NEET Exam > NEET Questions > A mixture of Benzaldehyde and Formaldehyde on...
Start Learning for Free
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :
- a)Benzyl alcohol and methyl alcohol
- b)Benzyl alcohol and sodium formate
- c)Sodium benzoate and methyl alcohol
- d)Sodium benzoate and sodium formate
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaO...
Crossed Cannizzaro's reaction where formaldehyde is oxidized and benzaldehyde is reduced. This occurs because aliphatic are oxidized much more readily compared to aromatic aldehydes
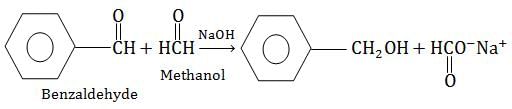

Most Upvoted Answer
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaO...
Reaction between Benzaldehyde and Formaldehyde with aqueous NaOH solution
Introduction:
Benzaldehyde and Formaldehyde are both aldehydes. Aldehydes are organic compounds that have a carbonyl group (C=O) at the end of the carbon chain. They are reactive compounds and undergo various chemical reactions. One of such reactions is with aqueous NaOH solution.
Reaction:
The reaction between Benzaldehyde and Formaldehyde with aqueous NaOH solution results in the formation of Benzyl alcohol and Sodium formate.
Explanation:
When Benzaldehyde and Formaldehyde are heated with aqueous NaOH solution, they undergo Cannizzaro’s reaction. Cannizzaro’s reaction is a self-oxidation and reduction reaction that takes place in the presence of a strong base. In this reaction, one molecule of aldehyde is oxidized to a carboxylic acid and the other molecule is reduced to an alcohol.
The reaction can be represented as follows:

In the above reaction, Benzaldehyde is oxidized to Benzoic acid and Formaldehyde is reduced to Benzyl alcohol.
The reaction also results in the formation of Sodium formate. Sodium formate is formed due to the oxidation of Formaldehyde to formic acid and its subsequent reaction with NaOH.
The reaction can be represented as follows:

Sodium formate is a white crystalline solid that is soluble in water. It is used in the textile and leather industries as a reducing agent and a mordant.
Conclusion:
The reaction between Benzaldehyde and Formaldehyde with aqueous NaOH solution results in the formation of Benzyl alcohol and Sodium formate. Cannizzaro’s reaction takes place in the presence of a strong base and involves the oxidation of one molecule of aldehyde to a carboxylic acid and the reduction of the other molecule to an alcohol.
Introduction:
Benzaldehyde and Formaldehyde are both aldehydes. Aldehydes are organic compounds that have a carbonyl group (C=O) at the end of the carbon chain. They are reactive compounds and undergo various chemical reactions. One of such reactions is with aqueous NaOH solution.
Reaction:
The reaction between Benzaldehyde and Formaldehyde with aqueous NaOH solution results in the formation of Benzyl alcohol and Sodium formate.
Explanation:
When Benzaldehyde and Formaldehyde are heated with aqueous NaOH solution, they undergo Cannizzaro’s reaction. Cannizzaro’s reaction is a self-oxidation and reduction reaction that takes place in the presence of a strong base. In this reaction, one molecule of aldehyde is oxidized to a carboxylic acid and the other molecule is reduced to an alcohol.
The reaction can be represented as follows:

In the above reaction, Benzaldehyde is oxidized to Benzoic acid and Formaldehyde is reduced to Benzyl alcohol.
The reaction also results in the formation of Sodium formate. Sodium formate is formed due to the oxidation of Formaldehyde to formic acid and its subsequent reaction with NaOH.
The reaction can be represented as follows:

Sodium formate is a white crystalline solid that is soluble in water. It is used in the textile and leather industries as a reducing agent and a mordant.
Conclusion:
The reaction between Benzaldehyde and Formaldehyde with aqueous NaOH solution results in the formation of Benzyl alcohol and Sodium formate. Cannizzaro’s reaction takes place in the presence of a strong base and involves the oxidation of one molecule of aldehyde to a carboxylic acid and the reduction of the other molecule to an alcohol.
Free Test
FREE
| Start Free Test |
Community Answer
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaO...
》》Yeah..option B is correct...because it is Crossed Canizzaro Reaction. Benzaldehyde and Formaldehyde react with 50% NAOH solution and gives Benzyl Alcohol (C6H5CH2OH) and sodium formate(HCOONA).see..there the reactant reduces to Benzyl Alcohol and oxidase to Sodim formate...so..here oxidation and reduction both occur..so it is called also Disproportionation Reaction..
=so..option B is correct answer...
=so..option B is correct answer...

|
Explore Courses for NEET exam
|

|
Question Description
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer?.
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer?.
Solutions for A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A mixture of Benzaldehyde and Formaldehyde on heating with aqueous NaOH solution gives :a)Benzyl alcohol and methyl alcoholb)Benzyl alcohol and sodium formatec)Sodium benzoate and methyl alcohold)Sodium benzoate and sodium formateCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























