NEET Exam > NEET Questions > I'm Millikan's oil drop experiment; oil dropl...
Start Learning for Free
I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ??
Verified Answer
I'm Millikan's oil drop experiment; oil droplet acquires which charge ...
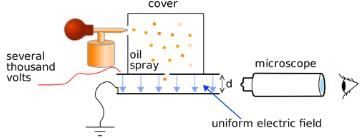
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community. In the experiment, Milliken allowed charged tiny oil droplets to pass through a hole into an electric field. By varying the strength of electric field the charge over an oil droplet was calculated, which always came as an integral value of ‘e.’

 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
I'm Millikan's oil drop experiment; oil droplet acquires which charge ...
Introduction:
Millikan's oil drop experiment was conducted by American physicist Robert A. Millikan in 1909 to determine the charge of an electron. This experiment played a crucial role in understanding the fundamental properties of electrons and their charge.
Oil Droplets and Charge:
In the oil drop experiment, tiny oil droplets were suspended in a chamber and subjected to an electric field. By carefully measuring the motion of these droplets, Millikan was able to determine the charge on them and hence calculate the charge of an electron.
Acquisition of Charge:
During the experiment, the oil droplets acquired charge primarily through two processes: frictional charging and ionization.
Frictional Charging:
When the oil droplets moved through the air in the chamber, they experienced friction with the air molecules. This friction resulted in the transfer of electrons between the air and the oil droplets. If more electrons were transferred to the droplets, they acquired a negative charge. Conversely, if electrons were transferred from the droplets to the air, they acquired a positive charge.
Ionization:
The oil droplets could also acquire charge through ionization. In the chamber, some air molecules were ionized by radiation, creating positive and negative ions. These ions could attach to the oil droplets, causing them to become positively or negatively charged depending on the type of ion.
Controlled Experiment:
To conduct a controlled experiment, Millikan initially allowed the oil droplets to fall freely in the chamber without applying an electric field. This ensured that any charge acquired by the droplets was solely due to frictional charging and ionization.
Electric Field and Charge Measurement:
Once the droplets were charged, Millikan applied an electric field in the chamber. By adjusting the strength of the electric field, he was able to observe the motion of the droplets. He carefully measured the terminal velocity of the droplets, which was the velocity at which the gravitational force on the droplet equaled the drag force due to the air resistance. By comparing the gravitational and drag forces, Millikan could determine the charge on the droplets.
Conclusion:
Based on Millikan's observations and measurements, he found that the charge on the oil droplets was quantized and always found to be a multiple of the elementary charge, which is the charge of a single electron (-1.6 x 10^-19 coulombs). This confirmed the existence of fundamental particles with discrete charges. Therefore, the oil droplets in Millikan's oil drop experiment could acquire either a positive or negative charge, depending on the transfer of electrons during frictional charging and the attachment of ions during ionization.
Millikan's oil drop experiment was conducted by American physicist Robert A. Millikan in 1909 to determine the charge of an electron. This experiment played a crucial role in understanding the fundamental properties of electrons and their charge.
Oil Droplets and Charge:
In the oil drop experiment, tiny oil droplets were suspended in a chamber and subjected to an electric field. By carefully measuring the motion of these droplets, Millikan was able to determine the charge on them and hence calculate the charge of an electron.
Acquisition of Charge:
During the experiment, the oil droplets acquired charge primarily through two processes: frictional charging and ionization.
Frictional Charging:
When the oil droplets moved through the air in the chamber, they experienced friction with the air molecules. This friction resulted in the transfer of electrons between the air and the oil droplets. If more electrons were transferred to the droplets, they acquired a negative charge. Conversely, if electrons were transferred from the droplets to the air, they acquired a positive charge.
Ionization:
The oil droplets could also acquire charge through ionization. In the chamber, some air molecules were ionized by radiation, creating positive and negative ions. These ions could attach to the oil droplets, causing them to become positively or negatively charged depending on the type of ion.
Controlled Experiment:
To conduct a controlled experiment, Millikan initially allowed the oil droplets to fall freely in the chamber without applying an electric field. This ensured that any charge acquired by the droplets was solely due to frictional charging and ionization.
Electric Field and Charge Measurement:
Once the droplets were charged, Millikan applied an electric field in the chamber. By adjusting the strength of the electric field, he was able to observe the motion of the droplets. He carefully measured the terminal velocity of the droplets, which was the velocity at which the gravitational force on the droplet equaled the drag force due to the air resistance. By comparing the gravitational and drag forces, Millikan could determine the charge on the droplets.
Conclusion:
Based on Millikan's observations and measurements, he found that the charge on the oil droplets was quantized and always found to be a multiple of the elementary charge, which is the charge of a single electron (-1.6 x 10^-19 coulombs). This confirmed the existence of fundamental particles with discrete charges. Therefore, the oil droplets in Millikan's oil drop experiment could acquire either a positive or negative charge, depending on the transfer of electrons during frictional charging and the attachment of ions during ionization.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ??
Question Description
I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ??.
I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ??.
Solutions for I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? defined & explained in the simplest way possible. Besides giving the explanation of
I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ??, a detailed solution for I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? has been provided alongside types of I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? theory, EduRev gives you an
ample number of questions to practice I'm Millikan's oil drop experiment; oil droplet acquires which charge + ve or -ve ?? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























