NEET Exam > NEET Questions > Nitrogen combines with metals to forma)Nitrit...
Start Learning for Free
Nitrogen combines with metals to form
- a)Nitrites
- b)Nitrates
- c)Nitrosyi chloride
- d)Nitrides
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi ch...
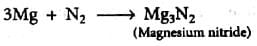
Most Upvoted Answer
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi ch...
Nitrogen combines with metals to form Nitrides.
Explanation:
When nitrogen combines with metals, it forms compounds called nitrides. Nitrides are a class of chemical compounds in which nitrogen is combined with a metal. These compounds are formed by the direct reaction between nitrogen gas (N2) and the metal.
Formation of Nitrides:
The formation of nitrides occurs through a process called nitriding. Nitriding involves the reaction of nitrogen with a metal to form a nitride compound. This reaction can occur at high temperatures or under certain specific conditions.
Properties of Nitrides:
- Nitrides are generally hard and refractory compounds.
- They have high melting points and are often used in high-temperature applications.
- Nitrides exhibit a wide range of electrical and thermal conductivity properties, depending on the specific metal involved.
- Some nitrides are chemically inert, while others can react with water or acids.
Examples of Nitrides:
- Titanium nitride (TiN): It is a hard, ceramic material with a gold appearance. It is commonly used as a coating for metal surfaces to improve their hardness and wear resistance.
- Aluminum nitride (AlN): It is a semiconductor material with excellent thermal conductivity. It is used in electronic applications, such as high-power LEDs and microelectronic devices.
- Boron nitride (BN): It is a compound consisting of boron and nitrogen atoms. It has a similar structure to carbon graphite and has properties such as high thermal conductivity and electrical insulation.
Conclusion:
In summary, when nitrogen combines with metals, it forms compounds called nitrides. Nitrides have various properties and applications depending on the specific metal involved. Examples of nitrides include titanium nitride, aluminum nitride, and boron nitride.
Explanation:
When nitrogen combines with metals, it forms compounds called nitrides. Nitrides are a class of chemical compounds in which nitrogen is combined with a metal. These compounds are formed by the direct reaction between nitrogen gas (N2) and the metal.
Formation of Nitrides:
The formation of nitrides occurs through a process called nitriding. Nitriding involves the reaction of nitrogen with a metal to form a nitride compound. This reaction can occur at high temperatures or under certain specific conditions.
Properties of Nitrides:
- Nitrides are generally hard and refractory compounds.
- They have high melting points and are often used in high-temperature applications.
- Nitrides exhibit a wide range of electrical and thermal conductivity properties, depending on the specific metal involved.
- Some nitrides are chemically inert, while others can react with water or acids.
Examples of Nitrides:
- Titanium nitride (TiN): It is a hard, ceramic material with a gold appearance. It is commonly used as a coating for metal surfaces to improve their hardness and wear resistance.
- Aluminum nitride (AlN): It is a semiconductor material with excellent thermal conductivity. It is used in electronic applications, such as high-power LEDs and microelectronic devices.
- Boron nitride (BN): It is a compound consisting of boron and nitrogen atoms. It has a similar structure to carbon graphite and has properties such as high thermal conductivity and electrical insulation.
Conclusion:
In summary, when nitrogen combines with metals, it forms compounds called nitrides. Nitrides have various properties and applications depending on the specific metal involved. Examples of nitrides include titanium nitride, aluminum nitride, and boron nitride.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer?
Question Description
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer?.
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Nitrogen combines with metals to forma)Nitritesb)Nitratesc)Nitrosyi chlorided)NitridesCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























