GATE Exam > GATE Questions > For a 2nd order phase transition, which of th...
Start Learning for Free
For a 2nd order phase transition, which of the following remains continuous?
- a)Specific heat
- b)Entropy
- c)Magnetic susceptibility
- d)Isothermal compressibility
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
For a 2nd order phase transition, which of the following remains conti...
For a 2nd order phase transition, the quantities which involves second order derivative of free energy are discontinuous whereas the quantities which involves first order derivative of free energy are continuous .Now, we have
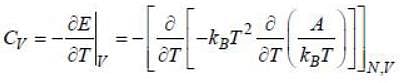
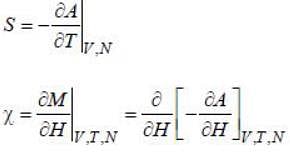
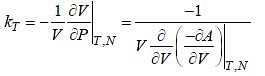
As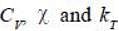 are 2nd order derivatives of free energy A, they are discontinuous. Moreover, entropy is continuous across 2nd order phase transition.
are 2nd order derivatives of free energy A, they are discontinuous. Moreover, entropy is continuous across 2nd order phase transition.
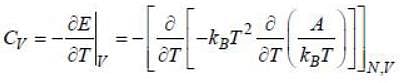
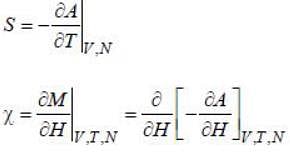
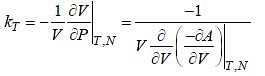
As
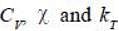 are 2nd order derivatives of free energy A, they are discontinuous. Moreover, entropy is continuous across 2nd order phase transition.
are 2nd order derivatives of free energy A, they are discontinuous. Moreover, entropy is continuous across 2nd order phase transition.Most Upvoted Answer
For a 2nd order phase transition, which of the following remains conti...
Explanation:
Second order phase transitions are continuous transitions from one phase to another without a sudden change in any thermodynamic quantity. Examples include liquid-gas transition, ferromagnetic-paramagnetic transition, and superconductor-normal transition.
Out of the given options, only entropy remains continuous during a second order phase transition. This can be explained as follows:
- Specific heat (option A): Specific heat is defined as the amount of heat required to raise the temperature of a substance by one degree. During a second order phase transition, the specific heat usually diverges (i.e., becomes infinite) at the transition point, indicating the presence of critical fluctuations. Therefore, specific heat is not continuous during a second order phase transition.
- Magnetic susceptibility (option C): Magnetic susceptibility is a measure of the response of a material to an applied magnetic field. During a second order phase transition, the magnetic susceptibility may diverge or become very large, indicating the presence of critical phenomena. Therefore, magnetic susceptibility is not continuous during a second order phase transition.
- Isothermal compressibility (option D): Isothermal compressibility is a measure of the change in volume of a substance in response to a change in pressure at constant temperature. During a second order phase transition, the isothermal compressibility may diverge or become very large, indicating the presence of critical fluctuations. Therefore, isothermal compressibility is not continuous during a second order phase transition.
- Entropy (option B): Entropy is a thermodynamic quantity that measures the degree of disorder or randomness in a system. During a second order phase transition, the entropy remains continuous, indicating that there is no sudden change in the degree of disorder or randomness in the system. Therefore, entropy is continuous during a second order phase transition.
To summarize, during a second order phase transition, specific heat, magnetic susceptibility, and isothermal compressibility may diverge or become very large, indicating the presence of critical phenomena. However, entropy remains continuous, indicating that there is no sudden change in the degree of disorder or randomness in the system.
Second order phase transitions are continuous transitions from one phase to another without a sudden change in any thermodynamic quantity. Examples include liquid-gas transition, ferromagnetic-paramagnetic transition, and superconductor-normal transition.
Out of the given options, only entropy remains continuous during a second order phase transition. This can be explained as follows:
- Specific heat (option A): Specific heat is defined as the amount of heat required to raise the temperature of a substance by one degree. During a second order phase transition, the specific heat usually diverges (i.e., becomes infinite) at the transition point, indicating the presence of critical fluctuations. Therefore, specific heat is not continuous during a second order phase transition.
- Magnetic susceptibility (option C): Magnetic susceptibility is a measure of the response of a material to an applied magnetic field. During a second order phase transition, the magnetic susceptibility may diverge or become very large, indicating the presence of critical phenomena. Therefore, magnetic susceptibility is not continuous during a second order phase transition.
- Isothermal compressibility (option D): Isothermal compressibility is a measure of the change in volume of a substance in response to a change in pressure at constant temperature. During a second order phase transition, the isothermal compressibility may diverge or become very large, indicating the presence of critical fluctuations. Therefore, isothermal compressibility is not continuous during a second order phase transition.
- Entropy (option B): Entropy is a thermodynamic quantity that measures the degree of disorder or randomness in a system. During a second order phase transition, the entropy remains continuous, indicating that there is no sudden change in the degree of disorder or randomness in the system. Therefore, entropy is continuous during a second order phase transition.
To summarize, during a second order phase transition, specific heat, magnetic susceptibility, and isothermal compressibility may diverge or become very large, indicating the presence of critical phenomena. However, entropy remains continuous, indicating that there is no sudden change in the degree of disorder or randomness in the system.

|
Explore Courses for GATE exam
|

|
Similar GATE Doubts
For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer?
Question Description
For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer?.
For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer?.
Solutions for For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For a 2nd order phase transition, which of the following remains continuous?a)Specific heatb)Entropyc)Magnetic susceptibilityd)Isothermal compressibilityCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















