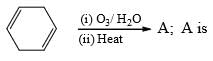All Exams >
Chemistry >
Organic Chemistry >
All Questions
All questions of Organic Named Reactions & Reagents for Chemistry Exam
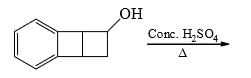
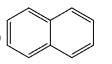
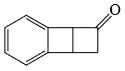
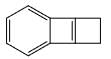
The major product formed in the following reaction is: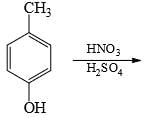
- a)
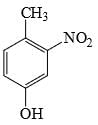
- b)
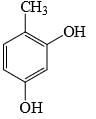
- c)

- d)
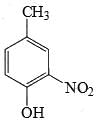
Correct answer is option 'D'. Can you explain this answer?
The major product formed in the following reaction is:

a)

b)

c)

d)


|
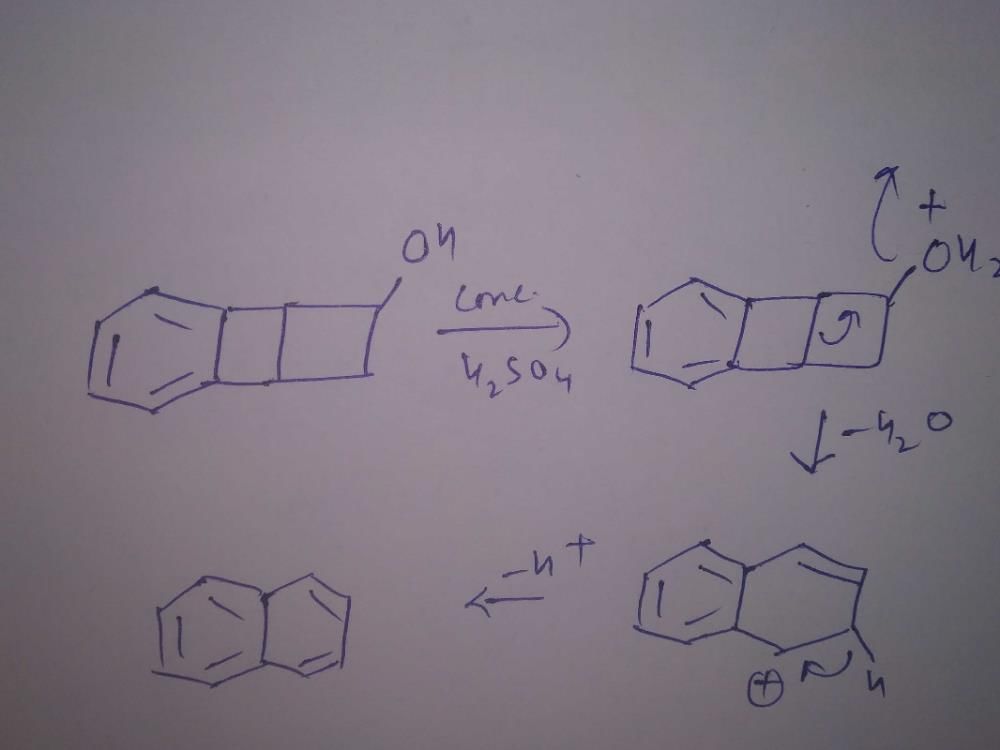
Saurabh Chavan answered |
OH group is stronger EDG than R(-CH3). No2 is EWG then it will give more electron density at OH group.
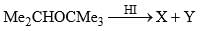 Predict the nature of product and the type of reaction involved in their formation.
Predict the nature of product and the type of reaction involved in their formation.
- a)Me2CHI and Me3COH, formed by SN1 reaction
- b)Me2CHOH and Me3CI formed by SN1 reaction
- c)Me2CHI and Me3COH, formed by SN2 reaction
- d)Me2CHOH and Me3Cl, formed by SN2 reaction
Correct answer is option 'B'. Can you explain this answer?
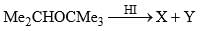
Predict the nature of product and the type of reaction involved in their formation.
a)
Me2CHI and Me3COH, formed by SN1 reaction
b)
Me2CHOH and Me3CI formed by SN1 reaction
c)
Me2CHI and Me3COH, formed by SN2 reaction
d)
Me2CHOH and Me3Cl, formed by SN2 reaction

|
Pie Academy answered |

A carboxylic acid can best be converted into acid chloride by using:- a)PCl5
- b)SOCl2
- c)HCl
- d)ClCOCOCl
Correct answer is option 'D'. Can you explain this answer?
A carboxylic acid can best be converted into acid chloride by using:
a)
PCl5
b)
SOCl2
c)
HCl
d)
ClCOCOCl

|
Veda Institute answered |
Use of SOCl2 and CICOCOCI forms gaseous by products which can be easily removed, giving better yield of RCOCI. Further, oxalyl chIoride is particularly easy to use becasue any excess of it can be easily evaporated due to its low b.p. (62∘C)

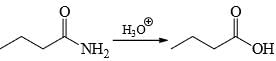
Choose the reaction(s) that will not proceed as shown hereunder:- a)
- b)
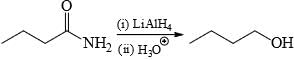
- c)
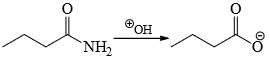
- d)
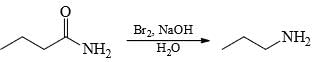
Correct answer is option 'B'. Can you explain this answer?
Choose the reaction(s) that will not proceed as shown hereunder:
a)

b)
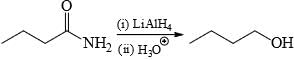
c)

d)
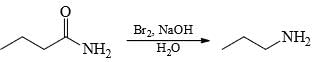

|
Pie Academy answered |
Reduction of amide by LAH gives amine but hydrolysis of amine not give alcohol.
Hence B is the answer.
Which of the following statement is false:- a)Cannizzaro reaction is given by aldehydes in presence of alkali
- b)Aldol condensation is given by aldehydes in presence of alkali
- c)Aldol condensation is given by aldehydes and ketones in presence of acids
- d)None of these.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is false:
a)
Cannizzaro reaction is given by aldehydes in presence of alkali
b)
Aldol condensation is given by aldehydes in presence of alkali
c)
Aldol condensation is given by aldehydes and ketones in presence of acids
d)
None of these.

|
Shivani Mehta answered |
False Statement in Organic Chemistry
The false statement in organic chemistry is:
Cannizzaro reaction is given by aldehydes in the presence of alkali.
Explanation
Cannizzaro reaction is a redox reaction that occurs in aldehydes having no alpha-hydrogen atoms. In this reaction, one molecule of aldehyde is oxidized to a carboxylic acid, and the other molecule of the same aldehyde is reduced to an alcohol in the presence of a strong base. This reaction is not given by all aldehydes, only those without alpha-hydrogen atoms.
Aldol Condensation
Aldol condensation is a reaction between two carbonyl compounds, such as aldehydes or ketones, in the presence of a base or an acid catalyst. The reaction leads to the formation of a β-hydroxy carbonyl compound. Aldol condensation can be carried out using aldehydes or ketones or a combination of both.
Aldol Condensation with Aldehydes and Ketones
Aldol condensation can also occur between aldehydes and ketones in the presence of an acid catalyst. In this case, the reaction is called crossed aldol condensation. The acid catalyst promotes the formation of an enol intermediate, which then reacts with the carbonyl group of the other reactant to form a β-hydroxy carbonyl compound.
Conclusion
The false statement in organic chemistry is Cannizzaro reaction is given by aldehydes in the presence of alkali. The correct statement is that Cannizzaro reaction is only given by aldehydes without alpha-hydrogen atoms. Aldol condensation is a reaction between two carbonyl compounds, such as aldehydes or ketones, in the presence of a base or an acid catalyst. Aldol condensation can also occur between aldehydes and ketones in the presence of an acid catalyst, called crossed aldol condensation.
The false statement in organic chemistry is:
Cannizzaro reaction is given by aldehydes in the presence of alkali.
Explanation
Cannizzaro reaction is a redox reaction that occurs in aldehydes having no alpha-hydrogen atoms. In this reaction, one molecule of aldehyde is oxidized to a carboxylic acid, and the other molecule of the same aldehyde is reduced to an alcohol in the presence of a strong base. This reaction is not given by all aldehydes, only those without alpha-hydrogen atoms.
Aldol Condensation
Aldol condensation is a reaction between two carbonyl compounds, such as aldehydes or ketones, in the presence of a base or an acid catalyst. The reaction leads to the formation of a β-hydroxy carbonyl compound. Aldol condensation can be carried out using aldehydes or ketones or a combination of both.
Aldol Condensation with Aldehydes and Ketones
Aldol condensation can also occur between aldehydes and ketones in the presence of an acid catalyst. In this case, the reaction is called crossed aldol condensation. The acid catalyst promotes the formation of an enol intermediate, which then reacts with the carbonyl group of the other reactant to form a β-hydroxy carbonyl compound.
Conclusion
The false statement in organic chemistry is Cannizzaro reaction is given by aldehydes in the presence of alkali. The correct statement is that Cannizzaro reaction is only given by aldehydes without alpha-hydrogen atoms. Aldol condensation is a reaction between two carbonyl compounds, such as aldehydes or ketones, in the presence of a base or an acid catalyst. Aldol condensation can also occur between aldehydes and ketones in the presence of an acid catalyst, called crossed aldol condensation.
Which of the following could result as a product in the aldol condensation reaction?- a)4-methyl-3-penten-2-on
- b)4-methyl-4-penten-2-one
- c)4-methyl-5-hexen-2-one
- d)3-methyl-4-hexen-2-one
Correct answer is option 'A'. Can you explain this answer?
Which of the following could result as a product in the aldol condensation reaction?
a)
4-methyl-3-penten-2-on
b)
4-methyl-4-penten-2-one
c)
4-methyl-5-hexen-2-one
d)
3-methyl-4-hexen-2-one

|
Maulik Verma answered |
It is an α, β-unsaturated ketone which can be formed in an aldol condensation followed by dehydration.
Predict the compounds A and B in the following reactions: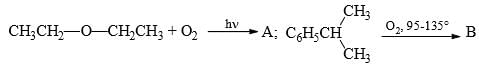
- a)
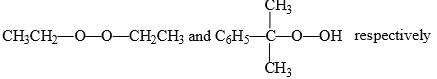
- b)
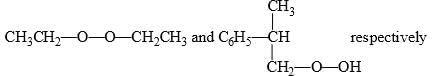
- c)
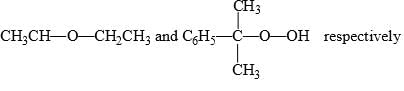
- d)
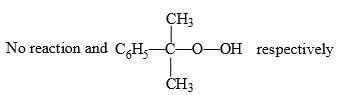
Correct answer is option 'C'. Can you explain this answer?
Predict the compounds A and B in the following reactions:

a)
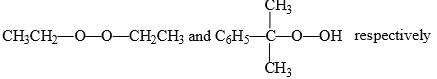
b)
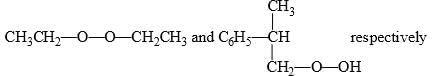
c)

d)


|
Asf Institute answered |
Option C correct. Due to Photochemical conditions O-O bond of Oxygen will be cleaved.
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/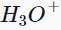 ?
?
- a)4-heptanone
- b)Ethyl benzoate
- c)4,4-dimethylcyclohexanone
- d)3-methylpentanal
Correct answer is option 'D'. Can you explain this answer?
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/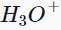 ?
?
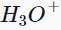 ?
?a)
4-heptanone
b)
Ethyl benzoate
c)
4,4-dimethylcyclohexanone
d)
3-methylpentanal

|
Ameya Reddy answered |
Reaction of Methylmagnesium bromide with Carbonyl Compounds
Carbonyl compounds contain a carbonyl group (C=O) and can undergo nucleophilic addition reactions with nucleophiles such as methylmagnesium bromide (CH3MgBr) to form alcohols. The reaction proceeds through the formation of an unstable intermediate called an alkoxy magnesium halide, which is then hydrolyzed to form the alcohol.
Reaction of Methylmagnesium bromide with 4-Heptanone
4-heptanone is a ketone with the formula C7H14O. When it is treated with methylmagnesium bromide, a Grignard reagent, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C7H14O + CH3MgBr → C7H13OMgBr + CH4
C7H13OMgBr + H2O → C7H16O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2,3,3-tetramethylbutan-2-ol.
Reaction of Methylmagnesium bromide with 3-Methylpentanal
3-methylpentanal is an aldehyde with the formula C6H12O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C6H12O + CH3MgBr → C6H11OMgBr + CH4
C6H11OMgBr + H2O → C6H14O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2-dimethyl-3-pentanol.
Reaction of Methylmagnesium bromide with Ethyl benzoate
Ethyl benzoate is an ester with the formula C9H10O2. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C9H10O2 + CH3MgBr → C9H9OMgBr + C2H5Br
C9H9OMgBr + H2O → C9H12O2 + MgBrOH
The alcohol formed is a tertiary alcohol, 2-phenylethanol.
Reaction of Methylmagnesium bromide with 4,4-Dimethylcyclohexanone
4,4-dimethylcyclohexanone is a ketone with the formula C10H18O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C10H18O + CH3MgBr → C10H17OMgBr + CH4
C10H17OMgBr + H2O → C10H20O + MgBrOH
The alcohol formed is a tertiary alcohol, 4
Carbonyl compounds contain a carbonyl group (C=O) and can undergo nucleophilic addition reactions with nucleophiles such as methylmagnesium bromide (CH3MgBr) to form alcohols. The reaction proceeds through the formation of an unstable intermediate called an alkoxy magnesium halide, which is then hydrolyzed to form the alcohol.
Reaction of Methylmagnesium bromide with 4-Heptanone
4-heptanone is a ketone with the formula C7H14O. When it is treated with methylmagnesium bromide, a Grignard reagent, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C7H14O + CH3MgBr → C7H13OMgBr + CH4
C7H13OMgBr + H2O → C7H16O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2,3,3-tetramethylbutan-2-ol.
Reaction of Methylmagnesium bromide with 3-Methylpentanal
3-methylpentanal is an aldehyde with the formula C6H12O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C6H12O + CH3MgBr → C6H11OMgBr + CH4
C6H11OMgBr + H2O → C6H14O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2-dimethyl-3-pentanol.
Reaction of Methylmagnesium bromide with Ethyl benzoate
Ethyl benzoate is an ester with the formula C9H10O2. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C9H10O2 + CH3MgBr → C9H9OMgBr + C2H5Br
C9H9OMgBr + H2O → C9H12O2 + MgBrOH
The alcohol formed is a tertiary alcohol, 2-phenylethanol.
Reaction of Methylmagnesium bromide with 4,4-Dimethylcyclohexanone
4,4-dimethylcyclohexanone is a ketone with the formula C10H18O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C10H18O + CH3MgBr → C10H17OMgBr + CH4
C10H17OMgBr + H2O → C10H20O + MgBrOH
The alcohol formed is a tertiary alcohol, 4
Which of the following compounds is not formed in iodoform reaction of acetone?- a)CH3COCH2I
- b)CH3COCHI2
- c)CH3COCl3
- d)ICH2COCH2I
Correct answer is option 'D'. Can you explain this answer?
Which of the following compounds is not formed in iodoform reaction of acetone?
a)
CH3COCH2I
b)
CH3COCHI2
c)
CH3COCl3
d)
ICH2COCH2I

|
Saranya Mehta answered |
In the iodoform reaction, acetone (CH3COCH3) is treated with iodine and a base to form iodoform (CHI3) and an alkoxide ion. The reaction proceeds through a series of steps, starting with the formation of an enolate ion and followed by halogenation and deprotonation. Let's analyze each option to determine which compound is not formed in the iodoform reaction of acetone.
a) CH3COCH2I
In the iodoform reaction, the enolate ion of acetone is halogenated. In this case, the iodine atom replaces one of the hydrogen atoms, resulting in the formation of CH3COCH2I. This compound can be formed in the iodoform reaction, so option a) is not the correct answer.
b) CH3COCHI2
Similar to option a), in the iodoform reaction, the iodine atom can replace two hydrogen atoms on acetone, resulting in the formation of CH3COCHI2. This compound can also be formed in the iodoform reaction, so option b) is not the correct answer.
c) CH3COCl3
In the iodoform reaction, the iodine atom is required for the halogenation step. Chlorine atoms cannot replace hydrogen atoms in acetone during the reaction. Therefore, CH3COCl3 is not formed in the iodoform reaction. This compound is the correct answer.
d) ICH2COCH2I
This compound is not directly formed in the iodoform reaction of acetone. The iodoform reaction involves the substitution of iodine atoms for hydrogen atoms. In this case, there are no hydrogen atoms adjacent to the carbonyl group. Therefore, this compound is not formed in the iodoform reaction.
a) CH3COCH2I
In the iodoform reaction, the enolate ion of acetone is halogenated. In this case, the iodine atom replaces one of the hydrogen atoms, resulting in the formation of CH3COCH2I. This compound can be formed in the iodoform reaction, so option a) is not the correct answer.
b) CH3COCHI2
Similar to option a), in the iodoform reaction, the iodine atom can replace two hydrogen atoms on acetone, resulting in the formation of CH3COCHI2. This compound can also be formed in the iodoform reaction, so option b) is not the correct answer.
c) CH3COCl3
In the iodoform reaction, the iodine atom is required for the halogenation step. Chlorine atoms cannot replace hydrogen atoms in acetone during the reaction. Therefore, CH3COCl3 is not formed in the iodoform reaction. This compound is the correct answer.
d) ICH2COCH2I
This compound is not directly formed in the iodoform reaction of acetone. The iodoform reaction involves the substitution of iodine atoms for hydrogen atoms. In this case, there are no hydrogen atoms adjacent to the carbonyl group. Therefore, this compound is not formed in the iodoform reaction.
Alkyl halides can be converted into Grignard reagents by _______________- a)Boiling them with Mg ribbon in alcoholic solution
- b)Warming them with magnesium powder in dry ether
- c)Refluxing them with MgCl2 solution
- d)Warming them with Mgcl2
Correct answer is option 'B'. Can you explain this answer?
Alkyl halides can be converted into Grignard reagents by _______________
a)
Boiling them with Mg ribbon in alcoholic solution
b)
Warming them with magnesium powder in dry ether
c)
Refluxing them with MgCl2 solution
d)
Warming them with Mgcl2

|
Pioneer Academy answered |
Alkyl halides can be converted into Grignard reagents by warming them with magnesium powder in dry ether.
RX + Mg + Dry ether → R−Mg−X
RX + Mg + Dry ether → R−Mg−X
1-Methylcyclopentene can be converted into 2-methylcyclopentanol by:- a)Acid-catalyzed hydration
- b)Hydroboration oxidation
- c)Epoxide formation followed by reduction with LiAlH4
- d)Oxymercuration-demercuration
Correct answer is option 'B'. Can you explain this answer?
1-Methylcyclopentene can be converted into 2-methylcyclopentanol by:
a)
Acid-catalyzed hydration
b)
Hydroboration oxidation
c)
Epoxide formation followed by reduction with LiAlH4
d)
Oxymercuration-demercuration

|
Rishabh Mehta answered |
Conversion of 1-Methylcyclopentene into 2-methylcyclopentanol
Hydroboration-oxidation is a reaction in which an alkene reacts with borane (BH3) to form a trialkylborane. This trialkylborane then reacts with hydrogen peroxide (H2O2) and a base to form an alcohol. This reaction is used to convert alkenes into alcohols.
Steps involved in the conversion of 1-Methylcyclopentene into 2-methylcyclopentanol are as follows:
Step 1: Hydroboration of 1-Methylcyclopentene
1-Methylcyclopentene reacts with borane (BH3) to form trialkylborane.
Step 2: Oxidation of trialkylborane
Trialkylborane reacts with hydrogen peroxide (H2O2) and a base to form an alcohol.
2-methylcyclopentanol is obtained as a product of the reaction.
Thus, the correct answer is option 'B' that is hydroboration oxidation.
Hydroboration-oxidation is a reaction in which an alkene reacts with borane (BH3) to form a trialkylborane. This trialkylborane then reacts with hydrogen peroxide (H2O2) and a base to form an alcohol. This reaction is used to convert alkenes into alcohols.
Steps involved in the conversion of 1-Methylcyclopentene into 2-methylcyclopentanol are as follows:
Step 1: Hydroboration of 1-Methylcyclopentene
1-Methylcyclopentene reacts with borane (BH3) to form trialkylborane.
Step 2: Oxidation of trialkylborane
Trialkylborane reacts with hydrogen peroxide (H2O2) and a base to form an alcohol.
2-methylcyclopentanol is obtained as a product of the reaction.
Thus, the correct answer is option 'B' that is hydroboration oxidation.
Which of the following does not reduce C6H5NO2 to aniline:- a)Sn/HCl
- b)SnCl2/HCl
- c)Zn/HCl
- d)LiAlH4
Correct answer is option 'B'. Can you explain this answer?
Which of the following does not reduce C6H5NO2 to aniline:
a)
Sn/HCl
b)
SnCl2/HCl
c)
Zn/HCl
d)
LiAlH4

|
Rashi Choudhury answered |
Explanation:
Reduction of nitrobenzene (C6H5NO2) to aniline (C6H5NH2) can be done with various reducing agents. Among the given options, all except SnCl2/HCl can reduce nitrobenzene to aniline.
a) Sn/HCl: Tin in hydrochloric acid reduces nitrobenzene to aniline.
b) SnCl2/HCl: This reducing agent cannot reduce nitrobenzene to aniline. Instead, it reduces it to azoxybenzene (C6H5N2O2).
c) Zn/HCl: Zinc in hydrochloric acid reduces nitrobenzene to aniline.
d) LiAlH4: Lithium aluminum hydride reduces nitrobenzene to aniline.
Conclusion:
Among the given options, SnCl2/HCl does not reduce nitrobenzene to aniline. It reduces it to azoxybenzene (C6H5N2O2).
Reduction of nitrobenzene (C6H5NO2) to aniline (C6H5NH2) can be done with various reducing agents. Among the given options, all except SnCl2/HCl can reduce nitrobenzene to aniline.
a) Sn/HCl: Tin in hydrochloric acid reduces nitrobenzene to aniline.
b) SnCl2/HCl: This reducing agent cannot reduce nitrobenzene to aniline. Instead, it reduces it to azoxybenzene (C6H5N2O2).
c) Zn/HCl: Zinc in hydrochloric acid reduces nitrobenzene to aniline.
d) LiAlH4: Lithium aluminum hydride reduces nitrobenzene to aniline.
Conclusion:
Among the given options, SnCl2/HCl does not reduce nitrobenzene to aniline. It reduces it to azoxybenzene (C6H5N2O2).
What could be reagents A and B for the following reactions:
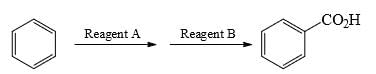
- a) Reagent A: CH3CH2Cl/ AlCl3; reagent B: Na2Cr2O7, heat
- b) Reagent A: CH3COCl/ AlCl3; reagent B: Na2Cr2O7, heat
- c) Reagent A: HNO3,H2SO4; reagent B: RCO3H, heat
- d) Reagent A: CH3COCl/ AlCl3; reagent B: H2, Ni, heat
Correct answer is option 'A'. Can you explain this answer?
What could be reagents A and B for the following reactions:
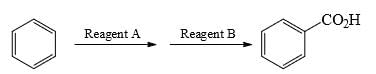
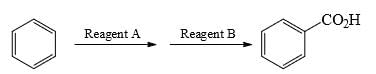
a)
Reagent A: CH3CH2Cl/ AlCl3; reagent B: Na2Cr2O7, heat
b)
Reagent A: CH3COCl/ AlCl3; reagent B: Na2Cr2O7, heat
c)
Reagent A: HNO3,H2SO4; reagent B: RCO3H, heat
d)
Reagent A: CH3COCl/ AlCl3; reagent B: H2, Ni, heat
|
|
Subha Som answered |
Cr2O7 2- strong oxidizing agent it can oxidized alkyl gr to carboxyl
A Grignard’s reagent may be made by reacting magnesium with which of the following compound?- a)Methyl amine
- b)Diethyl ether
- c)Ethyl iodide
- d)Ethyl alcohol
Correct answer is option 'C'. Can you explain this answer?
A Grignard’s reagent may be made by reacting magnesium with which of the following compound?
a)
Methyl amine
b)
Diethyl ether
c)
Ethyl iodide
d)
Ethyl alcohol

|
Asf Institute answered |
A Grignard’s reagent can be formed by reacting magnesium with Ethyl iodide, as shown below;
C2H5I + Mg + Dryether → C2H5 − Mg − I (Ethylmagnesium iodide)
C2H5I + Mg + Dryether → C2H5 − Mg − I (Ethylmagnesium iodide)
How acetophenone can be converted to phenol by reaction?- a)m-CPBA followed by base catalysed hydrolysis
- b)conc. HNO3
- c)iodine and NaOH
- d)Singlet oxygen followed by base catalysed hydrolysis product
Correct answer is option 'A'. Can you explain this answer?
How acetophenone can be converted to phenol by reaction?
a)
m-CPBA followed by base catalysed hydrolysis
b)
conc. HNO3
c)
iodine and NaOH
d)
Singlet oxygen followed by base catalysed hydrolysis product

|
Pioneer Academy answered |


m-CPBA can convert ketone into amide and after ester hydrolysis it is reduced into phenol.
How can we prepare RR’ R” OH by the action of excess of a suitable Grignard reagent on which of the following reactants?
- a)ketone or an ester
- b)ester or an alcohol
- c)aldehyde or a ketone
- d)nitrile or an aldehyde
Correct answer is option 'A'. Can you explain this answer?
How can we prepare RR’ R” OH by the action of excess of a suitable Grignard reagent on which of the following reactants?
a)
ketone or an ester
b)
ester or an alcohol
c)
aldehyde or a ketone
d)
nitrile or an aldehyde
|
|
Biswa Nath answered |
The answer will be D not A
Which of the following structure is more stable:
- a)
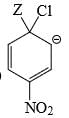
- b)
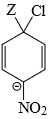
- c)
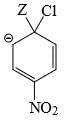
- d)
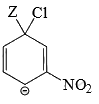
Correct answer is option 'B'. Can you explain this answer?
Which of the following structure is more stable:
a)

b)

c)

d)

|
|
Pooja Choudhury answered |
The negative charge is delocalized and NO2 shows -I and -M effect and hence stabilizes -ve charge.
Which of the following is most reactive towards aqueous HBr:a. 1- Phenyl-2-propanolb. 1-Phenyl-1-propanolc. 3-Phenyl-1-propanold. 2-Phenyl-1-propanolCorrect answer is option 'B'. Can you explain this answer?

|
Siddharth Majumdar answered |
Reactivity towards Aqueous HBr
To determine which compound is most reactive towards aqueous HBr, we need to consider the stability of the carbocation intermediates that are formed during the reaction. The more stable the carbocation, the more reactive the compound will be towards HBr.
Stability of Carbocation Intermediates
To determine the stability of the carbocation intermediates, we need to consider the following factors:
- Resonance: Compounds that can form resonance structures with the positive charge are more stable.
- Inductive effects: Compounds with electron-donating groups near the positive charge are more stable.
- Steric hindrance: Compounds with bulky groups near the positive charge are less stable.
Based on these factors, we can rank the stability of the carbocation intermediates in the following order:
3-Phenyl-1-propanol > 2-Phenyl-1-propanol > 1-Phenyl-1-propanol > 1-Phenyl-2-propanol
Therefore, 1-Phenyl-1-propanol is the most reactive towards aqueous HBr as it forms the least stable carbocation intermediate.
Conclusion
In conclusion, the stability of the carbocation intermediates determines the reactivity of compounds towards aqueous HBr. 1-Phenyl-1-propanol is the most reactive towards HBr as it forms the least stable carbocation intermediate.
To determine which compound is most reactive towards aqueous HBr, we need to consider the stability of the carbocation intermediates that are formed during the reaction. The more stable the carbocation, the more reactive the compound will be towards HBr.
Stability of Carbocation Intermediates
To determine the stability of the carbocation intermediates, we need to consider the following factors:
- Resonance: Compounds that can form resonance structures with the positive charge are more stable.
- Inductive effects: Compounds with electron-donating groups near the positive charge are more stable.
- Steric hindrance: Compounds with bulky groups near the positive charge are less stable.
Based on these factors, we can rank the stability of the carbocation intermediates in the following order:
3-Phenyl-1-propanol > 2-Phenyl-1-propanol > 1-Phenyl-1-propanol > 1-Phenyl-2-propanol
Therefore, 1-Phenyl-1-propanol is the most reactive towards aqueous HBr as it forms the least stable carbocation intermediate.
Conclusion
In conclusion, the stability of the carbocation intermediates determines the reactivity of compounds towards aqueous HBr. 1-Phenyl-1-propanol is the most reactive towards HBr as it forms the least stable carbocation intermediate.
The compound having the formula, 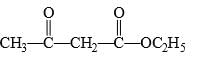
- a)Forms dioxime
- b)Undergoes Iodoform test
- c)Both (a) and (b)
- d)Neither of the two
Correct answer is option 'B'. Can you explain this answer?
The compound having the formula, 

a)
Forms dioxime
b)
Undergoes Iodoform test
c)
Both (a) and (b)
d)
Neither of the two

|
Sarbajeet Chakraborty answered |
It gives iodoform test successfully becoz it has active methylene group
Which of the following statement is true regarding amount of AlCl3 required during Friedel-Craft acetylation using acetyl chloride or acetic anhydride:- a)Both require same amount
- b)Acetylation with acetyl chloride requires more amount
- c)Acetylation with acetic anhydride requires more amount
- d)Nothing is definite
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is true regarding amount of AlCl3 required during Friedel-Craft acetylation using acetyl chloride or acetic anhydride:
a)
Both require same amount
b)
Acetylation with acetyl chloride requires more amount
c)
Acetylation with acetic anhydride requires more amount
d)
Nothing is definite
|
|
Sakshi Tiwari answered |
A
What will be the product for the given reactant and reagents?
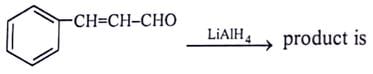
- a)
- b)
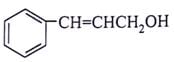
- c)
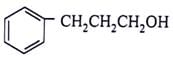
- d)
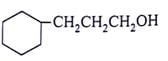
Correct answer is option 'C'. Can you explain this answer?
What will be the product for the given reactant and reagents?


a)
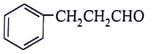
b)

c)
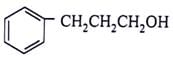
d)
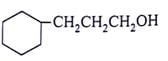

|
Pioneer Academy answered |
LiAlH4 will reduce double bond into single bond and aldehyde group into alcohol.
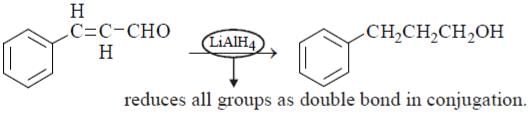

Which of the following carbonyl compounds does not give 2-methylpentan-2-ol upon reaction with methylmagnesium iodide in ether followed by a workup with aqueous ammonium chloride?- a)pentan-2-one
- b)b methyl propyl ketone
- c)ethyl butanoate
- d)ethyl 2-methylbutanoate
Correct answer is option 'D'. Can you explain this answer?
Which of the following carbonyl compounds does not give 2-methylpentan-2-ol upon reaction with methylmagnesium iodide in ether followed by a workup with aqueous ammonium chloride?
a)
pentan-2-one
b)
b methyl propyl ketone
c)
ethyl butanoate
d)
ethyl 2-methylbutanoate

|
Pranavi Mishra answered |
Carbonyl compounds are organic compounds that contain a carbonyl functional group (-C=O). When reacted with methylmagnesium iodide in ether followed by a workup with aqueous ammonium chloride, they form alcohols.
Explanation:
The reaction of carbonyl compounds with methylmagnesium iodide in ether is a nucleophilic addition reaction. The nucleophile attacks the electrophilic carbon of the carbonyl group, forming an alkoxide intermediate. This intermediate then reacts with a proton from the solvent (ether), forming an alcohol.
The reaction can be represented as follows:
Carbonyl compound + MeMgI in ether → alkoxide intermediate → alcohol
In this specific case, the carbonyl compounds given are:
a) pentan-2-one
b) 3-methylbutan-2-one
c) ethyl butanoate
d) ethyl 2-methylbutanoate
Out of these, only option d) ethyl 2-methylbutanoate will not give 2-methylpentan-2-ol upon reaction with methylmagnesium iodide in ether followed by a workup with aqueous ammonium chloride.
The reason for this is that ethyl 2-methylbutanoate has a bulky ethyl group attached to the carbonyl carbon, which makes it less reactive towards nucleophilic attack. This results in a slower reaction rate and a lower yield of the desired alcohol product.
In contrast, the other three carbonyl compounds have simpler alkyl groups attached to the carbonyl carbon, which makes them more reactive towards nucleophilic attack. This results in a faster reaction rate and a higher yield of the desired alcohol product (2-methylpentan-2-ol).
Explanation:
The reaction of carbonyl compounds with methylmagnesium iodide in ether is a nucleophilic addition reaction. The nucleophile attacks the electrophilic carbon of the carbonyl group, forming an alkoxide intermediate. This intermediate then reacts with a proton from the solvent (ether), forming an alcohol.
The reaction can be represented as follows:
Carbonyl compound + MeMgI in ether → alkoxide intermediate → alcohol
In this specific case, the carbonyl compounds given are:
a) pentan-2-one
b) 3-methylbutan-2-one
c) ethyl butanoate
d) ethyl 2-methylbutanoate
Out of these, only option d) ethyl 2-methylbutanoate will not give 2-methylpentan-2-ol upon reaction with methylmagnesium iodide in ether followed by a workup with aqueous ammonium chloride.
The reason for this is that ethyl 2-methylbutanoate has a bulky ethyl group attached to the carbonyl carbon, which makes it less reactive towards nucleophilic attack. This results in a slower reaction rate and a lower yield of the desired alcohol product.
In contrast, the other three carbonyl compounds have simpler alkyl groups attached to the carbonyl carbon, which makes them more reactive towards nucleophilic attack. This results in a faster reaction rate and a higher yield of the desired alcohol product (2-methylpentan-2-ol).
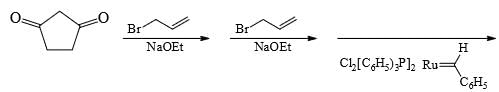
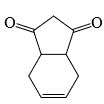
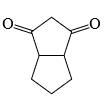
 A is optically active and C is one of the several aldol possible in the above reaction.Q. The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?
A is optically active and C is one of the several aldol possible in the above reaction.Q. The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?- a)2
- b)4
- c)6
- d)8
Correct answer is option 'D'. Can you explain this answer?

A is optically active and C is one of the several aldol possible in the above reaction.
Q.
The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?
a)
2
b)
4
c)
6
d)
8

|
Niti Saha answered |
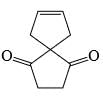
The major product formed in the following reaction is:
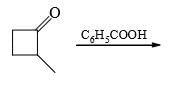
- a)
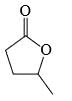
- b)
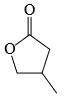
- c)
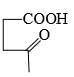
- d)
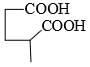
Correct answer is option 'A'. Can you explain this answer?
The major product formed in the following reaction is:


a)

b)

c)

d)


|
Saurabh Chavan answered |
Reagent is incorrect. it should be peracid. then give beayer villager reaction.
Which of the following gives effervescences of CO2 with NaHCO3 solution:- a)HCOOH
- b)2,4,6-trinitrophenol
- c)Both (a) and (b)
- d)None of these.
Correct answer is option 'C'. Can you explain this answer?
Which of the following gives effervescences of CO2 with NaHCO3 solution:
a)
HCOOH
b)
2,4,6-trinitrophenol
c)
Both (a) and (b)
d)
None of these.

|
Amar Chawla answered |
**Answer:**
The correct answer is option 'C', which means both HCOOH and 2,4,6-trinitrophenol give effervescences of CO2 with NaHCO3 solution.
**Explanation:**
When NaHCO3 (sodium bicarbonate) is added to a solution containing a compound that can release CO2, effervescence occurs. Let's analyze each option to see if they can release CO2 when reacted with NaHCO3.
a) HCOOH (formic acid):
Formic acid, HCOOH, is a weak acid. When it reacts with NaHCO3, it undergoes a neutralization reaction, resulting in the formation of sodium formate (NaHCOO) and the release of carbon dioxide gas (CO2).
HCOOH + NaHCO3 → NaHCOO + CO2 + H2O
b) 2,4,6-trinitrophenol (picric acid):
2,4,6-trinitrophenol, also known as picric acid, is a strong acid. Similar to formic acid, it can undergo a neutralization reaction with NaHCO3, resulting in the formation of sodium picrate (NaC6H2(NO2)3O7) and the release of carbon dioxide gas (CO2).
C6H2(NO2)3OH + NaHCO3 → NaC6H2(NO2)3O7 + CO2 + H2O
Therefore, both HCOOH and 2,4,6-trinitrophenol can release CO2 when reacted with NaHCO3 solution. Hence, option 'C' is the correct answer.
The correct answer is option 'C', which means both HCOOH and 2,4,6-trinitrophenol give effervescences of CO2 with NaHCO3 solution.
**Explanation:**
When NaHCO3 (sodium bicarbonate) is added to a solution containing a compound that can release CO2, effervescence occurs. Let's analyze each option to see if they can release CO2 when reacted with NaHCO3.
a) HCOOH (formic acid):
Formic acid, HCOOH, is a weak acid. When it reacts with NaHCO3, it undergoes a neutralization reaction, resulting in the formation of sodium formate (NaHCOO) and the release of carbon dioxide gas (CO2).
HCOOH + NaHCO3 → NaHCOO + CO2 + H2O
b) 2,4,6-trinitrophenol (picric acid):
2,4,6-trinitrophenol, also known as picric acid, is a strong acid. Similar to formic acid, it can undergo a neutralization reaction with NaHCO3, resulting in the formation of sodium picrate (NaC6H2(NO2)3O7) and the release of carbon dioxide gas (CO2).
C6H2(NO2)3OH + NaHCO3 → NaC6H2(NO2)3O7 + CO2 + H2O
Therefore, both HCOOH and 2,4,6-trinitrophenol can release CO2 when reacted with NaHCO3 solution. Hence, option 'C' is the correct answer.
In which of the following compounds, enol form exist? - a)C6H5COCH3
- b)C6H5CHO
- c)

- d)Both (a) and (c)
Correct answer is option 'D'. Can you explain this answer?
In which of the following compounds, enol form exist?
a)
C6H5COCH3
b)
C6H5CHO
c)
d)
Both (a) and (c)
|
|
Preeti Khanna answered |
Both option (a) and option (c) forms enol but option (b) does not form enol.
Which is not present in Grignard reagent?- a)Methyl group
- b)Magnesium
- c)Halogen
- d)−COOH group
Correct answer is option 'D'. Can you explain this answer?
Which is not present in Grignard reagent?
a)
Methyl group
b)
Magnesium
c)
Halogen
d)
−COOH group
|
|
Vivek Khatri answered |
Grignard reagents are made from halogenoalkanes (haloalkanes or alkyl halides) and introduces some of their reactions. A Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on a benzene ring) group.
Which of the following compounds gives a primary alcohol upon reaction with phenylmagnesium bromide?- a)2-methyloxirane
- b)ethylene oxide
- c)ethyl formate
- d)carbon dioxide
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds gives a primary alcohol upon reaction with phenylmagnesium bromide?
a)
2-methyloxirane
b)
ethylene oxide
c)
ethyl formate
d)
carbon dioxide

|
Ishaan Sengupta answered |
Primary alcohols are formed when a Grignard reagent reacts with an epoxide. In this case, the compound that gives a primary alcohol upon reaction with phenylmagnesium bromide is ethylene oxide.
- Epoxides are cyclic ethers with a three-membered ring containing an oxygen atom.
- Phenylmagnesium bromide (C6H5MgBr) is a Grignard reagent that contains a carbon-magnesium bond.
- Grignard reagents are highly reactive and can undergo nucleophilic addition reactions with a variety of electrophilic compounds.
- When phenylmagnesium bromide reacts with ethylene oxide, the oxygen atom of the epoxide is nucleophilically attacked by the carbon atom of the Grignard reagent.
- This results in the opening of the epoxide ring and the formation of a new carbon-oxygen bond.
- The product of this reaction is a primary alcohol, which has a hydroxyl group (-OH) directly attached to a carbon atom that is bonded to only one other carbon atom.
- In the case of ethylene oxide, the reaction with phenylmagnesium bromide would result in the formation of 1-phenylethanol.
- The hydroxyl group (-OH) is directly attached to the carbon atom that was previously part of the epoxide ring, which makes it a primary alcohol.
- The other compounds listed, 2-methyloxirane, ethyl formate, and carbon dioxide, do not have a carbon atom that can react with the Grignard reagent to form a primary alcohol.
- Therefore, only ethylene oxide gives a primary alcohol upon reaction with phenylmagnesium bromide.
- Epoxides are cyclic ethers with a three-membered ring containing an oxygen atom.
- Phenylmagnesium bromide (C6H5MgBr) is a Grignard reagent that contains a carbon-magnesium bond.
- Grignard reagents are highly reactive and can undergo nucleophilic addition reactions with a variety of electrophilic compounds.
- When phenylmagnesium bromide reacts with ethylene oxide, the oxygen atom of the epoxide is nucleophilically attacked by the carbon atom of the Grignard reagent.
- This results in the opening of the epoxide ring and the formation of a new carbon-oxygen bond.
- The product of this reaction is a primary alcohol, which has a hydroxyl group (-OH) directly attached to a carbon atom that is bonded to only one other carbon atom.
- In the case of ethylene oxide, the reaction with phenylmagnesium bromide would result in the formation of 1-phenylethanol.
- The hydroxyl group (-OH) is directly attached to the carbon atom that was previously part of the epoxide ring, which makes it a primary alcohol.
- The other compounds listed, 2-methyloxirane, ethyl formate, and carbon dioxide, do not have a carbon atom that can react with the Grignard reagent to form a primary alcohol.
- Therefore, only ethylene oxide gives a primary alcohol upon reaction with phenylmagnesium bromide.
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aldol condensation is an important reaction in organic chemistry, particularly for the formation of carbon-carbon bonds. This reaction typically involves the enolate ion derived from an aldehyde or ketone reacting with another carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone. This product can undergo dehydration to form an α,β-unsaturated carbonyl compound. The reaction is usually catalyzed by a base, although acid-catalyzed versions are also known. The presence of at least one α-hydrogen in the reactant is crucial for the aldol condensation to occur. Acetaldehyde, for example, can undergo aldol condensation to yield 3-hydroxybutanal, which can then dehydrate to form crotonaldehyde.
Ques: In the aldol condensation reaction of acetaldehyde (CH₃CHO), the initial product formed before dehydration is:
- a)2-Hydroxybutanal
- b)Crotonaldehyde
- c)3-Hydroxybutanal
- d)Acetone
Correct answer is option 'C'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aldol condensation is an important reaction in organic chemistry, particularly for the formation of carbon-carbon bonds. This reaction typically involves the enolate ion derived from an aldehyde or ketone reacting with another carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone. This product can undergo dehydration to form an α,β-unsaturated carbonyl compound. The reaction is usually catalyzed by a base, although acid-catalyzed versions are also known. The presence of at least one α-hydrogen in the reactant is crucial for the aldol condensation to occur. Acetaldehyde, for example, can undergo aldol condensation to yield 3-hydroxybutanal, which can then dehydrate to form crotonaldehyde.
Ques: In the aldol condensation reaction of acetaldehyde (CH₃CHO), the initial product formed before dehydration is:
a)
2-Hydroxybutanal
b)
Crotonaldehyde
c)
3-Hydroxybutanal
d)
Acetone
|
|
Amar Choudhary answered |
Understanding Aldol Condensation
Aldol condensation is a significant reaction in organic chemistry that allows for the formation of carbon-carbon bonds. When acetaldehyde is involved, the process can be broken down into key steps.
Formation of Enolate Ion
- The reaction begins with the formation of an enolate ion from acetaldehyde, which has at least one α-hydrogen.
- This enolate ion acts as a nucleophile, attacking another molecule of acetaldehyde.
Initial Product Formation
- The nucleophilic attack leads to the formation of a β-hydroxy aldehyde.
- In the case of acetaldehyde, the product formed is specifically 3-hydroxybutanal.
- This product has a hydroxyl group (-OH) and a carbonyl group (C=O) in its structure.
Dehydration Step
- The β-hydroxy aldehyde (3-hydroxybutanal) can undergo dehydration, resulting in the formation of an α,β-unsaturated carbonyl compound, which is crotonaldehyde.
- However, the question specifically asks for the product formed before this dehydration occurs.
Conclusion
- Therefore, the correct answer to the question is option 'C' - 3-hydroxybutanal, which is the initial product prior to dehydration in the aldol condensation of acetaldehyde.
Aldol condensation is a significant reaction in organic chemistry that allows for the formation of carbon-carbon bonds. When acetaldehyde is involved, the process can be broken down into key steps.
Formation of Enolate Ion
- The reaction begins with the formation of an enolate ion from acetaldehyde, which has at least one α-hydrogen.
- This enolate ion acts as a nucleophile, attacking another molecule of acetaldehyde.
Initial Product Formation
- The nucleophilic attack leads to the formation of a β-hydroxy aldehyde.
- In the case of acetaldehyde, the product formed is specifically 3-hydroxybutanal.
- This product has a hydroxyl group (-OH) and a carbonyl group (C=O) in its structure.
Dehydration Step
- The β-hydroxy aldehyde (3-hydroxybutanal) can undergo dehydration, resulting in the formation of an α,β-unsaturated carbonyl compound, which is crotonaldehyde.
- However, the question specifically asks for the product formed before this dehydration occurs.
Conclusion
- Therefore, the correct answer to the question is option 'C' - 3-hydroxybutanal, which is the initial product prior to dehydration in the aldol condensation of acetaldehyde.
Nitrobenzene can be reduced to aniline by:(I) H2/Ni(II) Sn/HCl(III) Zn/NaOH(IV) LiAlH4- a)I, II and III
- b)I and II
- c) I, II and IV
- d)Only IV
Correct answer is option 'B'. Can you explain this answer?
Nitrobenzene can be reduced to aniline by:
(I) H2/Ni
(II) Sn/HCl
(III) Zn/NaOH
(IV) LiAlH4
a)
I, II and III
b)
I and II
c)
I, II and IV
d)
Only IV

|
Niharika Kulkarni answered |
Correct Answer :- B
Explanation : When nitrobenzene is reduced with a metal and acid (Sn/HCl, Zn/HCl etc.), aniline is obtained. The intermediate products nitrosobenzene and phenylhydroxylamine are never isolated because these are reduced more rapidly than nitrobenzene.
However, the catalytic hydrogenation of nitrobenzene gives aniline.
PhNO2 (H2+Ni)------------->PhNH2
Azobenzene is formed when nitrobenzene is reduced by LiAlH4.
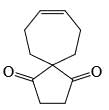
Choose the reaction sequence that could be used to perform the following transformation: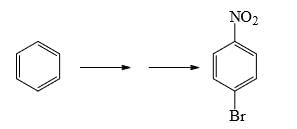
- a)
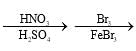
- b)
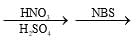
- c)

- d)
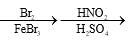
Correct answer is option 'D'. Can you explain this answer?
Choose the reaction sequence that could be used to perform the following transformation:

a)
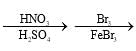
b)
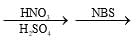
c)

d)

|
|
Nayak Satyaprakash Shyam answered |
Br has +m effect due to which ortho and para position available for No2
Fehling’s solution can be used for distinguishing between:- a)CH3CHO and C6H5CHO
- b)CH3CHO and CH3COCH2OH
- c)Both (a) and (b)
- d)None of these.
Correct answer is option 'A'. Can you explain this answer?
Fehling’s solution can be used for distinguishing between:
a)
CH3CHO and C6H5CHO
b)
CH3CHO and CH3COCH2OH
c)
Both (a) and (b)
d)
None of these.

|
Anisha Banerjee answered |
Fehling refers to a chemical reagent known as Fehling's solution, which is used to test for the presence of reducing sugars in a solution. It consists of two separate solutions, Fehling's A and Fehling's B, which are mixed together before use. Fehling's A contains copper(II) sulfate, while Fehling's B contains sodium potassium tartrate and sodium hydroxide.
When heated with a reducing sugar, such as glucose or fructose, in the presence of Fehling's solution, a redox reaction occurs. The reducing sugar is oxidized, while the copper(II) ions in Fehling's A are reduced to copper(I) oxide, which forms a brick-red precipitate. This precipitate indicates the presence of a reducing sugar in the solution.
Fehling's solution is commonly used in laboratory settings to identify and quantify reducing sugars in various samples, such as food products or biological fluids. It is particularly useful for detecting sugars like glucose, fructose, and lactose.
When heated with a reducing sugar, such as glucose or fructose, in the presence of Fehling's solution, a redox reaction occurs. The reducing sugar is oxidized, while the copper(II) ions in Fehling's A are reduced to copper(I) oxide, which forms a brick-red precipitate. This precipitate indicates the presence of a reducing sugar in the solution.
Fehling's solution is commonly used in laboratory settings to identify and quantify reducing sugars in various samples, such as food products or biological fluids. It is particularly useful for detecting sugars like glucose, fructose, and lactose.
Which is the mildest reducing agent which reduces only carbonyl group in presence of nitro, carboxyl, double bond and ester groups?- a)LiAIH4
- b)Na-NH3
- c)NaBH4
- d)H2-Ni
Correct answer is option 'C'. Can you explain this answer?
Which is the mildest reducing agent which reduces only carbonyl group in presence of nitro, carboxyl, double bond and ester groups?
a)
LiAIH4
b)
Na-NH3
c)
NaBH4
d)
H2-Ni
|
|
Vivek Khatri answered |
NaBH4 is mild reducing agent for this conversion. It is very effective for the reduction of aldehydes and ketones to alcohols. By itself, it will generally not reduce esters, carboxylic acids, or amides (although it will reduce acyl chlorides to alcohols).
Which can be deduced correctly regarding keto-enol tautomerism in general?- a)Increasing temperature increases the enol content at equilibrium
- b)Mono-enols are usually more stable than dienols
- c)Enols of ketones are generally more stable than enols of aliphatic aldehydes
- d)Keto-enol taytomerism is catalysed by both acidic and basic catalys
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which can be deduced correctly regarding keto-enol tautomerism in general?
a)
Increasing temperature increases the enol content at equilibrium
b)
Mono-enols are usually more stable than dienols
c)
Enols of ketones are generally more stable than enols of aliphatic aldehydes
d)
Keto-enol taytomerism is catalysed by both acidic and basic catalys

|
Ishita Deshpande answered |
Increasing temperature increases equilibrium content of less stable enol tautomers.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Which of the following reagents, when treated with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol?
- a)Ethanol
- b)Diethyl ether
- c)Ethanal
- d)Oxirane
Correct answer is option 'D'. Can you explain this answer?
Which of the following reagents, when treated with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol?
a)
Ethanol
b)
Diethyl ether
c)
Ethanal
d)
Oxirane
|
|
Vivek Khatri answered |
When is treated Diethylether with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol.
Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just “ether”). The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 – 30 minutes.
CH3CH2Br + Mg + Diethyl ether → CH3CH2MgBr
Everything must be perfectly dry because Grignard reagents react with water.
Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just “ether”). The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 – 30 minutes.
CH3CH2Br + Mg + Diethyl ether → CH3CH2MgBr
Everything must be perfectly dry because Grignard reagents react with water.
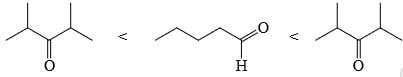
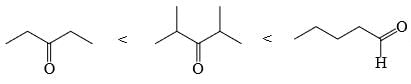
Choose order that has the following compounds correctly arranged with respect to increasing rate of reaction with LiAlH4 (most reactive compound on the right).- a)
- b)
- c)
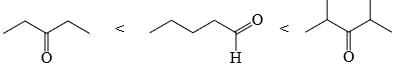
- d)
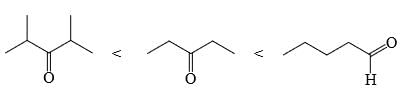
Correct answer is option 'D'. Can you explain this answer?
Choose order that has the following compounds correctly arranged with respect to increasing rate of reaction with LiAlH4 (most reactive compound on the right).
a)

b)

c)

d)


|
Sudipta Pahari answered |
D is the correct answer.aldehyde is more reactive than ketone
The ethereal linkage (—C—O—C—) is cleaved by: - a)HBr
- b)HNO3
- c)Both (a) and (b)
- d)None
Correct answer is option 'A'. Can you explain this answer?
The ethereal linkage (—C—O—C—) is cleaved by:
a)
HBr
b)
HNO3
c)
Both (a) and (b)
d)
None

|
Akash Kulkarni answered |
Also known as the ethereal connection or ethereal bond) is a metaphysical concept that describes a connection between two or more individuals or entities on a spiritual or energetic level.
According to this concept, the ethereal linkage is not dependent on physical proximity or communication, but rather on the alignment of energy, consciousness, or souls. It is believed to transcend time and space, allowing for a profound connection between individuals even when they are physically separated.
The ethereal linkage is often associated with deep emotional, mental, or spiritual connections. It is believed to enable individuals to understand and connect with each other on a profound level, often leading to a sense of unity, empathy, and shared experiences.
This concept is prevalent in various spiritual and esoteric traditions, such as certain forms of mysticism, New Age philosophies, and some belief systems focused on energy work or psychic abilities. It is often used to explain the bond between soulmates, twin flames, or spiritual allies.
The ethereal linkage is considered to be a powerful force that can facilitate growth, healing, and transformation. It is believed that individuals connected through this ethereal bond can support each other's spiritual journeys, provide guidance, and contribute to each other's personal and collective evolution.
However, it is important to note that the ethereal linkage is a concept that is not scientifically proven and may be interpreted differently by different individuals and belief systems. It is a subjective and personal experience that relies on intuition, spirituality, and metaphysical understandings.
According to this concept, the ethereal linkage is not dependent on physical proximity or communication, but rather on the alignment of energy, consciousness, or souls. It is believed to transcend time and space, allowing for a profound connection between individuals even when they are physically separated.
The ethereal linkage is often associated with deep emotional, mental, or spiritual connections. It is believed to enable individuals to understand and connect with each other on a profound level, often leading to a sense of unity, empathy, and shared experiences.
This concept is prevalent in various spiritual and esoteric traditions, such as certain forms of mysticism, New Age philosophies, and some belief systems focused on energy work or psychic abilities. It is often used to explain the bond between soulmates, twin flames, or spiritual allies.
The ethereal linkage is considered to be a powerful force that can facilitate growth, healing, and transformation. It is believed that individuals connected through this ethereal bond can support each other's spiritual journeys, provide guidance, and contribute to each other's personal and collective evolution.
However, it is important to note that the ethereal linkage is a concept that is not scientifically proven and may be interpreted differently by different individuals and belief systems. It is a subjective and personal experience that relies on intuition, spirituality, and metaphysical understandings.
One Integer Value Correct TypeDirection (Q. Nos. 23-27) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Consider the following aldol condensation reaction,Butanone + NaOH (Dil.) → AldolsQ. How many different isomers (including stereoisomers) are formed above?
Correct answer is '6'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 23-27) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Consider the following aldol condensation reaction,
Butanone + NaOH (Dil.) → Aldols
Q.
How many different isomers (including stereoisomers) are formed above?
|
|
Abhijeet Menon answered |
CH3CH2CH2COCH3 + CH3CHOHCH2Na → CH3CH2CH=C(CH3)CHOH + NaOH
23. How many moles of NaOH are required to react completely with 1 mole of butanone?
Answer: 1 mole
24. What is the total number of moles of reactants required to produce 1 mole of the product?
Answer: 2 moles (1 mole of butanone + 1 mole of CH3CHOHCH2Na)
25. What is the molecular formula of the product?
Answer: C6H10O
26. What is the name of the product?
Answer: 3-methyl-2-penten-2-ol
27. How many stereoisomers are possible for the product?
Answer: 2 stereoisomers (cis and trans)
23. How many moles of NaOH are required to react completely with 1 mole of butanone?
Answer: 1 mole
24. What is the total number of moles of reactants required to produce 1 mole of the product?
Answer: 2 moles (1 mole of butanone + 1 mole of CH3CHOHCH2Na)
25. What is the molecular formula of the product?
Answer: C6H10O
26. What is the name of the product?
Answer: 3-methyl-2-penten-2-ol
27. How many stereoisomers are possible for the product?
Answer: 2 stereoisomers (cis and trans)
Statement I : When a mixture of ethanal and propanal is treated with aqueous Na2CO3, four aldol (excluding stereoisomers) are formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'C'. Can you explain this answer?
Statement I : When a mixture of ethanal and propanal is treated with aqueous Na2CO3, four aldol (excluding stereoisomers) are formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Rishika Chauhan answered |
It would be true only if both carbonyls are capable of forming enolates, i.e. possesses α-H
Only One Option Correct TypeDirection (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. In hexane-2,4-dione, how many different mono-enols are possible?- a)2
- b)3
- c)4
- d)7
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
In hexane-2,4-dione, how many different mono-enols are possible?
a)
2
b)
3
c)
4
d)
7
|
|
Devanshi Mehta answered |
Possible Mono-enols in Hexane-2,4-dione
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
Chapter doubts & questions for Organic Named Reactions & Reagents - Organic Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Organic Named Reactions & Reagents - Organic Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Organic Chemistry
39 videos|92 docs|46 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup