NEET Exam > NEET Questions > Which of the following explains why propene ...
Start Learning for Free
Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?
- a)Br- is better nucleophile than CN−
- b)HBr is a better source of a proton as it is a stronger acid than HCN
- c)HCN attacks preferentially via lone pair of nitrogen
- d)The C-Br bond being stronger is formed easily as compared to C-CN bond
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Which of the following explains why propene undergoes electrophilic a...
Explanation:
Electrophilic Addition Reactions:
Electrophilic addition reactions involve the addition of an electrophile to a molecule, resulting in the formation of a new bond. In this type of reaction, the electrophile attacks the electron-rich region of the molecule, such as a double or triple bond.
Electrophilic Addition of HBr to Propene:
Propene (C₃H₆) has a carbon-carbon double bond, which contains a π bond with high electron density. HBr can act as an electrophile because the hydrogen atom is partially positive due to the difference in electronegativity between hydrogen and bromine.
When HBr reacts with propene, the π bond of propene is attacked by the partially positive hydrogen atom of HBr. The hydrogen atom is transferred to one of the carbon atoms, resulting in the formation of a new bond between carbon and hydrogen. The bromine atom becomes negatively charged and forms a bond with the other carbon atom, resulting in the formation of a C-Br bond.
Electrophilic Addition of HCN to Propene:
On the other hand, propene does not undergo electrophilic addition with HCN. HCN is a weak acid and is not a strong electrophile. The carbon atom in HCN is partially positive due to the difference in electronegativity between carbon and nitrogen, but it is not as electrophilic as the hydrogen atom in HBr.
The carbon atom in HCN is less likely to attack the electron-rich π bond of propene compared to the hydrogen atom in HBr. Therefore, HCN does not undergo electrophilic addition with propene.
Why HBr is a Stronger Acid compared to HCN:
HBr is a stronger acid compared to HCN because the H-Br bond is weaker than the H-CN bond. The strength of an acid is determined by the stability of its conjugate base. In the case of HBr, the bromide ion (Br-) is more stable than the cyanide ion (CN-). This is because the Br- ion is larger and can disperse the negative charge over a larger area, making it more stable. Therefore, HBr readily donates a proton (H+) and acts as a stronger acid compared to HCN.
Conclusion:
In summary, propene undergoes electrophilic addition with HBr because HBr is a stronger acid compared to HCN. The partially positive hydrogen atom in HBr acts as an electrophile and attacks the π bond of propene, resulting in the addition of H and Br to propene. HCN, on the other hand, is not as electrophilic as HBr and does not undergo electrophilic addition with propene.
Electrophilic Addition Reactions:
Electrophilic addition reactions involve the addition of an electrophile to a molecule, resulting in the formation of a new bond. In this type of reaction, the electrophile attacks the electron-rich region of the molecule, such as a double or triple bond.
Electrophilic Addition of HBr to Propene:
Propene (C₃H₆) has a carbon-carbon double bond, which contains a π bond with high electron density. HBr can act as an electrophile because the hydrogen atom is partially positive due to the difference in electronegativity between hydrogen and bromine.
When HBr reacts with propene, the π bond of propene is attacked by the partially positive hydrogen atom of HBr. The hydrogen atom is transferred to one of the carbon atoms, resulting in the formation of a new bond between carbon and hydrogen. The bromine atom becomes negatively charged and forms a bond with the other carbon atom, resulting in the formation of a C-Br bond.
Electrophilic Addition of HCN to Propene:
On the other hand, propene does not undergo electrophilic addition with HCN. HCN is a weak acid and is not a strong electrophile. The carbon atom in HCN is partially positive due to the difference in electronegativity between carbon and nitrogen, but it is not as electrophilic as the hydrogen atom in HBr.
The carbon atom in HCN is less likely to attack the electron-rich π bond of propene compared to the hydrogen atom in HBr. Therefore, HCN does not undergo electrophilic addition with propene.
Why HBr is a Stronger Acid compared to HCN:
HBr is a stronger acid compared to HCN because the H-Br bond is weaker than the H-CN bond. The strength of an acid is determined by the stability of its conjugate base. In the case of HBr, the bromide ion (Br-) is more stable than the cyanide ion (CN-). This is because the Br- ion is larger and can disperse the negative charge over a larger area, making it more stable. Therefore, HBr readily donates a proton (H+) and acts as a stronger acid compared to HCN.
Conclusion:
In summary, propene undergoes electrophilic addition with HBr because HBr is a stronger acid compared to HCN. The partially positive hydrogen atom in HBr acts as an electrophile and attacks the π bond of propene, resulting in the addition of H and Br to propene. HCN, on the other hand, is not as electrophilic as HBr and does not undergo electrophilic addition with propene.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following explains why propene undergoes electrophilic a...
HBr HBr being better source of proton. It gives a H+H+ and a Br− Br- ion.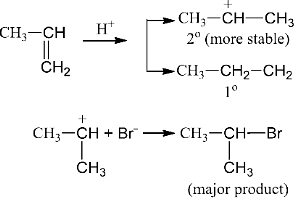
HBr ⟶ H+ + Br−

Thus, H+ attack the π bond of propene to form carbonium ion as,
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer?
Question Description
Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer?.
Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following explains why propene undergoes electrophilic addition with HBr, but not with HCN?a)Br- is better nucleophile than CN−b)HBr is a better source of a proton as it is a stronger acid than HCNc)HCN attacks preferentially via lone pair of nitrogend)The C-Br bond being stronger is formed easily as compared to C-CN bondCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























