NEET Exam > NEET Questions > In Millikans experiment, an oil drop having c...
Start Learning for Free
In Millikan's experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance 'd'. The weight of the drop is
- a)q V/d
- b)q d/V
- c)q /Vd
- d)q Vd
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
In Millikans experiment, an oil drop having charge q gets stationary o...
Millikan's Experiment and the Weight of Oil Drop
Millikan's oil drop experiment was conducted to determine the charge on an electron. In this experiment, oil drops were allowed to fall through a small hole in the top of a metal cylinder. The oil drops were charged by friction as they passed through the hole.
The oil drops then entered a region of uniform electric field between two parallel plates. The electric field caused the oil drops to be attracted to the bottom plate. By adjusting the voltage across the plates, the electric force on the oil drop could be balanced by the gravitational force on the drop, allowing the drop to remain stationary.
The weight of the oil drop can be calculated using the following formula:
Weight = Mass x Gravity
where Mass = Density x Volume
1. Finding the Volume of the Oil Drop
The volume of the oil drop can be determined by measuring the diameter of the drop using a microscope and calculating the volume using the formula for the volume of a sphere:
Volume = (4/3) x π x (Radius)^3
2. Finding the Mass of the Oil Drop
The mass of the oil drop can be determined using the density of the oil and the volume of the drop:
Mass = Density x Volume
3. Finding the Charge on the Oil Drop
The charge on the oil drop can be calculated by measuring the voltage required to stop the drop from falling under the influence of gravity and the electric field. The electric force on the drop is given by:
Electric Force = Charge x Electric Field
The electric field can be calculated from the voltage and the distance between the plates:
Electric Field = Voltage / Plate Separation
By equating the electric force to the gravitational force, the charge on the oil drop can be calculated:
Charge = Weight / Electric Field
4. Finding the Weight of the Oil Drop
Using the formula for the weight of the oil drop:
Weight = Mass x Gravity
Substituting the value of mass and gravity, we get:
Weight = Density x Volume x Gravity
However, the weight of the oil drop is balanced by the electric force on the drop, which is given by:
Electric Force = Charge x Electric Field
Substituting the value of electric field, we get:
Electric Force = Charge x Voltage / Plate Separation
Setting these two equations equal to each other and solving for the weight, we get:
Weight = Charge x Voltage / Plate Separation x Gravity
Simplifying this equation, we get:
Weight = Charge x Voltage / Plate Separation / g
Since Charge x Voltage / Plate Separation is equal to the electric force on the oil drop, we can write:
Weight = Electric Force / g
Substituting the value of electric force from the equation above, we get:
Weight = (Density x Volume x Electric Field) / g
Finally, substituting the values of Volume, Density, Electric Field, and g, we get:
Weight = q V / d
where q is the charge on the oil drop, V is the voltage across the plates, and d is the distance between the plates.
Thus, the correct answer is option 'A': q V / d.
Millikan's oil drop experiment was conducted to determine the charge on an electron. In this experiment, oil drops were allowed to fall through a small hole in the top of a metal cylinder. The oil drops were charged by friction as they passed through the hole.
The oil drops then entered a region of uniform electric field between two parallel plates. The electric field caused the oil drops to be attracted to the bottom plate. By adjusting the voltage across the plates, the electric force on the oil drop could be balanced by the gravitational force on the drop, allowing the drop to remain stationary.
The weight of the oil drop can be calculated using the following formula:
Weight = Mass x Gravity
where Mass = Density x Volume
1. Finding the Volume of the Oil Drop
The volume of the oil drop can be determined by measuring the diameter of the drop using a microscope and calculating the volume using the formula for the volume of a sphere:
Volume = (4/3) x π x (Radius)^3
2. Finding the Mass of the Oil Drop
The mass of the oil drop can be determined using the density of the oil and the volume of the drop:
Mass = Density x Volume
3. Finding the Charge on the Oil Drop
The charge on the oil drop can be calculated by measuring the voltage required to stop the drop from falling under the influence of gravity and the electric field. The electric force on the drop is given by:
Electric Force = Charge x Electric Field
The electric field can be calculated from the voltage and the distance between the plates:
Electric Field = Voltage / Plate Separation
By equating the electric force to the gravitational force, the charge on the oil drop can be calculated:
Charge = Weight / Electric Field
4. Finding the Weight of the Oil Drop
Using the formula for the weight of the oil drop:
Weight = Mass x Gravity
Substituting the value of mass and gravity, we get:
Weight = Density x Volume x Gravity
However, the weight of the oil drop is balanced by the electric force on the drop, which is given by:
Electric Force = Charge x Electric Field
Substituting the value of electric field, we get:
Electric Force = Charge x Voltage / Plate Separation
Setting these two equations equal to each other and solving for the weight, we get:
Weight = Charge x Voltage / Plate Separation x Gravity
Simplifying this equation, we get:
Weight = Charge x Voltage / Plate Separation / g
Since Charge x Voltage / Plate Separation is equal to the electric force on the oil drop, we can write:
Weight = Electric Force / g
Substituting the value of electric force from the equation above, we get:
Weight = (Density x Volume x Electric Field) / g
Finally, substituting the values of Volume, Density, Electric Field, and g, we get:
Weight = q V / d
where q is the charge on the oil drop, V is the voltage across the plates, and d is the distance between the plates.
Thus, the correct answer is option 'A': q V / d.
Free Test
FREE
| Start Free Test |
Community Answer
In Millikans experiment, an oil drop having charge q gets stationary o...
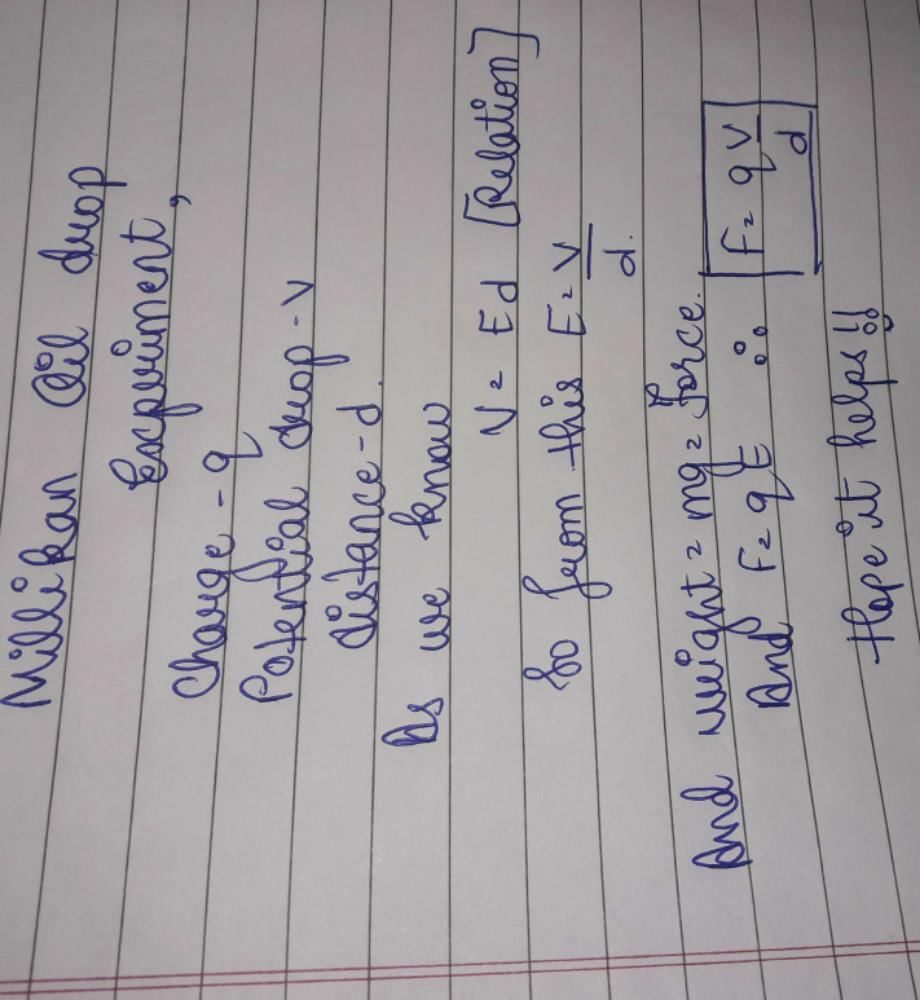
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer?
Question Description
In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer?.
In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer?.
Solutions for In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In Millikans experiment, an oil drop having charge q gets stationary on applying a potential differnce V in between two plates separated by a distance d. The weight of the drop isa)q V/db)q d/Vc)q /Vdd)q VdCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























