NEET Exam > NEET Questions > When propene reacts with HBr in the presence ...
Start Learning for Free
When propene reacts with HBr in the presence of peroxide, it gives rise to
- a)Allyl bromide
- b)Isopropyl bromide
- c)n-propyl bromide
- d)3-bromopropane
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
When propene reacts with HBr in the presence of peroxide, it gives ris...
Explanation of the Reaction of Propene with HBr in the Presence of Peroxide
The reaction of propene with hydrogen bromide in the presence of peroxide follows the rule of anti-Markovnikov addition. This rule states that the hydrogen (H) from HBr will add to the carbon with the most hydrogen atoms already attached, and the bromine (Br) will add to the other carbon. Peroxide promotes this anti-Markovnikov addition.
In the case of propene (CH3-CH=CH2), the hydrogen from HBr will add to the terminal carbon, which already has two hydrogens. The bromine will add to the middle carbon.
Here are the steps of the reaction:
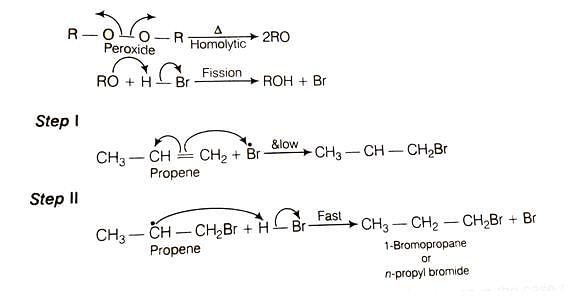
The reaction of propene with hydrogen bromide in the presence of peroxide follows the rule of anti-Markovnikov addition. This rule states that the hydrogen (H) from HBr will add to the carbon with the most hydrogen atoms already attached, and the bromine (Br) will add to the other carbon. Peroxide promotes this anti-Markovnikov addition.
In the case of propene (CH3-CH=CH2), the hydrogen from HBr will add to the terminal carbon, which already has two hydrogens. The bromine will add to the middle carbon.
Here are the steps of the reaction:

Free Test
FREE
| Start Free Test |
Community Answer
When propene reacts with HBr in the presence of peroxide, it gives ris...
Propene, also known as propylene, is an unsaturated hydrocarbon with the chemical formula C3H6. When propene reacts with HBr (hydrogen bromide) in the presence of peroxide, it undergoes a radical addition reaction to form n-propyl bromide (n-C3H7Br).
Here is a detailed explanation of the reaction:
1. Radical Initiation:
The presence of peroxide (often represented as ROOR, where R is an alkyl group) initiates the reaction by undergoing homolytic cleavage to produce two alkyl radicals, represented as R•. In this case, the peroxide could be tert-butyl peroxide (C(CH3)3OO•).
ROOR → 2R•
2. Radical Propagation:
The alkyl radical (R•) reacts with propene (C3H6) to form a more stable secondary radical intermediate.
R• + C3H6 → RCH2CH2•
The secondary radical intermediate then reacts with HBr to produce the alkyl bromide.
RCH2CH2• + HBr → RCH2CH2Br
This reaction step can occur at any position on the propene molecule, resulting in different possible alkyl bromide products.
3. Radical Termination:
The reaction can also undergo radical termination steps where two alkyl radicals combine to form a non-radical species. These termination steps play a role in consuming any excess alkyl radicals and stopping the chain reaction.
R• + R• → R-R
In the case of propene reacting with HBr in the presence of peroxide, the major product formed is n-propyl bromide (n-C3H7Br). This is because the secondary radical intermediate reacts preferentially with HBr at the terminal carbon of propene, resulting in the formation of n-propyl bromide. The other possible products, such as allyl bromide (C3H5Br), isopropyl bromide (C3H7Br), and 3-bromopropane (C3H7Br), are less favored due to the stability of the radical intermediate and the reactivity of HBr at the terminal carbon.
In summary, when propene reacts with HBr in the presence of peroxide, the major product obtained is n-propyl bromide (n-C3H7Br). This reaction follows a radical mechanism, initiated by the homolytic cleavage of peroxide and proceeds through radical propagation steps to form the alkyl bromide product.
Here is a detailed explanation of the reaction:
1. Radical Initiation:
The presence of peroxide (often represented as ROOR, where R is an alkyl group) initiates the reaction by undergoing homolytic cleavage to produce two alkyl radicals, represented as R•. In this case, the peroxide could be tert-butyl peroxide (C(CH3)3OO•).
ROOR → 2R•
2. Radical Propagation:
The alkyl radical (R•) reacts with propene (C3H6) to form a more stable secondary radical intermediate.
R• + C3H6 → RCH2CH2•
The secondary radical intermediate then reacts with HBr to produce the alkyl bromide.
RCH2CH2• + HBr → RCH2CH2Br
This reaction step can occur at any position on the propene molecule, resulting in different possible alkyl bromide products.
3. Radical Termination:
The reaction can also undergo radical termination steps where two alkyl radicals combine to form a non-radical species. These termination steps play a role in consuming any excess alkyl radicals and stopping the chain reaction.
R• + R• → R-R
In the case of propene reacting with HBr in the presence of peroxide, the major product formed is n-propyl bromide (n-C3H7Br). This is because the secondary radical intermediate reacts preferentially with HBr at the terminal carbon of propene, resulting in the formation of n-propyl bromide. The other possible products, such as allyl bromide (C3H5Br), isopropyl bromide (C3H7Br), and 3-bromopropane (C3H7Br), are less favored due to the stability of the radical intermediate and the reactivity of HBr at the terminal carbon.
In summary, when propene reacts with HBr in the presence of peroxide, the major product obtained is n-propyl bromide (n-C3H7Br). This reaction follows a radical mechanism, initiated by the homolytic cleavage of peroxide and proceeds through radical propagation steps to form the alkyl bromide product.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer?
Question Description
When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer?.
When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer?.
Solutions for When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When propene reacts with HBr in the presence of peroxide, it gives rise toa)Allyl bromideb)Isopropyl bromidec)n-propyl bromided)3-bromopropaneCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















