tables and Irreducible Representations:
Using group theory, we can construct a character table for the benzene molecule based on its symmetry elements. The character table lists the symmetry operations and their corresponding irreducible representations.
The irreducible representations are labeled with Greek letters, such as Γ, Σ, and Π. Each mode of vibration in the molecule corresponds to an irreducible representation.
3. Normal Modes of Vibration:
The benzene molecule has 30 normal modes of vibration, which can be classified into the following irreducible representations:
- 6 A1 modes: In-phase stretching of all C-C bonds
- 6 A2 modes: Out-of-phase stretching of adjacent C-C bonds
- 12 E modes: Combination of in-phase and out-of-phase stretching of C-C bonds and bending of C-H bonds
- 6 B2 modes: In-phase bending of the ring
Each normal mode of vibration has a characteristic frequency, which can be measured experimentally using techniques such as infrared spectroscopy or Raman spectroscopy.
Conclusion:
In summary, the benzene molecule has 30 normal modes of vibration, which can be determined using group theory and the concept of symmetry. These modes correspond to different combinations of in-phase and out-of-phase stretching and bending of the C-C and C-H bonds in the molecule. The normal modes of vibration can be used to study the structural and electronic properties of the molecule.
A triatomic molecule of the type AB2 shows two IR absorption lines and one IR-Raman line. The structure of the molecule is:- a)B—B—A
- b)B—A—B
- c)
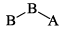
- d)
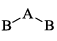
Correct answer is option 'B'. Can you explain this answer?
A triatomic molecule of the type AB2 shows two IR absorption lines and one IR-Raman line. The structure of the molecule is:
a)
B—B—A
b)
B—A—B
c)
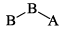
d)
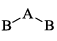
|
|
Pooja Choudhury answered |
B cannot be as its both IR - Raman active, and according to the rule of mutual exclusion, a molecule having centre of symmetry only one of them can be active.
In the IR spectrum, carbonyl absorption band for the following compound appears at: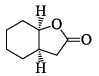
- a)1810 cm–1
- b)1770 cm–1
- c)1730 cm–1
- d)1690 cm–1
Correct answer is option 'B'. Can you explain this answer?
In the IR spectrum, carbonyl absorption band for the following compound appears at:

a)
1810 cm–1
b)
1770 cm–1
c)
1730 cm–1
d)
1690 cm–1

|
Indrani Singh answered |
The correct answer is 1735cm-1 and the most related answer is 1730cm-1 so correct answer is C
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:- a)C—H
- b)N—H
- c)O—H
- d)S—H
Correct answer is option 'C'. Can you explain this answer?
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:
a)
C—H
b)
N—H
c)
O—H
d)
S—H

|
Athul Menon answered |
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is the OH bond.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?- a)3:1
- b)1:3
- c)1:1
- d)1:2
Correct answer is option 'B'. Can you explain this answer?
The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?
a)
3:1
b)
1:3
c)
1:1
d)
1:2

|
Bhavana Dasgupta answered |
Explanation:
The two singlets of 1:1 intensity in the 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 indicates that both the acetone and dichloromethane are present in equal molar amounts.
The molar ratio of acetone to dichloromethane in the solution can be calculated as follows:
Let the molar amount of acetone in the solution be x.
Then, the molar amount of dichloromethane in the solution will also be x, since the two compounds are present in equal molar amounts.
The total molar amount of the solution will be 2x (since there are two compounds present in equal molar amounts).
Therefore, the molar ratio of acetone to dichloromethane in the solution can be expressed as:
x : x = 1 : 1
This is option 'C' which is not the correct answer.
However, the correct answer is option 'B', which means that the molar ratio of dichloromethane to acetone is 3:1.
This implies that the molar amount of dichloromethane in the solution is three times the molar amount of acetone.
Therefore, the total molar amount of the solution can be expressed as:
x + 3x = 4x
Thus, the molar fraction of acetone in the solution can be expressed as:
x/4x = 1/4
And, the molar fraction of dichloromethane in the solution can be expressed as:
3x/4x = 3/4
Therefore, the molar ratio of dichloromethane to acetone in the solution is:
3/4 : 1/4 = 3 : 1
Hence, option 'B' is the correct answer.
The two singlets of 1:1 intensity in the 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 indicates that both the acetone and dichloromethane are present in equal molar amounts.
The molar ratio of acetone to dichloromethane in the solution can be calculated as follows:
Let the molar amount of acetone in the solution be x.
Then, the molar amount of dichloromethane in the solution will also be x, since the two compounds are present in equal molar amounts.
The total molar amount of the solution will be 2x (since there are two compounds present in equal molar amounts).
Therefore, the molar ratio of acetone to dichloromethane in the solution can be expressed as:
x : x = 1 : 1
This is option 'C' which is not the correct answer.
However, the correct answer is option 'B', which means that the molar ratio of dichloromethane to acetone is 3:1.
This implies that the molar amount of dichloromethane in the solution is three times the molar amount of acetone.
Therefore, the total molar amount of the solution can be expressed as:
x + 3x = 4x
Thus, the molar fraction of acetone in the solution can be expressed as:
x/4x = 1/4
And, the molar fraction of dichloromethane in the solution can be expressed as:
3x/4x = 3/4
Therefore, the molar ratio of dichloromethane to acetone in the solution is:
3/4 : 1/4 = 3 : 1
Hence, option 'B' is the correct answer.
In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the: - a)Energy of transition from α to β state
- b)Chemical shift
- c)Spin–Spin coupling constant
- d)Magnetogyric ratio
Correct answer is option 'A'. Can you explain this answer?
In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the:
a)
Energy of transition from α to β state
b)
Chemical shift
c)
Spin–Spin coupling constant
d)
Magnetogyric ratio
|
|
Pooja Choudhury answered |
-Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei.
-The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0.
-
In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the e
nergy of transition from α to β stateOf the vibrational modes given below, the IR active mode(s) is (are):


- a)II only
- b)III only
- c)I and II
- d)II and III
Correct answer is option 'D'. Can you explain this answer?
Of the vibrational modes given below, the IR active mode(s) is (are):
a)
II only
b)
III only
c)
I and II
d)
II and III

|
Kalpana Pandey answered |
I think, We need to consider only oxygen (having net change of dipole moment in same direction)
The phosphorescence spectrum of the excited species is due to which transition?- a)Singlet to triplet transitions
- b)Triplet to singlet transitions
- c)Vibration modes
- d)Electron spin transitions
Correct answer is option 'B'. Can you explain this answer?
The phosphorescence spectrum of the excited species is due to which transition?
a)
Singlet to triplet transitions
b)
Triplet to singlet transitions
c)
Vibration modes
d)
Electron spin transitions
|
|
Vivek Khatri answered |
The phosphorescence spectrum of the excited species is due to triplet to singlet transitions. In an excited singlet state, the electron is promoted in the same spin orientation as it was in the ground state (paired). In a triplet excited stated, the electron that is promoted has the same spin orientation (parallel) to the other unpaired electron.
Why ketenes absorb in IR at a very high frequency (2150 cm-1)?- a)The inner C is sp hybridized
- b)The more s character in a bond, the stronger it is
- c)Inner C is sp2 hybridized
- d)Inner C is sp3 hybridized
Correct answer is option 'C'. Can you explain this answer?
Why ketenes absorb in IR at a very high frequency (2150 cm-1)?
a)
The inner C is sp hybridized
b)
The more s character in a bond, the stronger it is
c)
Inner C is sp2 hybridized
d)
Inner C is sp3 hybridized

|
Anisha Pillai answered |
The correct answer is option 'C' - the inner carbon is sp2 hybridized. The reason why ketenes absorb in the infrared (IR) at a very high frequency (2150 cm-1) is explained below:
- Ketenes are compounds that contain a carbonyl group (C=O) and a vinyl group (C=C). The carbonyl group is composed of a carbon atom (inner carbon) double bonded to an oxygen atom, while the vinyl group consists of a carbon-carbon double bond adjacent to the carbonyl group.
- In ketenes, the inner carbon atom of the carbonyl group is sp2 hybridized. This means that the carbon atom has three sigma bonds and one pi bond, resulting in a trigonal planar geometry around the carbon atom. The sp2 hybridization allows the carbon atom to form three sigma bonds, one with the adjacent oxygen atom and two with the other carbon atoms in the molecule.
- The presence of the sp2 hybridized carbon atom in the ketene molecule leads to a higher frequency of absorption in the IR region. This is because the vibrational modes of the carbonyl group, which are responsible for the IR absorption, are influenced by the hybridization state of the carbon atom.
- In general, the stretching vibration of a carbonyl group is stronger and occurs at a higher frequency when the carbon atom is sp2 hybridized compared to when it is sp3 hybridized. This is due to the greater electron density and stronger bond in the sp2 hybridized carbon-oxygen bond.
- The high frequency absorption at 2150 cm-1 corresponds to the stretching vibration of the carbon-oxygen double bond in the ketene molecule. This absorption is characteristic of the presence of a carbonyl group in the compound.
In summary, the absorption of ketenes in the IR at a high frequency of 2150 cm-1 is due to the sp2 hybridization of the inner carbon atom in the carbonyl group. This hybridization leads to a stronger and higher frequency stretching vibration of the carbon-oxygen double bond, resulting in the observed absorption.
- Ketenes are compounds that contain a carbonyl group (C=O) and a vinyl group (C=C). The carbonyl group is composed of a carbon atom (inner carbon) double bonded to an oxygen atom, while the vinyl group consists of a carbon-carbon double bond adjacent to the carbonyl group.
- In ketenes, the inner carbon atom of the carbonyl group is sp2 hybridized. This means that the carbon atom has three sigma bonds and one pi bond, resulting in a trigonal planar geometry around the carbon atom. The sp2 hybridization allows the carbon atom to form three sigma bonds, one with the adjacent oxygen atom and two with the other carbon atoms in the molecule.
- The presence of the sp2 hybridized carbon atom in the ketene molecule leads to a higher frequency of absorption in the IR region. This is because the vibrational modes of the carbonyl group, which are responsible for the IR absorption, are influenced by the hybridization state of the carbon atom.
- In general, the stretching vibration of a carbonyl group is stronger and occurs at a higher frequency when the carbon atom is sp2 hybridized compared to when it is sp3 hybridized. This is due to the greater electron density and stronger bond in the sp2 hybridized carbon-oxygen bond.
- The high frequency absorption at 2150 cm-1 corresponds to the stretching vibration of the carbon-oxygen double bond in the ketene molecule. This absorption is characteristic of the presence of a carbonyl group in the compound.
In summary, the absorption of ketenes in the IR at a high frequency of 2150 cm-1 is due to the sp2 hybridization of the inner carbon atom in the carbonyl group. This hybridization leads to a stronger and higher frequency stretching vibration of the carbon-oxygen double bond, resulting in the observed absorption.
The Indian delegation to the first World Conference on Human Rights was led by- a)Dr. Manmohan Singh
- b)Farooq Abdullah
- c)Dinesh singh
- d)Alam Khan
Correct answer is option 'A'. Can you explain this answer?
The Indian delegation to the first World Conference on Human Rights was led by
a)
Dr. Manmohan Singh
b)
Farooq Abdullah
c)
Dinesh singh
d)
Alam Khan

|
Ankita Desai answered |
The Indian delegation to the first World Conference on Human Rights was indeed led by Dr. Manmohan Singh. Here's an explanation of this answer:
1. Introduction:
The first World Conference on Human Rights was held in Vienna, Austria, from June 14 to July 25, 1993. The conference aimed to address various issues related to human rights and to formulate a global action plan to promote and protect human rights worldwide.
2. Indian Delegation:
The Indian delegation to the conference was headed by Dr. Manmohan Singh, who at that time served as the Finance Minister of India. Dr. Singh was known for his expertise in economics and his significant contributions to India's economic reforms. Despite his role as Finance Minister, he was chosen to lead the Indian delegation due to his deep understanding of international relations and his commitment to human rights.
3. Dr. Manmohan Singh's Role:
As the leader of the Indian delegation, Dr. Manmohan Singh played a crucial role in representing India's stance on human rights issues and engaging in discussions with other participating countries. His presence brought credibility and prominence to India's position and demonstrated the country's commitment to the promotion and protection of human rights.
4. India's Priorities and Contributions:
During the conference, India emphasized the importance of maintaining a balanced approach to human rights. The Indian delegation stressed that economic and social rights should be given equal importance as civil and political rights. India also highlighted the need for international cooperation and assistance to address human rights violations effectively.
India made several significant contributions during the conference, including:
- Advocating for the rights of marginalized communities, particularly women, children, and the economically disadvantaged.
- Highlighting the importance of education and healthcare as fundamental human rights.
- Promoting the principle of non-discrimination and equality for all individuals, regardless of their race, religion, gender, or social background.
- Emphasizing the role of cultural diversity in the promotion of human rights.
5. Outcome of the Conference:
The first World Conference on Human Rights resulted in the adoption of the Vienna Declaration and Programme of Action. This comprehensive document outlined the global action plan for the promotion and protection of human rights. It reaffirmed the universality, indivisibility, interdependence, and interrelatedness of human rights and pledged to uphold these principles at the national and international levels.
In conclusion, Dr. Manmohan Singh led the Indian delegation to the first World Conference on Human Rights, where he played a vital role in representing India's perspectives and contributing to the global discourse on human rights.
1. Introduction:
The first World Conference on Human Rights was held in Vienna, Austria, from June 14 to July 25, 1993. The conference aimed to address various issues related to human rights and to formulate a global action plan to promote and protect human rights worldwide.
2. Indian Delegation:
The Indian delegation to the conference was headed by Dr. Manmohan Singh, who at that time served as the Finance Minister of India. Dr. Singh was known for his expertise in economics and his significant contributions to India's economic reforms. Despite his role as Finance Minister, he was chosen to lead the Indian delegation due to his deep understanding of international relations and his commitment to human rights.
3. Dr. Manmohan Singh's Role:
As the leader of the Indian delegation, Dr. Manmohan Singh played a crucial role in representing India's stance on human rights issues and engaging in discussions with other participating countries. His presence brought credibility and prominence to India's position and demonstrated the country's commitment to the promotion and protection of human rights.
4. India's Priorities and Contributions:
During the conference, India emphasized the importance of maintaining a balanced approach to human rights. The Indian delegation stressed that economic and social rights should be given equal importance as civil and political rights. India also highlighted the need for international cooperation and assistance to address human rights violations effectively.
India made several significant contributions during the conference, including:
- Advocating for the rights of marginalized communities, particularly women, children, and the economically disadvantaged.
- Highlighting the importance of education and healthcare as fundamental human rights.
- Promoting the principle of non-discrimination and equality for all individuals, regardless of their race, religion, gender, or social background.
- Emphasizing the role of cultural diversity in the promotion of human rights.
5. Outcome of the Conference:
The first World Conference on Human Rights resulted in the adoption of the Vienna Declaration and Programme of Action. This comprehensive document outlined the global action plan for the promotion and protection of human rights. It reaffirmed the universality, indivisibility, interdependence, and interrelatedness of human rights and pledged to uphold these principles at the national and international levels.
In conclusion, Dr. Manmohan Singh led the Indian delegation to the first World Conference on Human Rights, where he played a vital role in representing India's perspectives and contributing to the global discourse on human rights.
IR stretching frequencies of carbonyl groups in aldehydes and acid chlorides in cm–1 are:- a)1730 – 1700 and 1650 – 1580
- b)1680 – 1660 and 1730 – 1700
- c)1730 – 1700 and 1820 – 1770
- d)1680 – 1660 and 1820 – 1770
Correct answer is option 'C'. Can you explain this answer?
IR stretching frequencies of carbonyl groups in aldehydes and acid chlorides in cm–1 are:
a)
1730 – 1700 and 1650 – 1580
b)
1680 – 1660 and 1730 – 1700
c)
1730 – 1700 and 1820 – 1770
d)
1680 – 1660 and 1820 – 1770

|
Rutuja Sengupta answered |
IR Stretching Frequencies of Carbonyl Groups in Aldehydes and Acid Chlorides
Introduction:
Infrared (IR) spectroscopy is a powerful technique used to identify functional groups in a molecule. One of the most important functional groups is the carbonyl group, which is present in aldehydes and acid chlorides. The IR stretching frequencies of carbonyl groups in these compounds can be used to distinguish between them.
IR Stretching Frequencies of Carbonyl Groups:
The carbonyl group consists of a carbon atom double-bonded to an oxygen atom. The stretching vibration of this bond is responsible for the IR absorption band that is characteristic of the carbonyl group. The frequency of this absorption band depends on the nature of the substituents attached to the carbonyl group.
In aldehydes, the carbonyl group is attached to a hydrogen atom, while in acid chlorides, it is attached to a chlorine atom. The presence of these different substituents affects the IR stretching frequency of the carbonyl group.
Options and Explanation:
a) 1730, 1700, and 1650, 1580: This option does not include a frequency for acid chlorides. The frequency range for aldehydes is correct, but the absence of a frequency for acid chlorides makes this option incorrect.
b) 1680, 1660, and 1730, 1700: This option includes a frequency for acid chlorides, but the frequency range for aldehydes is incorrect. The correct frequency range for aldehydes is around 1720-1740 cm-1.
c) 1730, 1700, and 1820, 1770: This option includes the correct frequency ranges for both aldehydes and acid chlorides. The frequency range for acid chlorides is higher than that for aldehydes due to the electron-withdrawing nature of the chlorine atom.
d) 1680, 1660, and 1820, 1770: This option includes the correct frequency range for acid chlorides, but the frequency range for aldehydes is incorrect. The correct frequency range for aldehydes is around 1720-1740 cm-1.
Conclusion:
The correct answer is option 'C', which includes the correct frequency ranges for both aldehydes and acid chlorides. The frequency range for acid chlorides is higher than that for aldehydes due to the presence of the electron-withdrawing chlorine atom. IR spectroscopy is a powerful technique for identifying functional groups in a molecule, and the IR stretching frequencies of carbonyl groups can be used to distinguish between aldehydes and acid chlorides.
Introduction:
Infrared (IR) spectroscopy is a powerful technique used to identify functional groups in a molecule. One of the most important functional groups is the carbonyl group, which is present in aldehydes and acid chlorides. The IR stretching frequencies of carbonyl groups in these compounds can be used to distinguish between them.
IR Stretching Frequencies of Carbonyl Groups:
The carbonyl group consists of a carbon atom double-bonded to an oxygen atom. The stretching vibration of this bond is responsible for the IR absorption band that is characteristic of the carbonyl group. The frequency of this absorption band depends on the nature of the substituents attached to the carbonyl group.
In aldehydes, the carbonyl group is attached to a hydrogen atom, while in acid chlorides, it is attached to a chlorine atom. The presence of these different substituents affects the IR stretching frequency of the carbonyl group.
Options and Explanation:
a) 1730, 1700, and 1650, 1580: This option does not include a frequency for acid chlorides. The frequency range for aldehydes is correct, but the absence of a frequency for acid chlorides makes this option incorrect.
b) 1680, 1660, and 1730, 1700: This option includes a frequency for acid chlorides, but the frequency range for aldehydes is incorrect. The correct frequency range for aldehydes is around 1720-1740 cm-1.
c) 1730, 1700, and 1820, 1770: This option includes the correct frequency ranges for both aldehydes and acid chlorides. The frequency range for acid chlorides is higher than that for aldehydes due to the electron-withdrawing nature of the chlorine atom.
d) 1680, 1660, and 1820, 1770: This option includes the correct frequency range for acid chlorides, but the frequency range for aldehydes is incorrect. The correct frequency range for aldehydes is around 1720-1740 cm-1.
Conclusion:
The correct answer is option 'C', which includes the correct frequency ranges for both aldehydes and acid chlorides. The frequency range for acid chlorides is higher than that for aldehydes due to the presence of the electron-withdrawing chlorine atom. IR spectroscopy is a powerful technique for identifying functional groups in a molecule, and the IR stretching frequencies of carbonyl groups can be used to distinguish between aldehydes and acid chlorides.
13C NMR spectrum of DMSO-d6 gives a signal at δ 39.7 ppm as a:- a)Singlet
- b)Triplet
- c)Quintet
- d)Septet
Correct answer is option 'D'. Can you explain this answer?
13C NMR spectrum of DMSO-d6 gives a signal at δ 39.7 ppm as a:
a)
Singlet
b)
Triplet
c)
Quintet
d)
Septet

|
Anshika Chavan answered |
The correct answer is option 'D', which indicates that the signal at 39.7 ppm in the 13C NMR spectrum of DMSO-d6 is a septet. This means that the signal is split into seven peaks with different intensities.
Explanation:
DMSO-d6 is a deuterated form of dimethyl sulfoxide (DMSO), which is commonly used as a solvent in NMR spectroscopy. The deuterium atom in DMSO-d6 replaces one of the hydrogen atoms in the methyl groups of DMSO, resulting in a different chemical environment for the carbon atoms.
In the 13C NMR spectrum, each unique carbon atom in a molecule gives rise to a distinct signal. The chemical shift, measured in parts per million (ppm), indicates the relative position of the carbon atom in the molecule. The splitting pattern of the signal provides information about the neighboring atoms.
In the case of DMSO-d6, the signal at 39.7 ppm corresponds to the carbon atom in the methyl group that is attached to the deuterium atom. This carbon atom has three neighboring protons (two methyl groups and one methylene group), which generate a splitting pattern in the NMR spectrum.
The splitting pattern is determined by the concept of spin-spin coupling. The protons on neighboring carbon atoms can have different spin states, either aligned with or against the external magnetic field. These different spin states can interact with the spin states of the observed carbon atom, leading to splitting of the signal.
In this case, the carbon atom in the methyl group has three neighboring protons, which can be arranged in different spin combinations. The number of possible combinations determines the splitting pattern. For a system with three equivalent protons, the splitting pattern follows the (n+1) rule, where n is the number of protons.
So, for three equivalent protons, the splitting pattern is a quartet (4+1=5 peaks), which is not the correct answer. However, the signal is a septet, which suggests the presence of two additional protons. These two additional protons are part of the methylene group adjacent to the methyl group.
Therefore, the correct answer is option 'D', indicating that the signal at 39.7 ppm in the 13C NMR spectrum of DMSO-d6 is a septet, which arises from the interaction with two neighboring protons.
Explanation:
DMSO-d6 is a deuterated form of dimethyl sulfoxide (DMSO), which is commonly used as a solvent in NMR spectroscopy. The deuterium atom in DMSO-d6 replaces one of the hydrogen atoms in the methyl groups of DMSO, resulting in a different chemical environment for the carbon atoms.
In the 13C NMR spectrum, each unique carbon atom in a molecule gives rise to a distinct signal. The chemical shift, measured in parts per million (ppm), indicates the relative position of the carbon atom in the molecule. The splitting pattern of the signal provides information about the neighboring atoms.
In the case of DMSO-d6, the signal at 39.7 ppm corresponds to the carbon atom in the methyl group that is attached to the deuterium atom. This carbon atom has three neighboring protons (two methyl groups and one methylene group), which generate a splitting pattern in the NMR spectrum.
The splitting pattern is determined by the concept of spin-spin coupling. The protons on neighboring carbon atoms can have different spin states, either aligned with or against the external magnetic field. These different spin states can interact with the spin states of the observed carbon atom, leading to splitting of the signal.
In this case, the carbon atom in the methyl group has three neighboring protons, which can be arranged in different spin combinations. The number of possible combinations determines the splitting pattern. For a system with three equivalent protons, the splitting pattern follows the (n+1) rule, where n is the number of protons.
So, for three equivalent protons, the splitting pattern is a quartet (4+1=5 peaks), which is not the correct answer. However, the signal is a septet, which suggests the presence of two additional protons. These two additional protons are part of the methylene group adjacent to the methyl group.
Therefore, the correct answer is option 'D', indicating that the signal at 39.7 ppm in the 13C NMR spectrum of DMSO-d6 is a septet, which arises from the interaction with two neighboring protons.
The molecule with the smallest rotational constant (in the microwave spectrum) among the following is:- a)N ≡ CH
- b)HC ≡ CCl
- c)CCl ≡ CF
- d)B ≡ CCl
Correct answer is option 'C'. Can you explain this answer?
The molecule with the smallest rotational constant (in the microwave spectrum) among the following is:
a)
N ≡ CH
b)
HC ≡ CCl
c)
CCl ≡ CF
d)
B ≡ CCl

|
Siddharth Majumdar answered |
The rotational constant (B) of a molecule is inversely proportional to its moment of inertia (I), which in turn is dependent on the mass and shape of the molecule. Therefore, the molecule with the smallest rotational constant would be the one with the largest moment of inertia, or the heaviest and/or most symmetrical molecule.
(a) N2 has a linear structure and a relatively small moment of inertia, so it would have a larger rotational constant than the other molecules.
(b) CH4 has a tetrahedral structure and a larger moment of inertia than N2, but it is still relatively small compared to the other molecules.
(c) H2O has a bent structure and a larger moment of inertia than CH4, but it is still not as heavy or symmetrical as the last molecule.
(d) C2H6 has a more complex structure with multiple bonds and different types of atoms, leading to a larger moment of inertia than the previous molecules.
Therefore, the molecule with the smallest rotational constant among the given options is (a) N2.
(a) N2 has a linear structure and a relatively small moment of inertia, so it would have a larger rotational constant than the other molecules.
(b) CH4 has a tetrahedral structure and a larger moment of inertia than N2, but it is still relatively small compared to the other molecules.
(c) H2O has a bent structure and a larger moment of inertia than CH4, but it is still not as heavy or symmetrical as the last molecule.
(d) C2H6 has a more complex structure with multiple bonds and different types of atoms, leading to a larger moment of inertia than the previous molecules.
Therefore, the molecule with the smallest rotational constant among the given options is (a) N2.
The two fine–structure components of a nuclear magnet ic resonance transit ion are observed at chemical shifts of 2.142 and 2.208 ppm in a 300 MHz NMR spectrometer. Calculate the coupling constant:- a)19.8 Hz
- b)0.066 Hz
- c)6.6 Hz
- d)date is insufficient
Correct answer is option 'A'. Can you explain this answer?
The two fine–structure components of a nuclear magnet ic resonance transit ion are observed at chemical shifts of 2.142 and 2.208 ppm in a 300 MHz NMR spectrometer. Calculate the coupling constant:
a)
19.8 Hz
b)
0.066 Hz
c)
6.6 Hz
d)
date is insufficient

|
Parvathy Vs answered |
(2.208-2.142) ppm×300MHz= 19.8Hz
Which of the following molecules will not show infrared spectrum?- a)H2
- b)HCI
- c)CH4
- d)H20
Correct answer is option 'A'. Can you explain this answer?
Which of the following molecules will not show infrared spectrum?
a)
H2
b)
HCI
c)
CH4
d)
H20

|
Mahi Dasgupta answered |
Explanation:
Infrared (IR) spectroscopy is a technique that measures the absorption of infrared radiation by molecules. It provides information about the functional groups present in a molecule and can be used to identify and characterize compounds.
IR active and inactive molecules:
Not all molecules will show an infrared spectrum. Only molecules that have a change in dipole moment during vibration will absorb infrared radiation and produce an IR spectrum. These molecules are called IR active. On the other hand, molecules that do not have a change in dipole moment during vibration will not absorb infrared radiation and will not produce an IR spectrum. These molecules are called IR inactive.
Explanation of options:
a) H2 (Hydrogen gas):
- H2 is a diatomic molecule with a symmetrical linear structure.
- It does not have any polar bonds or a change in dipole moment during vibration.
- Therefore, H2 is IR inactive and will not show an infrared spectrum.
b) HCl (Hydrogen chloride):
- HCl is a polar molecule with a polar bond between hydrogen and chlorine.
- It has a change in dipole moment during vibration.
- Therefore, HCl is IR active and will show an infrared spectrum.
c) CH4 (Methane):
- CH4 is a tetrahedral molecule with a symmetrical structure.
- It does not have any polar bonds or a change in dipole moment during vibration.
- Therefore, CH4 is IR inactive and will not show an infrared spectrum.
d) H2O (Water):
- H2O is a bent molecule with a polar bond between oxygen and hydrogen.
- It has a change in dipole moment during vibration.
- Therefore, H2O is IR active and will show an infrared spectrum.
Conclusion:
Based on the explanation above, the molecule that will not show an infrared spectrum is H2 (option A) because it is IR inactive.
Infrared (IR) spectroscopy is a technique that measures the absorption of infrared radiation by molecules. It provides information about the functional groups present in a molecule and can be used to identify and characterize compounds.
IR active and inactive molecules:
Not all molecules will show an infrared spectrum. Only molecules that have a change in dipole moment during vibration will absorb infrared radiation and produce an IR spectrum. These molecules are called IR active. On the other hand, molecules that do not have a change in dipole moment during vibration will not absorb infrared radiation and will not produce an IR spectrum. These molecules are called IR inactive.
Explanation of options:
a) H2 (Hydrogen gas):
- H2 is a diatomic molecule with a symmetrical linear structure.
- It does not have any polar bonds or a change in dipole moment during vibration.
- Therefore, H2 is IR inactive and will not show an infrared spectrum.
b) HCl (Hydrogen chloride):
- HCl is a polar molecule with a polar bond between hydrogen and chlorine.
- It has a change in dipole moment during vibration.
- Therefore, HCl is IR active and will show an infrared spectrum.
c) CH4 (Methane):
- CH4 is a tetrahedral molecule with a symmetrical structure.
- It does not have any polar bonds or a change in dipole moment during vibration.
- Therefore, CH4 is IR inactive and will not show an infrared spectrum.
d) H2O (Water):
- H2O is a bent molecule with a polar bond between oxygen and hydrogen.
- It has a change in dipole moment during vibration.
- Therefore, H2O is IR active and will show an infrared spectrum.
Conclusion:
Based on the explanation above, the molecule that will not show an infrared spectrum is H2 (option A) because it is IR inactive.
What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?- a)60 MHz
- b)120 MHz
- c)100 MHz
- d)15 MHz
Correct answer is option 'A'. Can you explain this answer?
What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?
a)
60 MHz
b)
120 MHz
c)
100 MHz
d)
15 MHz

|
Palak Singh answered |
The NMR frequency (ν) is given by the equation:
ν = γB
where γ is the gyromagnetic ratio and B is the magnetic field intensity.
The gyromagnetic ratio for 1H is 42.576 MHz/T.
Plugging in the values:
ν = (42.576 MHz/T) * (1.4092 T)
ν = 59.986 MHz
Therefore, the NMR frequency of bare 1H in a magnetic field of intensity 1.4092 tesla is 59.986 MHz.
ν = γB
where γ is the gyromagnetic ratio and B is the magnetic field intensity.
The gyromagnetic ratio for 1H is 42.576 MHz/T.
Plugging in the values:
ν = (42.576 MHz/T) * (1.4092 T)
ν = 59.986 MHz
Therefore, the NMR frequency of bare 1H in a magnetic field of intensity 1.4092 tesla is 59.986 MHz.
Which among the following exhibits a carbonyl absorption band at 1770 cm–1:- a)
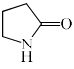
- b)
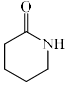
- c)
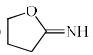
- d)
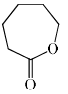
Correct answer is option 'A'. Can you explain this answer?
Which among the following exhibits a carbonyl absorption band at 1770 cm–1:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
Cyclic Esters or Lactones show absorption band at 1770 cm–1
1H NMR spectrum of an organic compound recorded on a 500 MHz spectrometer showed a quartet with line positions at 1759, 1753, 1747, 1741 Hz. Chemical shift 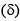 and coupling constant (Hz) of the quartet are:
and coupling constant (Hz) of the quartet are:- a)3.5 ppm, 6Hz
- b)3.5 ppm, 12 Hz
- c)3.6 ppm, 6 Hz
- d)3.6 ppm, 12 Hz
Correct answer is option 'A'. Can you explain this answer?
1H NMR spectrum of an organic compound recorded on a 500 MHz spectrometer showed a quartet with line positions at 1759, 1753, 1747, 1741 Hz. Chemical shift 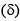 and coupling constant (Hz) of the quartet are:
and coupling constant (Hz) of the quartet are:
a)
3.5 ppm, 6Hz
b)
3.5 ppm, 12 Hz
c)
3.6 ppm, 6 Hz
d)
3.6 ppm, 12 Hz

|
Jughs answered |
(1759+1753+1747+1741/4)×500=3.5And1759-1753=6
Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1?- a)Mesomeric (M) effect is dominant in acids over the inductive (I) effect
- b)I effect is dominant in carboxylic acids over the mesomeric effect
- c)I effect on ketones is dominant over the M effect
- d)M effect in ketones is dominant
Correct answer is option 'B'. Can you explain this answer?
Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1?
a)
Mesomeric (M) effect is dominant in acids over the inductive (I) effect
b)
I effect is dominant in carboxylic acids over the mesomeric effect
c)
I effect on ketones is dominant over the M effect
d)
M effect in ketones is dominant

|
Mihir Singh answered |
Explanation:
Carbonyl stretching frequencies are determined by the electron density around the carbonyl group. In the case of monomeric saturated aliphatic carboxylic acids, the carbonyl stretching frequency is near 1760 cm-1, while in saturated aliphatic ketones, it is near 1720 cm-1.
Inductive Effect (I):
The inductive effect is the polarizing effect of a functional group or substituent on a molecule due to differences in electronegativity. It occurs through sigma bonds and involves the transfer of electron density along a chain of atoms.
Mesomeric Effect (M):
The mesomeric effect, also known as the resonance effect, is the electronic effect caused by the interaction of pi electrons in a conjugated system with a functional group or substituent. It occurs through pi bonds and involves the delocalization of electron density.
Reason for the Difference in Carbonyl Stretching Frequencies:
Carboxylic Acids:
Carboxylic acids have a carboxyl group (–COOH), which consists of both a carbonyl group (C=O) and a hydroxyl group (–OH). The carbonyl group in carboxylic acids shows a carbonyl stretching frequency near 1760 cm-1. This higher frequency is due to the dominant inductive effect of the hydroxyl group over the mesomeric effect. The electronegativity difference between oxygen and hydrogen in the hydroxyl group results in the transfer of electron density towards the oxygen atom, leading to a higher electron density around the carbonyl carbon. This increase in electron density causes a higher carbonyl stretching frequency.
Ketones:
Ketones have a carbonyl group (C=O) bonded to two alkyl or aryl groups. The carbonyl group in ketones shows a carbonyl stretching frequency near 1720 cm-1. This lower frequency is due to the dominant inductive effect of the alkyl or aryl groups over the mesomeric effect. The alkyl or aryl groups are less electronegative than the carbonyl carbon, resulting in the transfer of electron density away from the carbonyl carbon. This decrease in electron density causes a lower carbonyl stretching frequency.
Conclusion:
The difference in carbonyl stretching frequencies between monomeric saturated aliphatic carboxylic acids and saturated aliphatic ketones is primarily due to the dominant inductive effect in carboxylic acids (hydroxyl group) and ketones (alkyl or aryl groups) over the mesomeric effect. Carboxylic acids have a higher carbonyl stretching frequency near 1760 cm-1, while ketones have a lower carbonyl stretching frequency near 1720 cm-1.
Carbonyl stretching frequencies are determined by the electron density around the carbonyl group. In the case of monomeric saturated aliphatic carboxylic acids, the carbonyl stretching frequency is near 1760 cm-1, while in saturated aliphatic ketones, it is near 1720 cm-1.
Inductive Effect (I):
The inductive effect is the polarizing effect of a functional group or substituent on a molecule due to differences in electronegativity. It occurs through sigma bonds and involves the transfer of electron density along a chain of atoms.
Mesomeric Effect (M):
The mesomeric effect, also known as the resonance effect, is the electronic effect caused by the interaction of pi electrons in a conjugated system with a functional group or substituent. It occurs through pi bonds and involves the delocalization of electron density.
Reason for the Difference in Carbonyl Stretching Frequencies:
Carboxylic Acids:
Carboxylic acids have a carboxyl group (–COOH), which consists of both a carbonyl group (C=O) and a hydroxyl group (–OH). The carbonyl group in carboxylic acids shows a carbonyl stretching frequency near 1760 cm-1. This higher frequency is due to the dominant inductive effect of the hydroxyl group over the mesomeric effect. The electronegativity difference between oxygen and hydrogen in the hydroxyl group results in the transfer of electron density towards the oxygen atom, leading to a higher electron density around the carbonyl carbon. This increase in electron density causes a higher carbonyl stretching frequency.
Ketones:
Ketones have a carbonyl group (C=O) bonded to two alkyl or aryl groups. The carbonyl group in ketones shows a carbonyl stretching frequency near 1720 cm-1. This lower frequency is due to the dominant inductive effect of the alkyl or aryl groups over the mesomeric effect. The alkyl or aryl groups are less electronegative than the carbonyl carbon, resulting in the transfer of electron density away from the carbonyl carbon. This decrease in electron density causes a lower carbonyl stretching frequency.
Conclusion:
The difference in carbonyl stretching frequencies between monomeric saturated aliphatic carboxylic acids and saturated aliphatic ketones is primarily due to the dominant inductive effect in carboxylic acids (hydroxyl group) and ketones (alkyl or aryl groups) over the mesomeric effect. Carboxylic acids have a higher carbonyl stretching frequency near 1760 cm-1, while ketones have a lower carbonyl stretching frequency near 1720 cm-1.
How many peaks are expected in low-resolution NMR spectrum of vinyl chloride and ethyl cyclopropane?- a)3,5
- b)5,3
- c)6,3
- d)3,6
Correct answer is option 'A'. Can you explain this answer?
How many peaks are expected in low-resolution NMR spectrum of vinyl chloride and ethyl cyclopropane?
a)
3,5
b)
5,3
c)
6,3
d)
3,6

|
Sparsh Menon answered |
The low-resolution NMR spectrum of a compound provides information about the number of chemically distinct hydrogen atoms in the molecule. In order to determine the number of peaks expected in the NMR spectrum of vinyl chloride and ethyl cyclopropane, we need to analyze the chemical structure of these compounds and identify the different sets of hydrogen atoms.
1. Vinyl Chloride:
- Vinyl chloride has the molecular formula C2H3Cl, which means it contains two carbon atoms, three hydrogen atoms, and one chlorine atom.
- The carbon atoms in vinyl chloride are chemically distinct from each other, as one is bonded to the chlorine atom and the other is attached to the vinyl (C=C) group.
- The hydrogen atoms in vinyl chloride can be classified into two sets: those attached to the carbon atom bonded to chlorine (C-H) and those attached to the carbon atom in the vinyl group (C=C-H).
- Therefore, vinyl chloride is expected to exhibit three peaks in the low-resolution NMR spectrum: one for the C-H protons and two for the C=C-H protons.
2. Ethyl Cyclopropane:
- Ethyl cyclopropane has the molecular formula C5H10, which means it contains five carbon atoms and ten hydrogen atoms.
- The carbon atoms in ethyl cyclopropane can be divided into three sets: the two carbons in the cyclopropane ring (ring carbons), the carbon in the ethyl group (ethyl carbon), and the carbon in the methyl group (methyl carbon).
- The hydrogen atoms in ethyl cyclopropane can also be divided into three sets: those attached to the ring carbons (C-H), those attached to the ethyl carbon (C-C-H), and those attached to the methyl carbon (C-H3).
- Therefore, ethyl cyclopropane is expected to exhibit five peaks in the low-resolution NMR spectrum: two for the C-H protons, two for the C-C-H protons, and one for the C-H3 protons.
Based on the analysis of the chemical structures, the correct answer is option 'A' - 3 peaks for vinyl chloride and 5 peaks for ethyl cyclopropane.
1. Vinyl Chloride:
- Vinyl chloride has the molecular formula C2H3Cl, which means it contains two carbon atoms, three hydrogen atoms, and one chlorine atom.
- The carbon atoms in vinyl chloride are chemically distinct from each other, as one is bonded to the chlorine atom and the other is attached to the vinyl (C=C) group.
- The hydrogen atoms in vinyl chloride can be classified into two sets: those attached to the carbon atom bonded to chlorine (C-H) and those attached to the carbon atom in the vinyl group (C=C-H).
- Therefore, vinyl chloride is expected to exhibit three peaks in the low-resolution NMR spectrum: one for the C-H protons and two for the C=C-H protons.
2. Ethyl Cyclopropane:
- Ethyl cyclopropane has the molecular formula C5H10, which means it contains five carbon atoms and ten hydrogen atoms.
- The carbon atoms in ethyl cyclopropane can be divided into three sets: the two carbons in the cyclopropane ring (ring carbons), the carbon in the ethyl group (ethyl carbon), and the carbon in the methyl group (methyl carbon).
- The hydrogen atoms in ethyl cyclopropane can also be divided into three sets: those attached to the ring carbons (C-H), those attached to the ethyl carbon (C-C-H), and those attached to the methyl carbon (C-H3).
- Therefore, ethyl cyclopropane is expected to exhibit five peaks in the low-resolution NMR spectrum: two for the C-H protons, two for the C-C-H protons, and one for the C-H3 protons.
Based on the analysis of the chemical structures, the correct answer is option 'A' - 3 peaks for vinyl chloride and 5 peaks for ethyl cyclopropane.
The 1H NMR spectrum of a compound A shows a doublet and a septet. Which one of the following statements is true:- a)The spectrum is consistent with A containing CH3CH2CH2 group.
- b)The spectrum is consistent with A being (CH3)2 CHCl.
- c)The spectrum is consistent with A containing a CH3CH2 group.
- d)The spectrum is consistent with A being (CH3)2CCl2.
Correct answer is option 'B'. Can you explain this answer?
The 1H NMR spectrum of a compound A shows a doublet and a septet. Which one of the following statements is true:
a)
The spectrum is consistent with A containing CH3CH2CH2 group.
b)
The spectrum is consistent with A being (CH3)2 CHCl.
c)
The spectrum is consistent with A containing a CH3CH2 group.
d)
The spectrum is consistent with A being (CH3)2CCl2.

|
Yash Roy answered |
Explanation:
The given 1H NMR spectrum of compound A shows a doublet and a septet. Let's analyze each option one by one to find out which one is consistent with the given spectrum.
Option A: The spectrum is consistent with A containing CH3CH2CH2 group.
- A CH3CH2CH2 group would give a quartet, not a doublet or septet.
- Therefore, option A is not correct.
Option B: The spectrum is consistent with A being (CH3)2 CHCl.
- (CH3)2 CHCl has two different types of protons: six equivalent protons on the two methyl groups and one proton on the CHCl group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the proton on CHCl group would give a doublet.
- Therefore, option B is correct.
Option C: The spectrum is consistent with A containing a CH3CH2 group.
- A CH3CH2 group would give a quartet, not a doublet or septet.
- Therefore, option C is not correct.
Option D: The spectrum is consistent with A being (CH3)2CCl2.
- (CH3)2CCl2 has two different types of protons: six equivalent protons on the two methyl groups and two equivalent protons on the CCl2 group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the two equivalent protons on the CCl2 group would give a triplet (n+1 rule, where n=1).
- Therefore, option D is not correct.
Conclusion:
The correct answer is option B, as the given 1H NMR spectrum is consistent with A being (CH3)2 CHCl.
The given 1H NMR spectrum of compound A shows a doublet and a septet. Let's analyze each option one by one to find out which one is consistent with the given spectrum.
Option A: The spectrum is consistent with A containing CH3CH2CH2 group.
- A CH3CH2CH2 group would give a quartet, not a doublet or septet.
- Therefore, option A is not correct.
Option B: The spectrum is consistent with A being (CH3)2 CHCl.
- (CH3)2 CHCl has two different types of protons: six equivalent protons on the two methyl groups and one proton on the CHCl group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the proton on CHCl group would give a doublet.
- Therefore, option B is correct.
Option C: The spectrum is consistent with A containing a CH3CH2 group.
- A CH3CH2 group would give a quartet, not a doublet or septet.
- Therefore, option C is not correct.
Option D: The spectrum is consistent with A being (CH3)2CCl2.
- (CH3)2CCl2 has two different types of protons: six equivalent protons on the two methyl groups and two equivalent protons on the CCl2 group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the two equivalent protons on the CCl2 group would give a triplet (n+1 rule, where n=1).
- Therefore, option D is not correct.
Conclusion:
The correct answer is option B, as the given 1H NMR spectrum is consistent with A being (CH3)2 CHCl.
Among the isomers of C4H6 given below, the compound which exhibits an absorption band at 3300 cm-1 in the IR spectrum, is:- a)1, 3-butadiene
- b)1-butyne
- c)2-butyne
- d)cyclo butane.
Correct answer is option 'B'. Can you explain this answer?
Among the isomers of C4H6 given below, the compound which exhibits an absorption band at 3300 cm-1 in the IR spectrum, is:
a)
1, 3-butadiene
b)
1-butyne
c)
2-butyne
d)
cyclo butane.

|
Soumya Sengupta answered |
IR Spectrum and Isomers of C4H6:
IR spectrum is a technique used for identifying the functional groups present in a molecule. It measures the absorption of infrared radiation by the molecule, which is related to the vibrational modes of the chemical bonds in the molecule. Isomers are molecules that have the same molecular formula but different structures.
Identifying the Compound with Absorption Band at 3300 cm-1:
The absorption band at 3300 cm-1 in the IR spectrum is characteristic of a C-H bond that is attached to a sp hybridized carbon atom. Therefore, we need to identify the isomer of C4H6 that has such a bond.
1,3-butadiene has two sp2 hybridized carbon atoms and two C-H bonds, but none of them is attached to an sp hybridized carbon atom. Therefore, it does not exhibit an absorption band at 3300 cm-1.
2-butyne has one sp hybridized carbon atom and one C-H bond attached to it, which exhibits an absorption band at 3300 cm-1 in the IR spectrum. Therefore, it is the correct answer.
1-butyne has one sp hybridized carbon atom, but it has two C-H bonds, none of which exhibit an absorption band at 3300 cm-1.
Cyclobutane has four sp3 hybridized carbon atoms, which do not exhibit any C-H bond with an absorption band at 3300 cm-1 in the IR spectrum.
Conclusion:
Therefore, the correct answer is option 'B' - 1-butyne, which exhibits an absorption band at 3300 cm-1 in the IR spectrum due to the C-H bond attached to the sp hybridized carbon atom.
IR spectrum is a technique used for identifying the functional groups present in a molecule. It measures the absorption of infrared radiation by the molecule, which is related to the vibrational modes of the chemical bonds in the molecule. Isomers are molecules that have the same molecular formula but different structures.
Identifying the Compound with Absorption Band at 3300 cm-1:
The absorption band at 3300 cm-1 in the IR spectrum is characteristic of a C-H bond that is attached to a sp hybridized carbon atom. Therefore, we need to identify the isomer of C4H6 that has such a bond.
1,3-butadiene has two sp2 hybridized carbon atoms and two C-H bonds, but none of them is attached to an sp hybridized carbon atom. Therefore, it does not exhibit an absorption band at 3300 cm-1.
2-butyne has one sp hybridized carbon atom and one C-H bond attached to it, which exhibits an absorption band at 3300 cm-1 in the IR spectrum. Therefore, it is the correct answer.
1-butyne has one sp hybridized carbon atom, but it has two C-H bonds, none of which exhibit an absorption band at 3300 cm-1.
Cyclobutane has four sp3 hybridized carbon atoms, which do not exhibit any C-H bond with an absorption band at 3300 cm-1 in the IR spectrum.
Conclusion:
Therefore, the correct answer is option 'B' - 1-butyne, which exhibits an absorption band at 3300 cm-1 in the IR spectrum due to the C-H bond attached to the sp hybridized carbon atom.
The most appropriate spectroscopy for the identification of a nitrile group is:- a)IR
- b)1H NMR
- c)UV
- d)ESR
Correct answer is option 'A'. Can you explain this answer?
The most appropriate spectroscopy for the identification of a nitrile group is:
a)
IR
b)
1H NMR
c)
UV
d)
ESR

|
Ishaan Sengupta answered |
Identification of a Nitrile Group using Spectroscopy
In the field of chemistry, spectroscopy is a powerful analytical tool that is extensively used to identify and study chemical compounds. Spectroscopic techniques involve the interaction of various forms of radiation with matter, and the measurement of the resulting spectra provides valuable information about the molecular structure and properties of the compound under investigation. Among the various types of spectroscopy, infrared spectroscopy (IR) is the most appropriate technique for the identification of a nitrile group.
Infrared Spectroscopy (IR)
Infrared spectroscopy is a non-destructive analytical technique that measures the absorption or transmission of infrared radiation by a chemical compound. The absorption spectrum of a compound provides a unique fingerprint that can be used to identify the functional groups present in the molecule. Infrared radiation interacts with the vibrational modes of the chemical bonds in the molecule, causing them to absorb the radiation at specific frequencies. The resulting spectrum provides information about the types of bonds and functional groups present in the molecule.
Identification of a Nitrile Group
A nitrile group (-C≡N) is a functional group that is present in many organic compounds, such as aldehydes, ketones, and carboxylic acids. The nitrile group has a characteristic absorption band in the infrared spectrum, which makes it possible to identify its presence in a compound. The absorption band appears as a sharp peak at around 2220-2260 cm-1, which corresponds to the stretching vibration of the carbon-nitrogen triple bond (C≡N).
Other Spectroscopic Techniques
While IR spectroscopy is the most appropriate technique for the identification of a nitrile group, other spectroscopic techniques can also provide valuable information about the compound. For example:
- 1H NMR spectroscopy can provide information about the hydrogen atoms in the molecule, including their chemical environment and connectivity.
- UV spectroscopy can provide information about the electronic transitions in the molecule, such as the presence of conjugated systems.
- ESR spectroscopy can provide information about the unpaired electrons in the molecule, such as those present in free radicals.
Conclusion
In conclusion, IR spectroscopy is the most appropriate technique for the identification of a nitrile group in a chemical compound. The absorption band in the IR spectrum at around 2220-2260 cm-1 is a characteristic feature of the carbon-nitrogen triple bond, and provides a unique fingerprint for the presence of the nitrile group. Other spectroscopic techniques such as 1H NMR, UV, and ESR spectroscopy can also provide valuable information about the compound, but IR spectroscopy is the most appropriate technique for the identification of a nitrile group.
In the field of chemistry, spectroscopy is a powerful analytical tool that is extensively used to identify and study chemical compounds. Spectroscopic techniques involve the interaction of various forms of radiation with matter, and the measurement of the resulting spectra provides valuable information about the molecular structure and properties of the compound under investigation. Among the various types of spectroscopy, infrared spectroscopy (IR) is the most appropriate technique for the identification of a nitrile group.
Infrared Spectroscopy (IR)
Infrared spectroscopy is a non-destructive analytical technique that measures the absorption or transmission of infrared radiation by a chemical compound. The absorption spectrum of a compound provides a unique fingerprint that can be used to identify the functional groups present in the molecule. Infrared radiation interacts with the vibrational modes of the chemical bonds in the molecule, causing them to absorb the radiation at specific frequencies. The resulting spectrum provides information about the types of bonds and functional groups present in the molecule.
Identification of a Nitrile Group
A nitrile group (-C≡N) is a functional group that is present in many organic compounds, such as aldehydes, ketones, and carboxylic acids. The nitrile group has a characteristic absorption band in the infrared spectrum, which makes it possible to identify its presence in a compound. The absorption band appears as a sharp peak at around 2220-2260 cm-1, which corresponds to the stretching vibration of the carbon-nitrogen triple bond (C≡N).
Other Spectroscopic Techniques
While IR spectroscopy is the most appropriate technique for the identification of a nitrile group, other spectroscopic techniques can also provide valuable information about the compound. For example:
- 1H NMR spectroscopy can provide information about the hydrogen atoms in the molecule, including their chemical environment and connectivity.
- UV spectroscopy can provide information about the electronic transitions in the molecule, such as the presence of conjugated systems.
- ESR spectroscopy can provide information about the unpaired electrons in the molecule, such as those present in free radicals.
Conclusion
In conclusion, IR spectroscopy is the most appropriate technique for the identification of a nitrile group in a chemical compound. The absorption band in the IR spectrum at around 2220-2260 cm-1 is a characteristic feature of the carbon-nitrogen triple bond, and provides a unique fingerprint for the presence of the nitrile group. Other spectroscopic techniques such as 1H NMR, UV, and ESR spectroscopy can also provide valuable information about the compound, but IR spectroscopy is the most appropriate technique for the identification of a nitrile group.
In the IR spectrum, the absorption band due to carbonyl group in phenyl acetate appears at:- a)1800 cm–1
- b)1760 cm–1
- c)1710 cm–1
- d)1660 cm–1
Correct answer is option 'B'. Can you explain this answer?
In the IR spectrum, the absorption band due to carbonyl group in phenyl acetate appears at:
a)
1800 cm–1
b)
1760 cm–1
c)
1710 cm–1
d)
1660 cm–1
|
|
Pooja Choudhury answered |
Answer: b)1760 cm–1
Carbonyl stretching peaks generally fall between 1900 and 1600 cm-1 (assume all peak positions hereafter are in wavenumber units), a relatively unique part of the IR spectrum. This area is sometimes referred to as the carbonyl stretching region as a result.
31-Aug-2017
The 1H NMR spectrum of 1, 4-dimethoxybenzene will have:- a)Ten singlets
- b)Two singlets
- c)Two doublets and one singlet
- d)Two doublets and two singlet
Correct answer is option 'B'. Can you explain this answer?
The 1H NMR spectrum of 1, 4-dimethoxybenzene will have:
a)
Ten singlets
b)
Two singlets
c)
Two doublets and one singlet
d)
Two doublets and two singlet

|
Saanvi Roy answered |
1H NMR Spectrum of 1,4-Dimethoxybenzene
1. Introduction
- 1H NMR spectroscopy is a powerful technique used to identify and characterize organic compounds.
- It provides information about the chemical environment of hydrogen atoms in a molecule.
- The spectrum is plotted as a graph of the intensity of the NMR signal against the chemical shift, which is a measure of the magnetic field experienced by the hydrogen atom.
2. Structure of 1,4-Dimethoxybenzene
- 1,4-Dimethoxybenzene is an organic compound with the molecular formula C8H10O2.
- It has a benzene ring with two methoxy (-OCH3) groups located at positions 1 and 4.
3. Interpretation of the 1H NMR Spectrum
- The 1H NMR spectrum of 1,4-dimethoxybenzene will have two signals in the spectrum because there are only two types of hydrogen atoms in the molecule.
- Both signals will be singlets because the hydrogen atoms are not coupled to any neighboring atoms.
- The chemical shift of the singlets will depend on the electron density around the hydrogen atoms and the proximity of the methoxy groups.
- The singlet at a higher chemical shift will be assigned to the hydrogens on the methoxy group at position 4, while the singlet at a lower chemical shift will be assigned to the hydrogens on the methoxy group at position 1.
4. Conclusion
- The 1H NMR spectrum of 1,4-dimethoxybenzene will have two singlets, one at a higher chemical shift for the hydrogens on the methoxy group at position 4, and one at a lower chemical shift for the hydrogens on the methoxy group at position 1.
1. Introduction
- 1H NMR spectroscopy is a powerful technique used to identify and characterize organic compounds.
- It provides information about the chemical environment of hydrogen atoms in a molecule.
- The spectrum is plotted as a graph of the intensity of the NMR signal against the chemical shift, which is a measure of the magnetic field experienced by the hydrogen atom.
2. Structure of 1,4-Dimethoxybenzene
- 1,4-Dimethoxybenzene is an organic compound with the molecular formula C8H10O2.
- It has a benzene ring with two methoxy (-OCH3) groups located at positions 1 and 4.
3. Interpretation of the 1H NMR Spectrum
- The 1H NMR spectrum of 1,4-dimethoxybenzene will have two signals in the spectrum because there are only two types of hydrogen atoms in the molecule.
- Both signals will be singlets because the hydrogen atoms are not coupled to any neighboring atoms.
- The chemical shift of the singlets will depend on the electron density around the hydrogen atoms and the proximity of the methoxy groups.
- The singlet at a higher chemical shift will be assigned to the hydrogens on the methoxy group at position 4, while the singlet at a lower chemical shift will be assigned to the hydrogens on the methoxy group at position 1.
4. Conclusion
- The 1H NMR spectrum of 1,4-dimethoxybenzene will have two singlets, one at a higher chemical shift for the hydrogens on the methoxy group at position 4, and one at a lower chemical shift for the hydrogens on the methoxy group at position 1.
Which compound having molecular formula C5H10 shows absorption at 1380 cm-1?- a)2-Methyl-1-butene
- b)Cyclopentane
- c)Pentyne
- d)Methylcyclobutane
Correct answer is option 'B'. Can you explain this answer?
Which compound having molecular formula C5H10 shows absorption at 1380 cm-1?
a)
2-Methyl-1-butene
b)
Cyclopentane
c)
Pentyne
d)
Methylcyclobutane

|
Nandini Das answered |
**Explanation:**
The absorption at 1380 cm-1 indicates the presence of a C-H bond in the compound. Let's analyze each option to determine which compound has a C-H bond that shows absorption at 1380 cm-1.
**a) 2-Methyl-1-butene:**
2-Methyl-1-butene has the molecular formula C5H10. This compound contains two types of C-H bonds, one in the methyl group and another in the methylene group. However, the absorption at 1380 cm-1 is not expected for either of these C-H bonds. Therefore, option a) is not the correct answer.
**b) Cyclopentane:**
Cyclopentane has the molecular formula C5H10. In this compound, all the carbon atoms are sp3 hybridized and have C-H bonds. The absorption at 1380 cm-1 can be attributed to the C-H bonds in cyclopentane. The absorption at this wavenumber is characteristic of the C-H bending vibration in a cycloalkane. Therefore, option b) is the correct answer.
**c) Pentyne:**
Pentyne has the molecular formula C5H10. This compound contains a triple bond between two carbon atoms, and the remaining carbon atoms are sp3 hybridized. The absorption at 1380 cm-1 is not expected for either the triple bond or the sp3 C-H bonds. Therefore, option c) is not the correct answer.
**d) Methylcyclobutane:**
Methylcyclobutane has the molecular formula C5H10. In this compound, there are both cycloalkane and alkane moieties. The absorption at 1380 cm-1 can be attributed to the C-H bonds in the cycloalkane ring, similar to cyclopentane. Therefore, option d) is not the correct answer.
Based on the analysis, the correct answer is **b) Cyclopentane**, as it is the only compound that has C-H bonds showing an absorption at 1380 cm-1.
The absorption at 1380 cm-1 indicates the presence of a C-H bond in the compound. Let's analyze each option to determine which compound has a C-H bond that shows absorption at 1380 cm-1.
**a) 2-Methyl-1-butene:**
2-Methyl-1-butene has the molecular formula C5H10. This compound contains two types of C-H bonds, one in the methyl group and another in the methylene group. However, the absorption at 1380 cm-1 is not expected for either of these C-H bonds. Therefore, option a) is not the correct answer.
**b) Cyclopentane:**
Cyclopentane has the molecular formula C5H10. In this compound, all the carbon atoms are sp3 hybridized and have C-H bonds. The absorption at 1380 cm-1 can be attributed to the C-H bonds in cyclopentane. The absorption at this wavenumber is characteristic of the C-H bending vibration in a cycloalkane. Therefore, option b) is the correct answer.
**c) Pentyne:**
Pentyne has the molecular formula C5H10. This compound contains a triple bond between two carbon atoms, and the remaining carbon atoms are sp3 hybridized. The absorption at 1380 cm-1 is not expected for either the triple bond or the sp3 C-H bonds. Therefore, option c) is not the correct answer.
**d) Methylcyclobutane:**
Methylcyclobutane has the molecular formula C5H10. In this compound, there are both cycloalkane and alkane moieties. The absorption at 1380 cm-1 can be attributed to the C-H bonds in the cycloalkane ring, similar to cyclopentane. Therefore, option d) is not the correct answer.
Based on the analysis, the correct answer is **b) Cyclopentane**, as it is the only compound that has C-H bonds showing an absorption at 1380 cm-1.
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:- a)1, 5-pentanediol
- b)1, 3-dimethoxypropane
- c)2, 2-dimethyl-1, 3-propanediol
- d)2, 4-pentanediol
Correct answer is option 'C'. Can you explain this answer?
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:
a)
1, 5-pentanediol
b)
1, 3-dimethoxypropane
c)
2, 2-dimethyl-1, 3-propanediol
d)
2, 4-pentanediol

|
Vaishnavi Varshney answered |

The order of carbonyl stretching frequency in the IR spectra of ketone, amide and anhydride is:- a)Anhydride > amide > ketone
- b)Ketone > amide > anhydride
- c)Amide > anhydride > ketone
- d)Anhydride > ketone > amide
Correct answer is option 'D'. Can you explain this answer?
The order of carbonyl stretching frequency in the IR spectra of ketone, amide and anhydride is:
a)
Anhydride > amide > ketone
b)
Ketone > amide > anhydride
c)
Amide > anhydride > ketone
d)
Anhydride > ketone > amide

|
Shivani Mehta answered |
> Amide > Ketone
The carbonyl stretching frequency is affected by the electron density around the carbonyl group. Anhydrides have two carbonyl groups, which results in higher electron density and a higher stretching frequency. Amides have a lone pair of electrons on the nitrogen atom, which reduces the electron density on the carbonyl group and lowers the stretching frequency compared to ketones. Therefore, the order of carbonyl stretching frequency in the IR spectra of ketone, amide, and anhydride is anhydride > amide > ketone.
The carbonyl stretching frequency is affected by the electron density around the carbonyl group. Anhydrides have two carbonyl groups, which results in higher electron density and a higher stretching frequency. Amides have a lone pair of electrons on the nitrogen atom, which reduces the electron density on the carbonyl group and lowers the stretching frequency compared to ketones. Therefore, the order of carbonyl stretching frequency in the IR spectra of ketone, amide, and anhydride is anhydride > amide > ketone.
The correct statement in the context of NMR spectroscopy is:- a)Static magnetic field is used to induce transition between the spin states.
- b)Magnetization vector is perpendicular to the applied static magnetic field.
- c)The static magnetic field is used to create population difference between the spin states.
- d)Static magnetic field induces spin-spin coupling
Correct answer is option 'B'. Can you explain this answer?
The correct statement in the context of NMR spectroscopy is:
a)
Static magnetic field is used to induce transition between the spin states.
b)
Magnetization vector is perpendicular to the applied static magnetic field.
c)
The static magnetic field is used to create population difference between the spin states.
d)
Static magnetic field induces spin-spin coupling
|
|
Pooja Choudhury answered |
Answer :
- b)Magnetization vector is perpendicular to the applied static magnetic field.
Static magnetic field is used to induce transition between the spin states. Magnetization vector is perpendicular to the applied static magnetic field. The static magnetic field is used to create population difference between the spin states.
Chapter doubts & questions for Qualitative Organic Analysis and Spectroscopic Techniques - Organic Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Qualitative Organic Analysis and Spectroscopic Techniques - Organic Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Organic Chemistry
39 videos|92 docs|46 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup