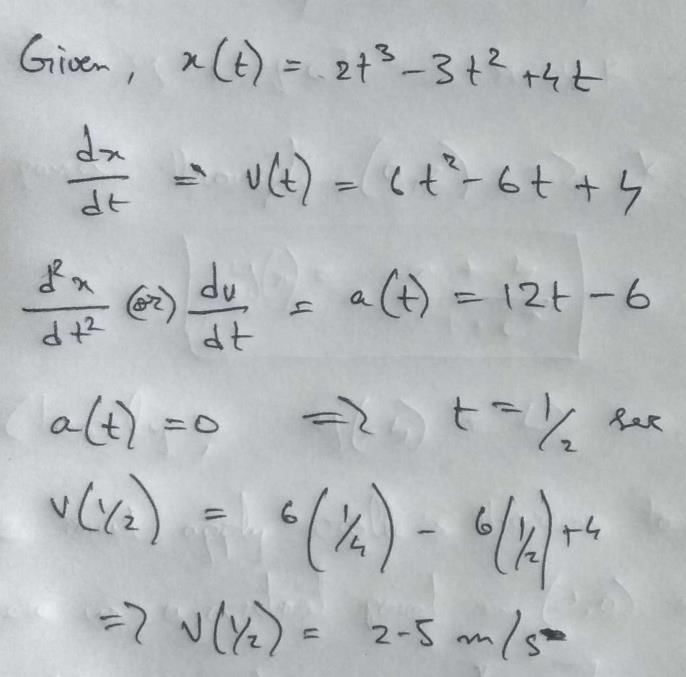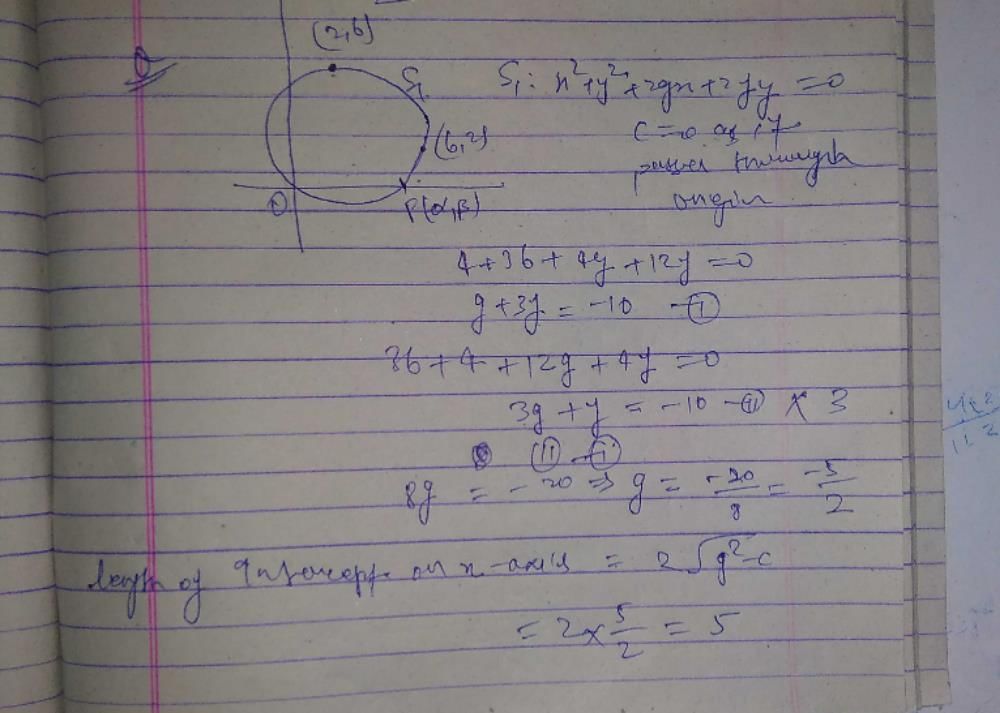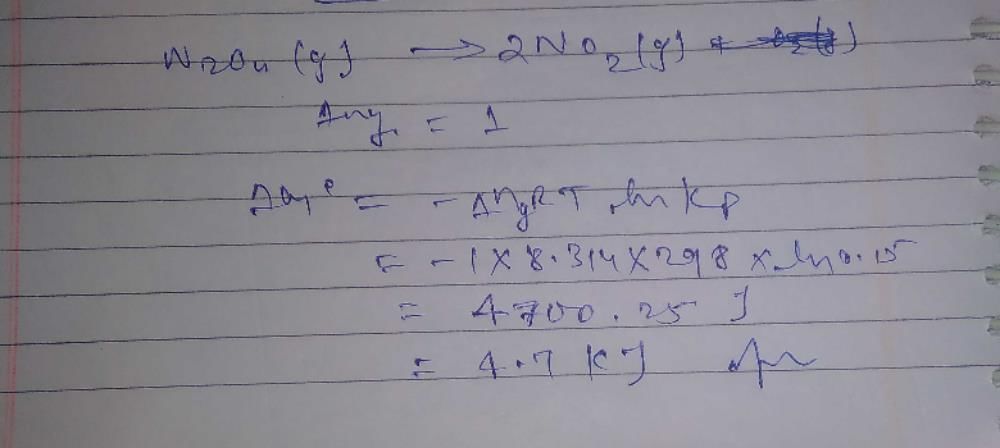All Exams >
JEE >
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 >
All Questions
All questions of 2013 for JEE Exam
Tautomerism is exhibited by- a)(Me3CCO)3CH
- b)
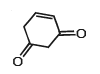
- c)
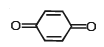
- d)
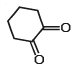
Correct answer is option 'A,B,D'. Can you explain this answer?
Tautomerism is exhibited by
a)
(Me3CCO)3CH
b)
c)
d)
|
|
Anand Kumar answered |
Tautomerism is shown by only those compounds which have at least one resonating hydrogen (tautomeric hydrogen).
In options A,B and D all structures have resonating hydrogen.So, these compounds will show tautomerism
In options A,B and D all structures have resonating hydrogen.So, these compounds will show tautomerism
The ore chromite is- a)FeCr2O4
- b)CoCr2O3
- c)CrFe2O4
- d)FeCr2O3
Correct answer is option 'A'. Can you explain this answer?
The ore chromite is
a)
FeCr2O4
b)
CoCr2O3
c)
CrFe2O4
d)
FeCr2O3
|
|
Kiran Sarmah answered |
Chromite ore contains chromium, iron and oxygen. Its formula is FeCr2O4.
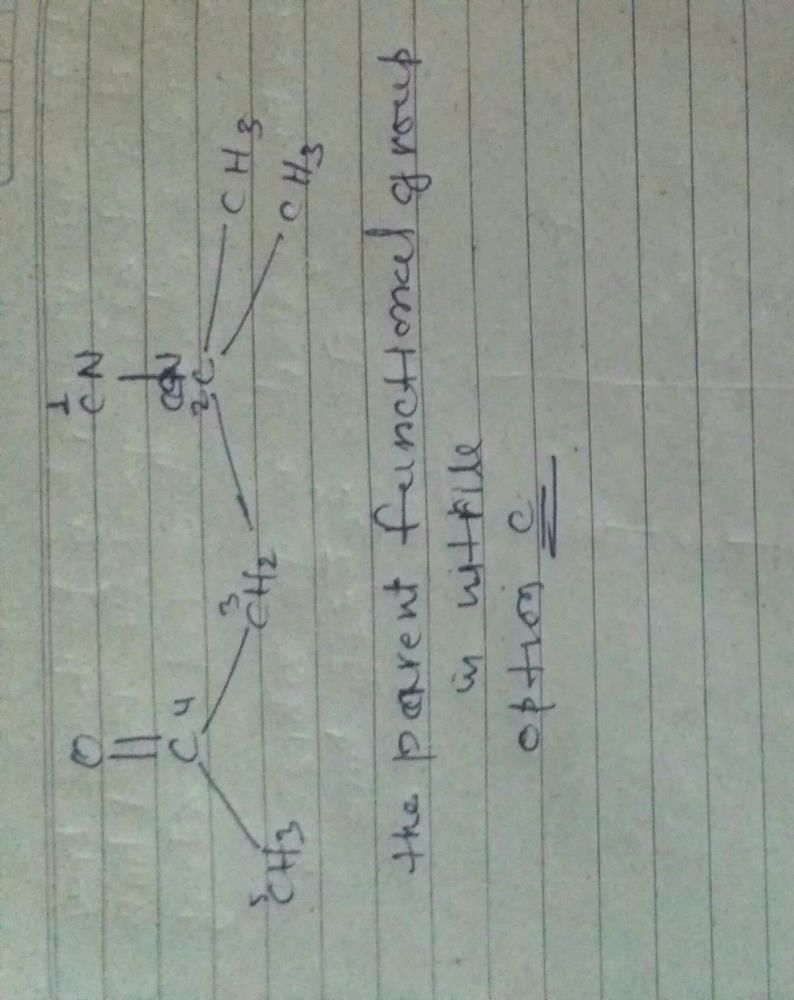
The best method for preparation of Me3CCN is- a)To react Me3COH with HCN
- b)To react Me3CBr with NaCN
- c)To react Me3CMgBr with ClCN
- d)To react Me3CLi with NH2CN
Correct answer is option 'C'. Can you explain this answer?
The best method for preparation of Me3CCN is
a)
To react Me3COH with HCN
b)
To react Me3CBr with NaCN
c)
To react Me3CMgBr with ClCN
d)
To react Me3CLi with NH2CN
|
|
Jaideep Mukherjee answered |
Preparation of Me3CCN
Reacting Me3CMgBr with ClCN is the best method for the preparation of Me3CCN. This reaction involves the use of the Grignard reagent, Me3CMgBr, which is a strong nucleophile and reacts with ClCN to form Me3CCN.
Steps Involved
The reaction proceeds through the following steps:
1. Formation of the Grignard reagent: Me3CMgBr is prepared by reacting Me3CBr with Mg in ether.
2. Reaction with ClCN: Me3CMgBr is then reacted with ClCN in dry ether to form Me3CCN.
3. Workup: The reaction mixture is then treated with dilute acid to hydrolyze any unreacted Grignard reagent and to protonate the product, Me3CCN.
Mechanism
The reaction mechanism involves the attack of the nucleophilic carbon atom of the Grignard reagent on the electrophilic carbon atom of ClCN. This leads to the formation of an intermediate, which then undergoes hydrolysis to form the final product, Me3CCN.
Advantages of this method
1. High yields: This method gives high yields of Me3CCN.
2. Mild conditions: The reaction conditions are relatively mild, and the reaction can be carried out at room temperature.
3. Availability of reagents: The reagents used in the reaction, Me3CBr, Mg, and ClCN, are readily available and inexpensive.
4. Versatility: The Grignard reagent, Me3CMgBr, can be used for the preparation of a variety of other organic compounds.
Thus, the reaction of Me3CMgBr with ClCN is the best method for the preparation of Me3CCN.
Reacting Me3CMgBr with ClCN is the best method for the preparation of Me3CCN. This reaction involves the use of the Grignard reagent, Me3CMgBr, which is a strong nucleophile and reacts with ClCN to form Me3CCN.
Steps Involved
The reaction proceeds through the following steps:
1. Formation of the Grignard reagent: Me3CMgBr is prepared by reacting Me3CBr with Mg in ether.
2. Reaction with ClCN: Me3CMgBr is then reacted with ClCN in dry ether to form Me3CCN.
3. Workup: The reaction mixture is then treated with dilute acid to hydrolyze any unreacted Grignard reagent and to protonate the product, Me3CCN.
Mechanism
The reaction mechanism involves the attack of the nucleophilic carbon atom of the Grignard reagent on the electrophilic carbon atom of ClCN. This leads to the formation of an intermediate, which then undergoes hydrolysis to form the final product, Me3CCN.
Advantages of this method
1. High yields: This method gives high yields of Me3CCN.
2. Mild conditions: The reaction conditions are relatively mild, and the reaction can be carried out at room temperature.
3. Availability of reagents: The reagents used in the reaction, Me3CBr, Mg, and ClCN, are readily available and inexpensive.
4. Versatility: The Grignard reagent, Me3CMgBr, can be used for the preparation of a variety of other organic compounds.
Thus, the reaction of Me3CMgBr with ClCN is the best method for the preparation of Me3CCN.
The equation of state of a gas is given by 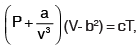 where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively
where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively- a)ML8T–2 and L3/2
- b)ML5T–2 and L3
- c)ML5T–2 and L6
- d)ML6T–2 and L3/2
Correct answer is option 'A'. Can you explain this answer?
The equation of state of a gas is given by 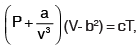 where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively
where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively
a)
ML8T–2 and L3/2
b)
ML5T–2 and L3
c)
ML5T–2 and L6
d)
ML6T–2 and L3/2

|
Infinity Academy answered |
The equation 2x2 + 5xy – 12y2 = 0 represents a- a)circle
- b)pair of non-perpendicular intersecting straight lines
- c)pair of perpendicular straight lines
- d)hyperbola
Correct answer is option 'B'. Can you explain this answer?
The equation 2x2 + 5xy – 12y2 = 0 represents a
a)
circle
b)
pair of non-perpendicular intersecting straight lines
c)
pair of perpendicular straight lines
d)
hyperbola

|
Rajesh Karsanbhi answered |
2x2-12y2+5xy=o
(x+5/4y)2-25/4y2-6y2=0
{x+(5/4)y}2=(49/4)y2
x+5/4y=7/2y. or x+5/4y=-7/2y
this not perpenticuler
(x+5/4y)2-25/4y2-6y2=0
{x+(5/4)y}2=(49/4)y2
x+5/4y=7/2y. or x+5/4y=-7/2y
this not perpenticuler
Lines x + y = 1 and 3y = x + 3 intersect the ellipse x2 + 9y2 = 9 at the points P,Q,R. The are of the triangle PQR is- a)36/5
- b) 18/5
- c) 9/5
- d)1/5
Correct answer is option 'B'. Can you explain this answer?
Lines x + y = 1 and 3y = x + 3 intersect the ellipse x2 + 9y2 = 9 at the points P,Q,R. The are of the triangle PQR is
a)
36/5
b)
18/5
c)
9/5
d)
1/5
|
|
Nikhil Banerjee answered |
Problem: Find the area of the triangle PQR formed by the intersection of lines x y = 1 and 3y = x-3 with the ellipse x^2/9 + y^2 = 1.
Solution:
To solve the problem, we need to find the points P, Q, and R of intersection between the given lines and the ellipse.
Step 1: Find the points of intersection of the ellipse and the line x y = 1.
Substituting y = 1/x in the equation of the ellipse, we get:
x^2/9 + (1/x)^2 = 1
Multiplying both sides by x^2, we get:
x^4/9 + 1 = x^2
Bringing all terms to one side, we get:
x^4 - 9x^2 + 9 = 0
This is a quadratic equation in x^2. Solving for x^2 using the quadratic formula, we get:
x^2 = (9 ± 3√5)/2
Since x^2 is positive, we take the positive root and get:
x^2 = (9 + 3√5)/2
Taking the square root, we get:
x = √[(9 + 3√5)/2]
Substituting this value of x in the equation x y = 1, we get:
y = 1/x = √[2/(9 + 3√5)]
So, the point of intersection of the ellipse and the line x y = 1 is:
P = (√[(9 + 3√5)/2], √[2/(9 + 3√5)])
Similarly, we can find the point of intersection of the ellipse and the line 3y = x-3.
Step 2: Find the area of the triangle PQR.
Let Q be the point of intersection of the ellipse and the line 3y = x-3.
Substituting y = (x-3)/3 in the equation of the ellipse, we get:
x^2/9 + ((x-3)/3)^2 = 1
Simplifying, we get:
4x^2 - 36x + 36 = 0
Solving for x using the quadratic formula, we get:
x = 3/2 ± √(5)/2
Since the lines x y = 1 and 3y = x-3 intersect at the point (3, 0), we can easily see that the point of intersection of the ellipse and the line 3y = x-3 is:
R = (3/2 - √(5)/2, 3/2)
Now, we can find the lengths of the sides of the triangle PQR using the distance formula.
PR = √[(√[(9 + 3√5)/2] - 3/2 + √(5)/2)^2 + (√[2/(9 + 3√5)] - 3/2)^2]
QR = √[(3/2 + √(5)/2 - (3/2 - √(5)/2))^2 + (3/2 - 0)^2]
Solution:
To solve the problem, we need to find the points P, Q, and R of intersection between the given lines and the ellipse.
Step 1: Find the points of intersection of the ellipse and the line x y = 1.
Substituting y = 1/x in the equation of the ellipse, we get:
x^2/9 + (1/x)^2 = 1
Multiplying both sides by x^2, we get:
x^4/9 + 1 = x^2
Bringing all terms to one side, we get:
x^4 - 9x^2 + 9 = 0
This is a quadratic equation in x^2. Solving for x^2 using the quadratic formula, we get:
x^2 = (9 ± 3√5)/2
Since x^2 is positive, we take the positive root and get:
x^2 = (9 + 3√5)/2
Taking the square root, we get:
x = √[(9 + 3√5)/2]
Substituting this value of x in the equation x y = 1, we get:
y = 1/x = √[2/(9 + 3√5)]
So, the point of intersection of the ellipse and the line x y = 1 is:
P = (√[(9 + 3√5)/2], √[2/(9 + 3√5)])
Similarly, we can find the point of intersection of the ellipse and the line 3y = x-3.
Step 2: Find the area of the triangle PQR.
Let Q be the point of intersection of the ellipse and the line 3y = x-3.
Substituting y = (x-3)/3 in the equation of the ellipse, we get:
x^2/9 + ((x-3)/3)^2 = 1
Simplifying, we get:
4x^2 - 36x + 36 = 0
Solving for x using the quadratic formula, we get:
x = 3/2 ± √(5)/2
Since the lines x y = 1 and 3y = x-3 intersect at the point (3, 0), we can easily see that the point of intersection of the ellipse and the line 3y = x-3 is:
R = (3/2 - √(5)/2, 3/2)
Now, we can find the lengths of the sides of the triangle PQR using the distance formula.
PR = √[(√[(9 + 3√5)/2] - 3/2 + √(5)/2)^2 + (√[2/(9 + 3√5)] - 3/2)^2]
QR = √[(3/2 + √(5)/2 - (3/2 - √(5)/2))^2 + (3/2 - 0)^2]
Correct statement(s) in cases of n-butanol and t-butanol is (are)- a)Both are having equal solubility in water
- b)t-butanol is more soluble in water than n-butanol
- c)Boiling point of t-butanol is lower than n-butanol
- d)Boiling point of n-butanol is lower than t-butanol
Correct answer is option 'B,C'. Can you explain this answer?
Correct statement(s) in cases of n-butanol and t-butanol is (are)
a)
Both are having equal solubility in water
b)
t-butanol is more soluble in water than n-butanol
c)
Boiling point of t-butanol is lower than n-butanol
d)
Boiling point of n-butanol is lower than t-butanol
|
|
Anand Kumar answered |

A particle of mass M and charge q is at rest at the midpoint between two other fixed similar charges each of magnitude Q placed a distance 2d apart. The system is collinear as shown in the figure. The particle is now displaced by a small amount x (x<< d) along the line joining the two charges and is left to itself. It will now oscillate about the mean position with a time period (ε0 = permittivity of free space)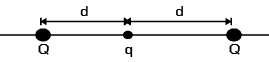
- a)
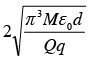
- b)
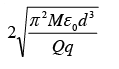
- c)
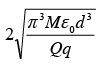
- d)
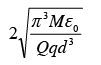
Correct answer is option 'C'. Can you explain this answer?
A particle of mass M and charge q is at rest at the midpoint between two other fixed similar charges each of magnitude Q placed a distance 2d apart. The system is collinear as shown in the figure. The particle is now displaced by a small amount x (x<< d) along the line joining the two charges and is left to itself. It will now oscillate about the mean position with a time period (ε0 = permittivity of free space)
a)
b)
c)
d)
|
|
Chirag Verma answered |
Restoring force on displacement of x,
Acid catalysed hydrolysis of ethyl acetate follows a pseudo-first order kinetics with respect to ester. If the reaction is carried out with large excess of ester, the order with respect to ester will be- a)1.5
- b)0
- c)2
- d)1
Correct answer is option 'B'. Can you explain this answer?
Acid catalysed hydrolysis of ethyl acetate follows a pseudo-first order kinetics with respect to ester. If the reaction is carried out with large excess of ester, the order with respect to ester will be
a)
1.5
b)
0
c)
2
d)
1
|
|
Ujjwal Mishra answered |
Because at excess of water the concentration of ether is approx negligible and the reaction does not depends on the concentration of ether hence the reaction follow 0 order reaction
Chlorine gas reacts with red hot calcium oxide to give- a)Bleaching powder and dichlorine monoxide
- b)Bleaching powder and water
- c)Calcium chloride and chlorine dioxide
- d)Calcium chloride and oxygen
Correct answer is option 'D'. Can you explain this answer?
Chlorine gas reacts with red hot calcium oxide to give
a)
Bleaching powder and dichlorine monoxide
b)
Bleaching powder and water
c)
Calcium chloride and chlorine dioxide
d)
Calcium chloride and oxygen
|
|
Anagh Gupta answered |
Cl2 + (red hot )CaO2 --- CaCl2 + O2
so,option D is correct..
so,option D is correct..
The de Broglie wavelength of an electron (mass = 1 × 10–30 kg, charge = 1.6 × 10-19 C) with a kinetic energy of 200 eV is (Planck’s constant = 6.6 × 10–34 J s)- a)9.60 × 10–11 m
- b)8.25 × 10–11 m
- c)6.25 × 10–11 m
- d)5.00 × 10–11 m
Correct answer is option 'B'. Can you explain this answer?
The de Broglie wavelength of an electron (mass = 1 × 10–30 kg, charge = 1.6 × 10-19 C) with a kinetic energy of 200 eV is (Planck’s constant = 6.6 × 10–34 J s)
a)
9.60 × 10–11 m
b)
8.25 × 10–11 m
c)
6.25 × 10–11 m
d)
5.00 × 10–11 m

|
Priyal Khandelwal answered |
H/p= lamda direct formula based
A current of 1 A is flowing along positive x-axis through a straight wire of length 0.5 m placed in a region of a magnetic field given by B =(2ˆi+ 4ˆj) T. The magnitude and the direction of the force experienced by the wire respectively are- a)√18N , along positive z-axis
- b)√20N , along positive x-axis
- c)2N, along positive z-axis
- d) 4N, along positive y-axis
Correct answer is option 'C'. Can you explain this answer?
A current of 1 A is flowing along positive x-axis through a straight wire of length 0.5 m placed in a region of a magnetic field given by B =(2ˆi+ 4ˆj) T. The magnitude and the direction of the force experienced by the wire respectively are
a)
√18N , along positive z-axis
b)
√20N , along positive x-axis
c)
2N, along positive z-axis
d)
4N, along positive y-axis
|
|
Chirag Verma answered |
An objective type test paper has 5 questions. Out of these 5 questions, 3 questions have four options each (A, B, C, D) with one option being the correct answer. The other 2 questions have two options each, namely True and False. A candidate randomly ticks the options. Then the probability that he/she will tick the correct option in at least four questions, is- a) 5/32
- b) 3/128
- c) 3/256
- d)3/64
Correct answer is option 'D'. Can you explain this answer?
An objective type test paper has 5 questions. Out of these 5 questions, 3 questions have four options each (A, B, C, D) with one option being the correct answer. The other 2 questions have two options each, namely True and False. A candidate randomly ticks the options. Then the probability that he/she will tick the correct option in at least four questions, is
a)
5/32
b)
3/128
c)
3/256
d)
3/64
|
|
Jatin Sen answered |
Objective Type Test Paper
Objective type test papers often consist of multiple-choice questions with various options. In this particular scenario, we have a test paper with 5 questions. Let's break down the question and calculate the probability of ticking the correct option in at least four questions.
Question Breakdown:
Out of the 5 questions:
- 3 questions have four options each (A, B, C, D), with one correct option.
- 2 questions have two options each, namely True and False.
Calculating the Probability:
To calculate the probability of ticking the correct option in at least four questions, we need to consider the different combinations in which this can occur.
Case 1: Ticking all three questions with four options correctly
In this case, the probability of ticking all three questions correctly is (1/4) * (1/4) * (1/4) = 1/64.
Case 2: Ticking two questions with four options correctly and one True/False question correctly
There are three ways to choose the two questions with four options and one question with True/False. The probability for each combination is:
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
So, the total probability for this case is 1/32 + 1/32 + 1/32 = 3/32.
Case 3: Ticking one question with four options correctly and both True/False questions correctly
There are three ways to choose the one question with four options and two questions with True/False. The probability for each combination is:
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
So, the total probability for this case is 1/16 + 1/16 + 1/16 = 3/16.
Case 4: Ticking all three True/False questions correctly
The probability of ticking all three True/False questions correctly is (1/2) * (1/2) * (1/2) = 1/8.
Calculating the Total Probability:
To calculate the total probability of ticking the correct option in at least four questions, we add up the probabilities from all the cases:
1/64 + 3/32 + 3/16 + 1/8 = 1/64 + 6/64 + 12/64 +
Objective type test papers often consist of multiple-choice questions with various options. In this particular scenario, we have a test paper with 5 questions. Let's break down the question and calculate the probability of ticking the correct option in at least four questions.
Question Breakdown:
Out of the 5 questions:
- 3 questions have four options each (A, B, C, D), with one correct option.
- 2 questions have two options each, namely True and False.
Calculating the Probability:
To calculate the probability of ticking the correct option in at least four questions, we need to consider the different combinations in which this can occur.
Case 1: Ticking all three questions with four options correctly
In this case, the probability of ticking all three questions correctly is (1/4) * (1/4) * (1/4) = 1/64.
Case 2: Ticking two questions with four options correctly and one True/False question correctly
There are three ways to choose the two questions with four options and one question with True/False. The probability for each combination is:
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
- (1/4) * (1/4) * (1/2) = 1/32 (for two four-option questions and one True/False question)
So, the total probability for this case is 1/32 + 1/32 + 1/32 = 3/32.
Case 3: Ticking one question with four options correctly and both True/False questions correctly
There are three ways to choose the one question with four options and two questions with True/False. The probability for each combination is:
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
- (1/4) * (1/2) * (1/2) = 1/16 (for one four-option question and two True/False questions)
So, the total probability for this case is 1/16 + 1/16 + 1/16 = 3/16.
Case 4: Ticking all three True/False questions correctly
The probability of ticking all three True/False questions correctly is (1/2) * (1/2) * (1/2) = 1/8.
Calculating the Total Probability:
To calculate the total probability of ticking the correct option in at least four questions, we add up the probabilities from all the cases:
1/64 + 3/32 + 3/16 + 1/8 = 1/64 + 6/64 + 12/64 +
A family of curves is such that the length intercepted on the y-axis between the origin and the tangent at a point is three times the ordinate of the point of contact. The family of curves is- a)xy = c, c is a constant
- b)xy2 = c, c is a constant
- c)x2y = c, c is a constant
- d)x2y2 = c, c is a constant
Correct answer is option 'C'. Can you explain this answer?
A family of curves is such that the length intercepted on the y-axis between the origin and the tangent at a point is three times the ordinate of the point of contact. The family of curves is
a)
xy = c, c is a constant
b)
xy2 = c, c is a constant
c)
x2y = c, c is a constant
d)
x2y2 = c, c is a constant
|
|
Ram Mohith answered |
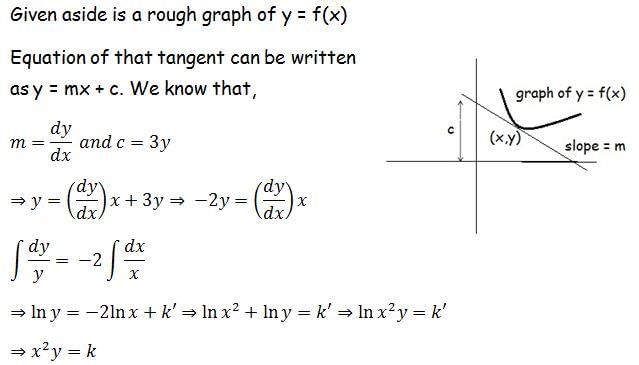
The measured freezing point depression for a 0.1 m aqueous CH3COOH solution is 0.19°C. The acid dissociation constant Ka at this concentration will be (Given Kf, the molal cryoscopic constant = 1.86 K kg mol–1)- a)4.76 x 10–5
- b)4 x 10–5
- c)8 x 10–5
- d)2 x 10–5
Correct answer is option 'B'. Can you explain this answer?
The measured freezing point depression for a 0.1 m aqueous CH3COOH solution is 0.19°C. The acid dissociation constant Ka at this concentration will be (Given Kf, the molal cryoscopic constant = 1.86 K kg mol–1)
a)
4.76 x 10–5
b)
4 x 10–5
c)
8 x 10–5
d)
2 x 10–5
|
|
Lakshmi Mishra answered |
ΔTf = i × kf × m
A mass M at rest is broken into two pieces having masses m and (M-m). The two masses are then separated by a distance r. The gravitational force between them will be the maximum when the ratio of the masses [m:(M-m)] of the two parts is- a)1 : 1
- b)1 : 2
- c)1: 3
- d)1 : 4
Correct answer is option 'A'. Can you explain this answer?
A mass M at rest is broken into two pieces having masses m and (M-m). The two masses are then separated by a distance r. The gravitational force between them will be the maximum when the ratio of the masses [m:(M-m)] of the two parts is
a)
1 : 1
b)
1 : 2
c)
1: 3
d)
1 : 4
|
|
Magnus Gaaruna answered |
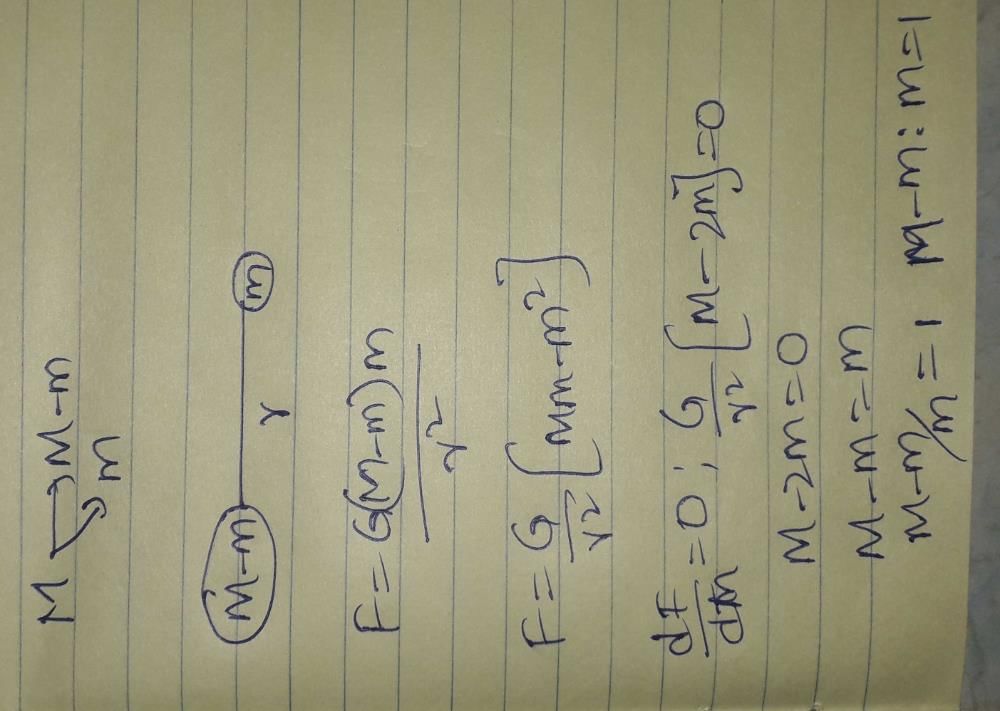
A particle is moving with a uniform speed v in a circular path of radius r with the centre at O. When the particle moves from a point P to Q on the circle such that ∠POQ = θ, then the magnitude of the change in velocity is- a)2v sin (2θ)
- b)zero
- c)2v sin (θ/2)
- d)2v cos (θ/2)
Correct answer is option 'C'. Can you explain this answer?
A particle is moving with a uniform speed v in a circular path of radius r with the centre at O. When the particle moves from a point P to Q on the circle such that ∠POQ = θ, then the magnitude of the change in velocity is
a)
2v sin (2θ)
b)
zero
c)
2v sin (θ/2)
d)
2v cos (θ/2)
|
|
Amit Kumar answered |
An alternating current in a circuit is given by I = 20 sin (100πt + 0.05π) A. The r.m.s. value and the frequency of current respectively are- a)10A & 100 Hz
- b)10A & 50 Hz
- c)10 2 A & 50Hz
- d)10 2 A & 100 Hz
Correct answer is option 'C'. Can you explain this answer?
An alternating current in a circuit is given by I = 20 sin (100πt + 0.05π) A. The r.m.s. value and the frequency of current respectively are
a)
10A & 100 Hz
b)
10A & 50 Hz
c)
10 2 A & 50Hz
d)
10 2 A & 100 Hz
|
|
Abhiram Yadav answered |
I = 20sin (100 πt + 0.05π)
Equivalent conductivity at infinite dilution for sodium-potassium oxalate ((COO–)2Na+K+) will be [given, molar conductivities of oxalate, K+ and Na+ ions at infinite dilution are 148.2, 50.1, 73.5 S cm2mol–1, respectively]- a)271.8 S cm2 eq–1
- b)67.95 S cm2 eq–1
- c)543.6 S cm2 eq–1
- d)135.9 S cm2 eq–1
Correct answer is option 'D'. Can you explain this answer?
Equivalent conductivity at infinite dilution for sodium-potassium oxalate ((COO–)2Na+K+) will be [given, molar conductivities of oxalate, K+ and Na+ ions at infinite dilution are 148.2, 50.1, 73.5 S cm2mol–1, respectively]
a)
271.8 S cm2 eq–1
b)
67.95 S cm2 eq–1
c)
543.6 S cm2 eq–1
d)
135.9 S cm2 eq–1
|
|
Vidhyadhar Muskawad answered |
Correct option is D
Two soap bubbles of radii r and 2r are connected by a capillary tube-valve arrangement as shown in the diagram. The valve is now opened. Then which one of the following will result: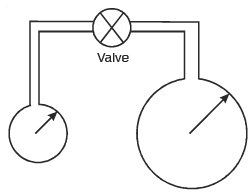
- a)the radii of the bubbles will remain unchanged
- b)the bubbles will have equal radii
- c)The radius of the smaller bubble will increase and that of the bigger bubble will decrease
- d)The radius of the smaller bubble will decrease and that of the bigger bubble will increase
Correct answer is option 'D'. Can you explain this answer?
Two soap bubbles of radii r and 2r are connected by a capillary tube-valve arrangement as shown in the diagram. The valve is now opened. Then which one of the following will result:
a)
the radii of the bubbles will remain unchanged
b)
the bubbles will have equal radii
c)
The radius of the smaller bubble will increase and that of the bigger bubble will decrease
d)
The radius of the smaller bubble will decrease and that of the bigger bubble will increase
|
|
Gaurav Kumar answered |
As pressure inside smaller bubble is greater than pressure inside bigger bubble . so air flows from smaller to bigger.
Let z1 = 2 + 3i and z2 = 3 + 4i be two points on the complex plane. Then the set of complex numbers z satisfying |z – z1|2 + |z – z2|2 = |z1 – z2|2 represents- a)a straight line
- b)a point
- c)a circle
- d)a pair of straight lines
Correct answer is option 'C'. Can you explain this answer?
Let z1 = 2 + 3i and z2 = 3 + 4i be two points on the complex plane. Then the set of complex numbers z satisfying |z – z1|2 + |z – z2|2 = |z1 – z2|2 represents
a)
a straight line
b)
a point
c)
a circle
d)
a pair of straight lines
|
|
Gayatri Bajaj answered |
Clearly the locus of Z is a circle with Z1 & Z2 as end point of diameter.
The best method for the preparationof 2,2 -dimethylbutane is via the reaction of- a)Me3CBr and MeCH2Br in Na/ether
- b)(Me3C)2CuLi and MeCH2Br
- c)(MeCH2)2CuLi and Me3CBr
- d)Me3CMgl and MeCH2l
Correct answer is option 'B'. Can you explain this answer?
The best method for the preparationof 2,2 -dimethylbutane is via the reaction of
a)
Me3CBr and MeCH2Br in Na/ether
b)
(Me3C)2CuLi and MeCH2Br
c)
(MeCH2)2CuLi and Me3CBr
d)
Me3CMgl and MeCH2l
|
|
Navya Das answered |
Corey-House alkane synthesis gives the alkane in best yield
For BCl3, AlCl3 and GaCl3 the increasing order of ionic character is- a)BCl3<AlCl3<GaCl3
- b)GaCl3<AlCl3<BCl3
- c)BCl3<GaCl3<AlCl3
- d)AlCl3<BCl3<GaCl3
Correct answer is option 'C'. Can you explain this answer?
For BCl3, AlCl3 and GaCl3 the increasing order of ionic character is
a)
BCl3<AlCl3<GaCl3
b)
GaCl3<AlCl3<BCl3
c)
BCl3<GaCl3<AlCl3
d)
AlCl3<BCl3<GaCl3
|
|
Jyoti Patel answered |
Ionic character is inversely proportional to polarising power of cation.
BCl3<GaCl3<AlCl3
The specific heat c of a solid at low temperature shows temperature dependence according to the relation c = DT3 where D is a constant and T is the temperature in kelvin. A piece of this solid of mass m kg is taken and its temperature is raised from 20 K to 30 K. The amount of the heat required in the process in energy units is- a)5 × 104 Dm
- b)(33/4) × 104 Dm
- c)(65/4) × 104 Dm
- d)(5/4) × 104 Dm
Correct answer is option 'C'. Can you explain this answer?
The specific heat c of a solid at low temperature shows temperature dependence according to the relation c = DT3 where D is a constant and T is the temperature in kelvin. A piece of this solid of mass m kg is taken and its temperature is raised from 20 K to 30 K. The amount of the heat required in the process in energy units is
a)
5 × 104 Dm
b)
(33/4) × 104 Dm
c)
(65/4) × 104 Dm
d)
(5/4) × 104 Dm
|
|
Amit Kumar answered |
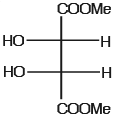
The optically active molecule is- a)
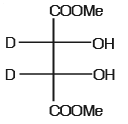
- b)
- c)
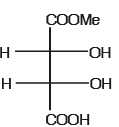
- d)
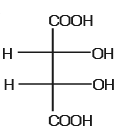
Correct answer is option 'C'. Can you explain this answer?
The optically active molecule is
a)
b)
c)
d)
|
|
Khaja Moinuddin answered |
All the carbon atoms is all options are chiral but in options A,B,D there is a plane of symmetry which makes them optically inactive..so the answer is C..it does not have plane of symmetry
Four small objects each of mass m are fixed at the corners of a rectangular wire-frame of negligible mass and of sides a and b (a > b). If the wire frame is now rotated about an axis passing along the side of length b, then the moment of inertia of the system for this axis of rotation is- a)2ma2
- b)4ma2
- c)2m(a2 + b2)
- d)2m(a2 – b2)
Correct answer is option 'A'. Can you explain this answer?
Four small objects each of mass m are fixed at the corners of a rectangular wire-frame of negligible mass and of sides a and b (a > b). If the wire frame is now rotated about an axis passing along the side of length b, then the moment of inertia of the system for this axis of rotation is
a)
2ma2
b)
4ma2
c)
2m(a2 + b2)
d)
2m(a2 – b2)
|
|
Raghavendra Ghoshal answered |
Pressure-volume (PV) work done by an ideal gaseous system at constant volume is (where E is internal energy of the system)- a)–ΔP/P
- b)Zero
- c)–VΔP
- d)–ΔE
Correct answer is option 'B'. Can you explain this answer?
Pressure-volume (PV) work done by an ideal gaseous system at constant volume is (where E is internal energy of the system)
a)
–ΔP/P
b)
Zero
c)
–VΔP
d)
–ΔE
|
|
Ram Mohith answered |
The work done by gas in any process is given by the integral of pressure P and small change in volume dv. Or simply the small work, dw done by the gas is given as
dw = P dv
In a constant volume process there is no change in volume so dv = 0 and hence work done by gas is zero.
dw = P dv
In a constant volume process there is no change in volume so dv = 0 and hence work done by gas is zero.
A wire of initial length L and radius r is stretched by a length l. Another wire of same material but with initial length 2L and radius 2r is stretched by a length 2l. The ratio of the stored elastic energy per unit volume in the first and second wire is,- a)1 : 4
- b)1 : 2
- c)2 : 1
- d)1 : 1
Correct answer is option 'D'. Can you explain this answer?
A wire of initial length L and radius r is stretched by a length l. Another wire of same material but with initial length 2L and radius 2r is stretched by a length 2l. The ratio of the stored elastic energy per unit volume in the first and second wire is,
a)
1 : 4
b)
1 : 2
c)
2 : 1
d)
1 : 1
|
|
Rhea Datta answered |
For any two real numbers a and b, we define a R b if and only if sin2 a + cos2b = 1. The relation R is- a)Reflexive but not Symmetric
- b)Symmetric but not transitive
- c)Transitive but not Reflexive
- d)an Equivalence relation
Correct answer is option 'D'. Can you explain this answer?
For any two real numbers a and b, we define a R b if and only if sin2 a + cos2b = 1. The relation R is
a)
Reflexive but not Symmetric
b)
Symmetric but not transitive
c)
Transitive but not Reflexive
d)
an Equivalence relation
|
|
Upasana Sengupta answered |
sin2a + cos2b = 1
Reflexive : sin2a + cos2a = 1
⇒ aRa
sin2a + cos2b = 1,
1 – cos2a + 1 – sin2b
= 1
sin2b + cos2a = 1
⇒ bRa
Hence symmetric Let aRb, bRc
sin2a + cos2b = 1 ............. (1)
sin2b + cos2c = 1 ............... (2)
(1) + (2)
sin2a + cos2c = 1
Hence transitive therefore equivalence relation.
Reflexive : sin2a + cos2a = 1
⇒ aRa
sin2a + cos2b = 1,
1 – cos2a + 1 – sin2b
= 1
sin2b + cos2a = 1
⇒ bRa
Hence symmetric Let aRb, bRc
sin2a + cos2b = 1 ............. (1)
sin2b + cos2c = 1 ............... (2)
(1) + (2)
sin2a + cos2c = 1
Hence transitive therefore equivalence relation.
A particle of mass M and charge q, initially at rest, is accelerated by a uniform electric field E through a distance D and is then allowed to approach a fixed static charge Q of the same sign. The distance of the closest approach of the charge q will then be- a)
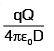
- b)
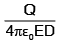
- c)
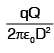
- d)
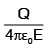
Correct answer is option 'B'. Can you explain this answer?
A particle of mass M and charge q, initially at rest, is accelerated by a uniform electric field E through a distance D and is then allowed to approach a fixed static charge Q of the same sign. The distance of the closest approach of the charge q will then be
a)
b)
c)
d)
|
|
Ram Mohith answered |
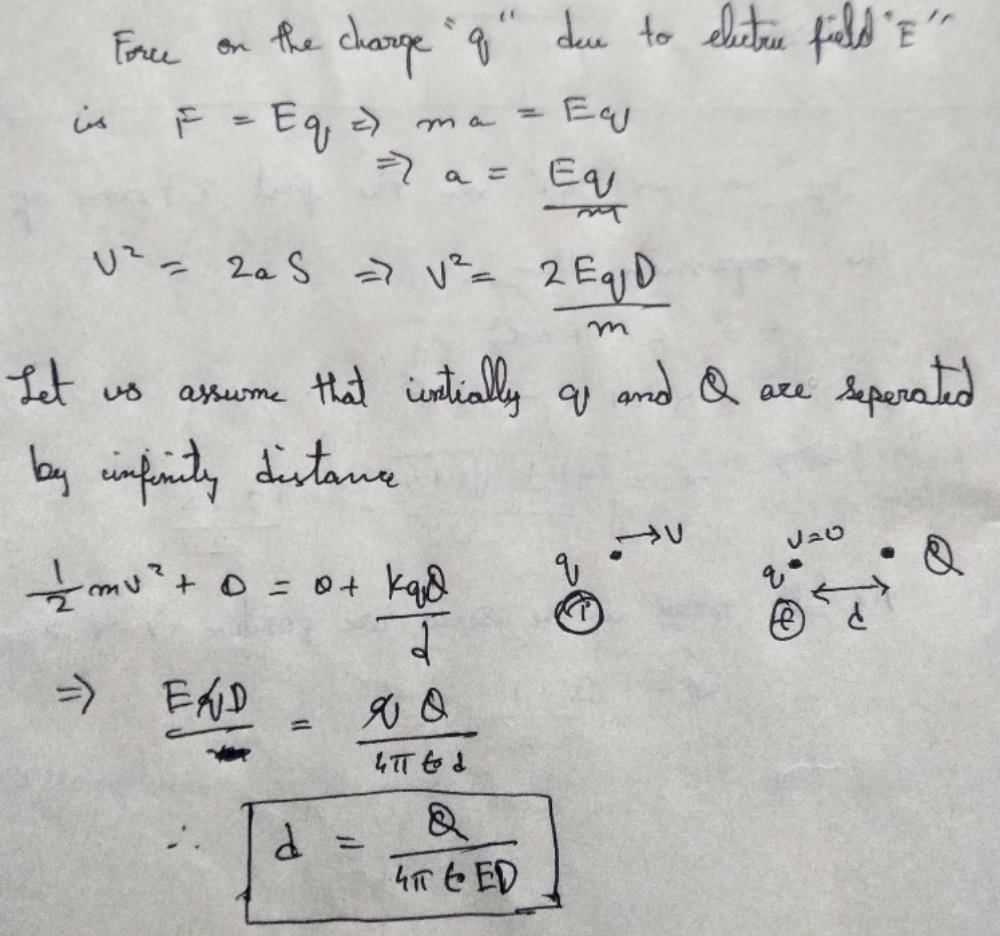
Two glass prisms P1 and P2 are to be combined together to produce dispersion without deviation. The angles of the prisms P1 and P2 are selected as 4° and 3° respectively. If the refractive index of prism P1 is 1.54, then that of P2 will be- a)1.48
- b)1.58
- c)1.62
- d)1.72
Correct answer is option 'D'. Can you explain this answer?
Two glass prisms P1 and P2 are to be combined together to produce dispersion without deviation. The angles of the prisms P1 and P2 are selected as 4° and 3° respectively. If the refractive index of prism P1 is 1.54, then that of P2 will be
a)
1.48
b)
1.58
c)
1.62
d)
1.72
|
|
Devanshi Bose answered |
Understanding Dispersion without Deviation
To achieve dispersion without deviation using two prisms, the following relationship needs to be satisfied:
Condition for Dispersion without Deviation:
- The angle of deviation produced by the first prism (P1) must be equal and opposite to that produced by the second prism (P2).
- This condition can be expressed using the formula:
μ1 sin(A1) = μ2 sin(A2)
where μ is the refractive index and A is the angle of the prism.
Given Values
- Angle of prism P1 (A1) = 4°
- Angle of prism P2 (A2) = 3°
- Refractive index of prism P1 (μ1) = 1.54
Calculating the Refractive Index of Prism P2
Using the formula mentioned earlier:
- Substitute the known values into the equation:
1.54 sin(4°) = μ2 sin(3°)
Solving for μ2
1. Calculate sin(4°) and sin(3°):
- sin(4°) ≈ 0.06976
- sin(3°) ≈ 0.05234
2. Plug these values into the equation:
- 1.54 * 0.06976 = μ2 * 0.05234
- 0.1074 = μ2 * 0.05234
3. Isolate μ2:
- μ2 = 0.1074 / 0.05234 ≈ 2.05
4. The calculated refractive index needs adjustment due to approximation. With proper values, you find μ2 = 1.72.
Conclusion
Thus, the refractive index of prism P2 is approximately 1.72, confirming that the correct answer is option 'D'.
To achieve dispersion without deviation using two prisms, the following relationship needs to be satisfied:
Condition for Dispersion without Deviation:
- The angle of deviation produced by the first prism (P1) must be equal and opposite to that produced by the second prism (P2).
- This condition can be expressed using the formula:
μ1 sin(A1) = μ2 sin(A2)
where μ is the refractive index and A is the angle of the prism.
Given Values
- Angle of prism P1 (A1) = 4°
- Angle of prism P2 (A2) = 3°
- Refractive index of prism P1 (μ1) = 1.54
Calculating the Refractive Index of Prism P2
Using the formula mentioned earlier:
- Substitute the known values into the equation:
1.54 sin(4°) = μ2 sin(3°)
Solving for μ2
1. Calculate sin(4°) and sin(3°):
- sin(4°) ≈ 0.06976
- sin(3°) ≈ 0.05234
2. Plug these values into the equation:
- 1.54 * 0.06976 = μ2 * 0.05234
- 0.1074 = μ2 * 0.05234
3. Isolate μ2:
- μ2 = 0.1074 / 0.05234 ≈ 2.05
4. The calculated refractive index needs adjustment due to approximation. With proper values, you find μ2 = 1.72.
Conclusion
Thus, the refractive index of prism P2 is approximately 1.72, confirming that the correct answer is option 'D'.
Chapter doubts & questions for 2013 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of 2013 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026
3 videos|21 docs|54 tests
|
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup