NEET Exam > NEET Questions > The ground state electronic configuration of ...
Start Learning for Free
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?
- a)Due to octahedral shape ofSatoms
- b)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited state
- c)Due to sp3 hybridisation of S atom which provides 6 electrons to 6 F atoms
- d)Due to presence of 3 sigma and 3 pi bonds between S and F
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The ground state electronic configuration of S is 3s23p4. How does it ...
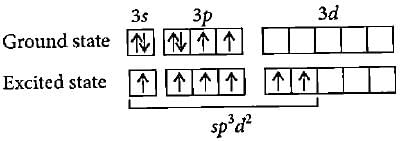
S can go into excited state with 6 unpaired electrons due to presence of vacant 3d-orbitals, which are overlapped by six F electrons.
Most Upvoted Answer
The ground state electronic configuration of S is 3s23p4. How does it ...
**Explanation:**
The compound SF6 is formed by the combination of sulfur (S) and six fluorine (F) atoms. To understand how this compound is formed, we need to consider the electronic configuration of sulfur.
The ground state electronic configuration of sulfur is 3s²3p⁴. This means that in the ground state, sulfur has 2 electrons in the 3s orbital and 4 electrons in the 3p orbital.
In order to form SF6, sulfur needs to share electrons with six fluorine atoms. Sulfur can achieve a stable configuration by either gaining two electrons or losing six electrons. However, neither of these options is favorable for sulfur.
**Formation of SF6 through Excited State:**
In order to accommodate the six fluorine atoms, sulfur undergoes excitation and promotes one electron from the 3s orbital to the 3d orbital. This gives sulfur an excited state electronic configuration of 3s¹3p³3d¹.
In the excited state, sulfur has access to the 3d orbital, which provides six unpaired electrons. These six unpaired electrons can then form bonds with the six fluorine atoms, resulting in the formation of SF6. Each fluorine atom shares one electron with sulfur, forming a single bond.
Therefore, the correct option is (b) due to the presence of vacant 3d orbitals which provide six unpaired electrons in the excited state. This allows sulfur to form bonds with six fluorine atoms, resulting in the compound SF6.
**Other Options:**
- Option (a) is incorrect because the octahedral shape of SF6 is a consequence of the six bonding pairs around the central sulfur atom, which is formed due to the presence of vacant 3d orbitals in the excited state.
- Option (c) is incorrect because the sp3 hybridization of sulfur only accounts for the formation of four sigma bonds in the compound SF4, not SF6.
- Option (d) is incorrect because the formation of sigma and pi bonds is related to the type of overlap between atomic orbitals, which is not the primary factor for the formation of SF6. The presence of vacant 3d orbitals in the excited state of sulfur is the key factor.
The compound SF6 is formed by the combination of sulfur (S) and six fluorine (F) atoms. To understand how this compound is formed, we need to consider the electronic configuration of sulfur.
The ground state electronic configuration of sulfur is 3s²3p⁴. This means that in the ground state, sulfur has 2 electrons in the 3s orbital and 4 electrons in the 3p orbital.
In order to form SF6, sulfur needs to share electrons with six fluorine atoms. Sulfur can achieve a stable configuration by either gaining two electrons or losing six electrons. However, neither of these options is favorable for sulfur.
**Formation of SF6 through Excited State:**
In order to accommodate the six fluorine atoms, sulfur undergoes excitation and promotes one electron from the 3s orbital to the 3d orbital. This gives sulfur an excited state electronic configuration of 3s¹3p³3d¹.
In the excited state, sulfur has access to the 3d orbital, which provides six unpaired electrons. These six unpaired electrons can then form bonds with the six fluorine atoms, resulting in the formation of SF6. Each fluorine atom shares one electron with sulfur, forming a single bond.
Therefore, the correct option is (b) due to the presence of vacant 3d orbitals which provide six unpaired electrons in the excited state. This allows sulfur to form bonds with six fluorine atoms, resulting in the compound SF6.
**Other Options:**
- Option (a) is incorrect because the octahedral shape of SF6 is a consequence of the six bonding pairs around the central sulfur atom, which is formed due to the presence of vacant 3d orbitals in the excited state.
- Option (c) is incorrect because the sp3 hybridization of sulfur only accounts for the formation of four sigma bonds in the compound SF4, not SF6.
- Option (d) is incorrect because the formation of sigma and pi bonds is related to the type of overlap between atomic orbitals, which is not the primary factor for the formation of SF6. The presence of vacant 3d orbitals in the excited state of sulfur is the key factor.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer?
Question Description
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer?.
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?a)Due to octahedral shape ofSatomsb)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited statec)Due to sp3 hybridisation of S atom whichprovides 6 electrons to 6 F atomsd)Due to presence of 3 sigma and 3 pi bonds between S and FCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























