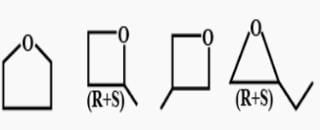
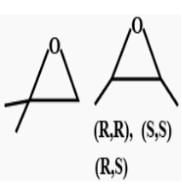
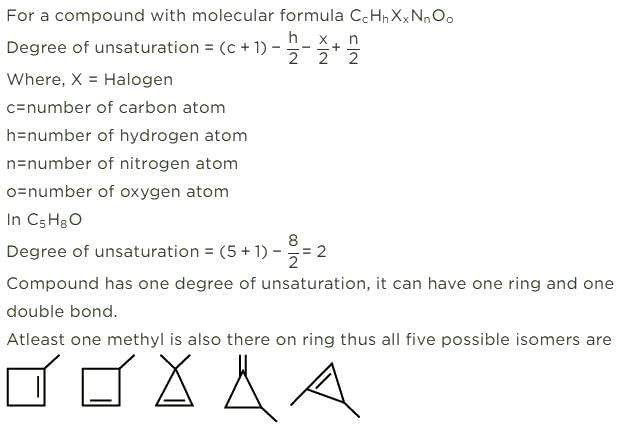
All questions of Hydrocarbon Isomers for Grade 9 Exam
Which among the following defines Meso forms of isomers?
- a)Meso form is optically inactive due to external compensation.
- b)The molecules of the meso isomers are chiral.
- c)It can be separated into optically active enantiomeric pairs.
- d)It is a single compound.
Correct answer is option 'D'. Can you explain this answer?
Which among the following defines Meso forms of isomers?
a)
Meso form is optically inactive due to external compensation.
b)
The molecules of the meso isomers are chiral.
c)
It can be separated into optically active enantiomeric pairs.
d)
It is a single compound.
|
|
Rohan Singh answered |
Meso forms of isomers are single compounds and their molecules are achiral hence they cannot be separated into pairs.
Additional Information: A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral.
If chlorocyclohexane is subjected to further chlorination, how many different isomers (geometrical plus structural only) of dichiorocyclohexane would be produced? - a)3
- b)5
- c)6
- d)7
Correct answer is option 'D'. Can you explain this answer?
If chlorocyclohexane is subjected to further chlorination, how many different isomers (geometrical plus structural only) of dichiorocyclohexane would be produced?
a)
3
b)
5
c)
6
d)
7

|
Learners Habitat answered |
The correct answer is option D
There will be three constitutional isomers i.e. 1,2 dichloro cyclohexane,1,3 dichloro cyclohexane and1,4 dichloro cyclohexane.Each constitutional isomer will have cis and trans configuration thereby making six isomers in all.Also there will be two optically active isomers.
You can understand it better through these infographics
1,2 dichloro cyclohexane
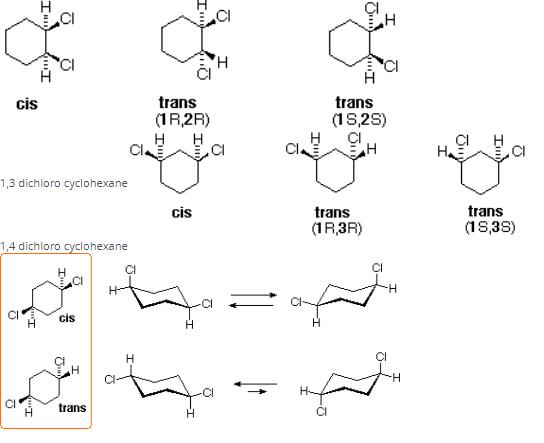
There will be three constitutional isomers i.e. 1,2 dichloro cyclohexane,1,3 dichloro cyclohexane and1,4 dichloro cyclohexane.Each constitutional isomer will have cis and trans configuration thereby making six isomers in all.Also there will be two optically active isomers.
You can understand it better through these infographics
1,2 dichloro cyclohexane

- a)3
- b)4
- c)5
- d)6
Correct answer is option 'D'. Can you explain this answer?
a)
3
b)
4
c)
5
d)
6
|
|
Krishna Iyer answered |
General formula of ketone = CnH2nO
12x+2x+16 = 100
x = 6
So, the formula of compound is C6H12O. We have to make ketone only. These are as follow
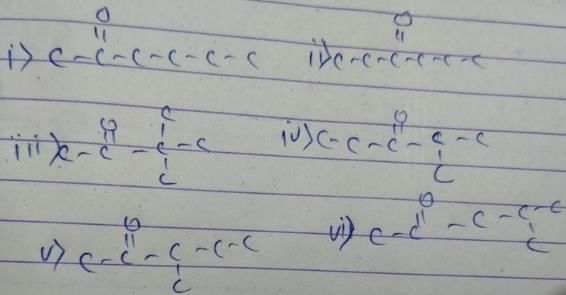
12x+2x+16 = 100
x = 6
So, the formula of compound is C6H12O. We have to make ketone only. These are as follow
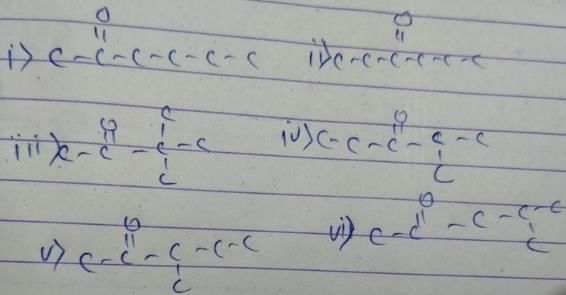
- a)3
- b)4
- c)5
- d)7
Correct answer is option 'A'. Can you explain this answer?
a)
3
b)
4
c)
5
d)
7
|
|
Riya Banerjee answered |
The compounds with each doubly bonded carbon attached to two different groups (like Cab=Cab, Cab=Ccd) exhibit geometrical isomerism i.e., cis and trans forms. The geometrical isomerism arises due to restricted rotation of double bond.
However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to one group each only.
The observed order of the stability of the cabocation is:- a)(CH3)2CH+ < (CH3)3C+ 3+ < CH3CH2+
- b)CH3+ < CH3CH2+ <(CH3)2CH+ < (CH3)3C+
- c)CH3CH2+ <(CH3)2CH+ < (CH3)3C+ < CH3+
- d)CH3+ < CH3CH2+ < (CH3)3C+ <(CH3)2CH+
Correct answer is option 'B'. Can you explain this answer?
The observed order of the stability of the cabocation is:
a)
(CH3)2CH+ < (CH3)3C+ 3+ < CH3CH2+
b)
CH3+ < CH3CH2+ <(CH3)2CH+ < (CH3)3C+
c)
CH3CH2+ <(CH3)2CH+ < (CH3)3C+ < CH3+
d)
CH3+ < CH3CH2+ < (CH3)3C+ <(CH3)2CH+
|
|
Rajesh Gupta answered |
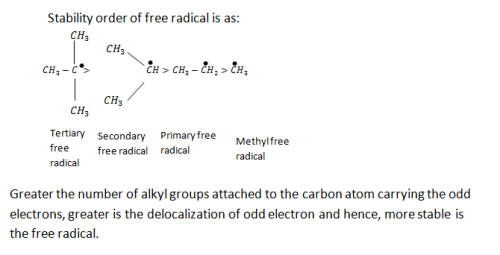
Alkyl groups directly attached to the +vely charged carbon stabilize the carbocations due to inductive and hyperconjugation effects.
Inductive effect:
→ Stability of carbocation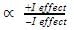
→ More number of +I group more stable carbocation.
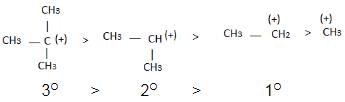
Hyperconjugation:
Inductive effect:
→ Stability of carbocation
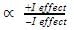
→ More number of +I group more stable carbocation.
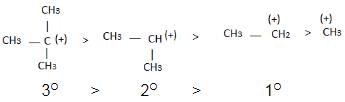
Hyperconjugation:
Stability
∝Number of canonical structures
∝Number of H (alpha hydrogen)
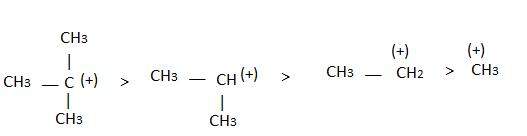
∝Number of canonical structures
∝Number of H (alpha hydrogen)
Which is true about conformers of 1,1,2,2-tetrachloroethane?Which is true about conformers of 1,1,2,2-tetrachloroethane?
a) The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
b) In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
c) In the most stable conformer, dihedral angle between hydrogen atoms is 60°
d) The most stable conformer is non-polar - a)The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
- b)In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
- c)In the most stable conformer, dihedral angle between hydrogen atoms is 60°
- d)The most stable conformer is non-polar
Correct answer is option 'D'. Can you explain this answer?
Which is true about conformers of 1,1,2,2-tetrachloroethane?
Which is true about conformers of 1,1,2,2-tetrachloroethane?
a) The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
b) In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
c) In the most stable conformer, dihedral angle between hydrogen atoms is 60°
d) The most stable conformer is non-polar
a) The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
b) In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
c) In the most stable conformer, dihedral angle between hydrogen atoms is 60°
d) The most stable conformer is non-polar
a)
The most stable conformer has dihedral angle of 60° between all adjacent chlorine atoms
b)
In the least stable conformer, two Cl-atoms are eclipsing one another while other two Cl-atoms are eclipsed to hydrogen atoms
c)
In the most stable conformer, dihedral angle between hydrogen atoms is 60°
d)
The most stable conformer is non-polar
|
|
Mira Joshi answered |
The correct answer is option D
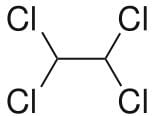
This molecule is non-polar two Cl atoms in one carbon atom cancel the polarity other two Cl atoms on the next carbon.
Also all the Cl atoms are at max distance from each other so max. Stability due to less repulsion between Cl atoms. Dihedral angle between H and Cl is 60°

This molecule is non-polar two Cl atoms in one carbon atom cancel the polarity other two Cl atoms on the next carbon.
Also all the Cl atoms are at max distance from each other so max. Stability due to less repulsion between Cl atoms. Dihedral angle between H and Cl is 60°

- a)3
- b)5
- c)7
- d)8
Correct answer is option 'C'. Can you explain this answer?
a)
3
b)
5
c)
7
d)
8
|
|
Neha Joshi answered |
The correct answer is Option B.
5 isomers are possible. C5H10(CnH2n). Molecules having the CnH2n formulas are most likely to be cyclic alkanes. The five isomers are:
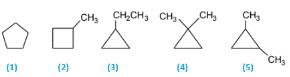
(1) Cyclopentane
(2) 1-Methylcyclobutane
(3) 1-Ethylcyclopropane
(4) 1,1-Dimethylcyclopropane
(5) 1,2-Dimethylcyclopropane
5 isomers are possible. C5H10(CnH2n). Molecules having the CnH2n formulas are most likely to be cyclic alkanes. The five isomers are:

(1) Cyclopentane
(2) 1-Methylcyclobutane
(3) 1-Ethylcyclopropane
(4) 1,1-Dimethylcyclopropane
(5) 1,2-Dimethylcyclopropane
Consider all possible isomeric ketones, including stereoisomers of Molecular weight 100. All these isomers are independently reacted with NaBH4
(Note stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are
[JEE Advanced 2014] - a)3
- b)4
- c)5
- d)6
Correct answer is option 'C'. Can you explain this answer?
Consider all possible isomeric ketones, including stereoisomers of Molecular weight 100. All these isomers are independently reacted with NaBH4
(Note stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are
(Note stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are
[JEE Advanced 2014]
a)
3
b)
4
c)
5
d)
6
|
|
Pooja Shah answered |
The general formula of isomeric ketone having molecular mass 100 is C6H12O [6×12+12×1+16].
The possible structure will be :
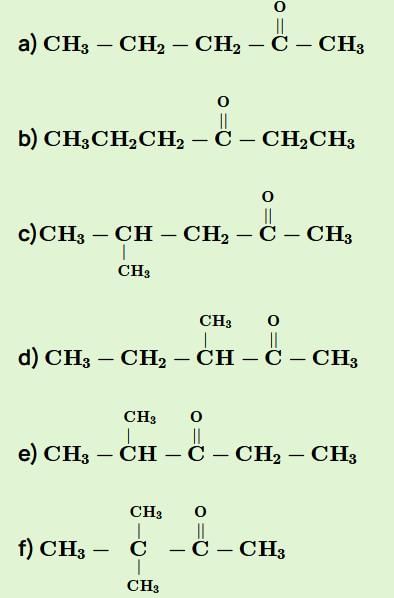
The number of ketones that gives racemic mixture with NaBH4 is 5 as the ketone with chiral center will give diastereoisomer with NaBH4
The isomer of ethanol is:
- a)Dimethyl ether
- b)Diethyl ether
- c)Methanol
- d)Acetone
Correct answer is option 'A'. Can you explain this answer?
The isomer of ethanol is:
a)
Dimethyl ether
b)
Diethyl ether
c)
Methanol
d)
Acetone
|
|
Pranavi Kulkarni answered |
The isomer of ethanol(CH3-CH2-OH) is dimethyl ether(CH3-O-CH3). This is a functional isomer of ethanol.
Explanation:
- Functional isomers are the ones with the same molecular formula but different functional groups.
- The molecular formula for dimethyl ether is C2H6O similar to ethanol.
- Dimethyl ether has the structural formula CH3OCH3 and ethanol has the structural formula CH3CH2OH.
- Therefore, dimethyl ether is the functional isomer of ethanol as both have the same molecular formula but one is ether and the other is alcohol.
 [JEE Main 2014 Online Exam]
[JEE Main 2014 Online Exam]- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
[JEE Main 2014 Online Exam]
a)
a
b)
b
c)
c
d)
d
|
|
Siddharth Mehra answered |
The correct answer is option 'B' - sp2 and sp.
Explanation:
In allene (C3H4), there are two carbon atoms, each with three sigma bonds. To determine the hybridization of the carbon atoms, we need to look at their bonding and lone pairs of electrons.
1. Hybridization of the first carbon atom:
- The first carbon atom is bonded to two other carbon atoms and one hydrogen atom.
- It has three sigma bonds, which means it will have three hybrid orbitals.
- The carbon atom also has one lone pair of electrons.
- The presence of three sigma bonds and one lone pair indicates sp2 hybridization.
- Therefore, the first carbon atom is sp2 hybridized.
2. Hybridization of the second carbon atom:
- The second carbon atom is also bonded to two other carbon atoms and one hydrogen atom.
- It also has three sigma bonds, which means it will have three hybrid orbitals.
- The carbon atom does not have any lone pairs of electrons.
- The presence of only three sigma bonds indicates sp hybridization.
- Therefore, the second carbon atom is sp hybridized.
In summary, in allene (C3H4), the type of hybridization of the carbon atoms is sp2 and sp.
Explanation:
In allene (C3H4), there are two carbon atoms, each with three sigma bonds. To determine the hybridization of the carbon atoms, we need to look at their bonding and lone pairs of electrons.
1. Hybridization of the first carbon atom:
- The first carbon atom is bonded to two other carbon atoms and one hydrogen atom.
- It has three sigma bonds, which means it will have three hybrid orbitals.
- The carbon atom also has one lone pair of electrons.
- The presence of three sigma bonds and one lone pair indicates sp2 hybridization.
- Therefore, the first carbon atom is sp2 hybridized.
2. Hybridization of the second carbon atom:
- The second carbon atom is also bonded to two other carbon atoms and one hydrogen atom.
- It also has three sigma bonds, which means it will have three hybrid orbitals.
- The carbon atom does not have any lone pairs of electrons.
- The presence of only three sigma bonds indicates sp hybridization.
- Therefore, the second carbon atom is sp hybridized.
In summary, in allene (C3H4), the type of hybridization of the carbon atoms is sp2 and sp.
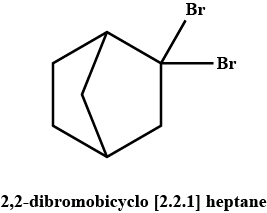
A pair of enantiomers is possible for _______ isomer of 2,2-dibromobicyclo [2.2.1] heptane.
- a)cis
- b)trans
- c)both cis and trans
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?
A pair of enantiomers is possible for _______ isomer of 2,2-dibromobicyclo [2.2.1] heptane.
a)
cis
b)
trans
c)
both cis and trans
d)
none of the above
|
|
Tejas Verma answered |
Only one pair of enantiomers is possible for cis-2,2-dibromobicyclo [2.2.1] heptane. The trans arrangement of one carbon bridge is structurally impossible. Such a molecule would have too much strain.
How many structural isomers are possible with molecular formula C4H10O ?- a)3
- b)5
- c)7
- d)8
Correct answer is option 'C'. Can you explain this answer?
How many structural isomers are possible with molecular formula C4H10O ?
a)
3
b)
5
c)
7
d)
8
|
|
Preeti Iyer answered |
The formula that these isomers contain no rings or double bonds. The isomers must be alcohols and ethers.
Let's start by drawing the alcohols.
Start with four carbons in a row and put an OH group in every possible position.
This gives us
1. CH3CH2CH2CH2OH, butan 1-ol
and
2. CH3CH2CH(OH)CH3, butan-2-ol
Now use a 3-carbon chain with a CH3 on the middle carbon.
This gives
(CH3)2CHCH2OH, 2-methylpropan-1-ol
and
(CH3)3COH, 2-methylpropan-2-ol.
Now for the ethers.
Let's start with five atoms in a row.
This gives
CH3CH2CH2OCH3, 1-methoxypropane
and
CH3CH2OCH2CH3 , ethoxyethane
Finally, the only choice for a branched-chain ether is
(CH3)2CHOCH3 , 2-methoxpropane.
And there are your seven isomers of C4H10O.
Direction (Q. Nos. 1 - 9) This section contains 9 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
- a)3
- b)5
- c)7
- d)9
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1 - 9) This section contains 9 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
a)
3
b)
5
c)
7
d)
9

|
Kamna Science Academy answered |
The number of optically active isomers = 2n−1−2n−1/2 =23−1−23−1/2 =2.
Heterolytic cleavage is a way to cleave the:- a)Non-ionic bonds
- b)Ionic bonds
- c)Covalent bonds
- d)Polar bonds
Correct answer is option 'C'. Can you explain this answer?
Heterolytic cleavage is a way to cleave the:
a)
Non-ionic bonds
b)
Ionic bonds
c)
Covalent bonds
d)
Polar bonds
|
|
Rahul Bansal answered |
In heterolytic cleavage, a covalent bond breaks in such a way that one fragment gets both of the shared electrons. In homolytic cleavage, a covalent bond breaks in such a way that each fragment gets one of the shared electrons. The word heterolytic comes from the Greek heteros, "different", and lysis, "loosening".
C3H6O represents an aldehyde and a ketone, is a type of:
- a)Chain isomerism
- b)Functional group isomerism
- c)Metamerism
- d)Position isomerism
Correct answer is option 'B'. Can you explain this answer?
C3H6O represents an aldehyde and a ketone, is a type of:
a)
Chain isomerism
b)
Functional group isomerism
c)
Metamerism
d)
Position isomerism
|
|
Preeti Iyer answered |
The aldehyde and ketone that can be represented by molecular formula C3H6O are propanal and propanone respectively.
Aldehyde - Propanal - CH3CH2CHO
Ketone - Propanone - CH3COCH3
These two carbon compounds are called functional isomers. They do not have the same physical properties since their functional group is different.
When hydrogen is present on one side of C=O group and other side is R, (RCHO), then it is an aldehyde group. When R is present on both sides of C=O group, then it is a ketone.
The number of optical isomers possible for 2, 3-pentanediol is:
- a)2
- b)3
- c)4
- d)5
Correct answer is option 'C'. Can you explain this answer?
The number of optical isomers possible for 2, 3-pentanediol is:
a)
2
b)
3
c)
4
d)
5
|
|
Preeti Iyer answered |
2 optical centers.
Total optically active isomers =22
=4
Total optically active isomers =22
=4
Which compound below is capable of showing geometrical isomerism ?- a)CH3 - CH = C = CH - CH3
- b)CH3 - CH = C = C = CH - CH3
- c)
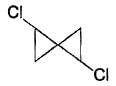
- d)
Correct answer is option 'B'. Can you explain this answer?
Which compound below is capable of showing geometrical isomerism ?
a)
CH3 - CH = C = CH - CH3
b)
CH3 - CH = C = C = CH - CH3
c)

d)


|
Kamna Science Academy answered |
The correct answer is option B
CH3 – CH = C = C = CH – CH3 will show geometrical isomerism because it is odd cumulene (3 pie bond) and odd cumulene always shows geometrical isomerism
CH3 – CH = C = C = CH – CH3 will show geometrical isomerism because it is odd cumulene (3 pie bond) and odd cumulene always shows geometrical isomerism
Which of the following statements are correct?
I. A pair of positional isomers differs in the position of the same functional group.
II. A pair of. structural isomers have the same relative molar mass.
Ill. A pair of functional group isomers belongs to different homologous series.
- a)I, II and Ill
- b)I and Ill
- c)II and III
- d)I and II
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements are correct?
I. A pair of positional isomers differs in the position of the same functional group.
II. A pair of. structural isomers have the same relative molar mass.
Ill. A pair of functional group isomers belongs to different homologous series.
II. A pair of. structural isomers have the same relative molar mass.
Ill. A pair of functional group isomers belongs to different homologous series.
a)
I, II and Ill
b)
I and Ill
c)
II and III
d)
I and II
|
|
Preeti Iyer answered |
According to me all statements are correct. For statement I, positional isomers have the same functional group. They just differ in the position of that group.
Statenmen II- Structural isomers have the same relative molar mass. Actually relative mass means the mass of one isomer is samee relative to another. Structural isomers differ in the structure of molecules. However they have the same molecular formula and hence; same molar mass.
Statement III- According to me, this statement is correct. As homologous series must contain the same functional group with the same physical & chemical properties. Also functional group isomers differ in the functional group attached with them. So, functional group isomers belong to different homologous series.
Statenmen II- Structural isomers have the same relative molar mass. Actually relative mass means the mass of one isomer is samee relative to another. Structural isomers differ in the structure of molecules. However they have the same molecular formula and hence; same molar mass.
Statement III- According to me, this statement is correct. As homologous series must contain the same functional group with the same physical & chemical properties. Also functional group isomers differ in the functional group attached with them. So, functional group isomers belong to different homologous series.
Which among the following is not an aromatic compound(in specific)- a)Naphthalene
- b)Aniline
- c)Pyridine
- d)Tropolone
Correct answer is option 'C'. Can you explain this answer?
Which among the following is not an aromatic compound(in specific)
a)
Naphthalene
b)
Aniline
c)
Pyridine
d)
Tropolone

|
Sai Mishra answered |
Pyridine is heterocyclic aromatic compound. Whereas naphthalene and aniline are benzenoid aromatic compounds and tropolone is a non-benzenoid aromatic compound.
How many different isomers of alkene with molecular formula C7H14, on catalytic hydrogenation, can give 3-methyl hexane?
Correct answer is '9'. Can you explain this answer?
How many different isomers of alkene with molecular formula C7H14, on catalytic hydrogenation, can give 3-methyl hexane?
|
|
Pranavi Banerjee answered |
Explanation:
Isomers are compounds with the same molecular formula but different arrangements of atoms in space. In this case, we are looking for isomers of an alkene with the molecular formula C7H14 that can give 3-methyl hexane on catalytic hydrogenation.
Step 1: Draw the structural formula of 3-methyl hexane
3-methyl hexane has the structural formula:

Step 2: Draw the structural formula of the alkene
The alkene has the molecular formula C7H14, which means it has two fewer hydrogen atoms than 3-methyl hexane. Therefore, we can start by drawing all the possible ways to add a double bond to a chain of seven carbon atoms.

Step 3: Identify the isomers that can give 3-methyl hexane on catalytic hydrogenation
We can now check each of these isomers to see if they can give 3-methyl hexane on catalytic hydrogenation. If an isomer can give 3-methyl hexane, it means that it has a methyl group (CH3) attached to the third carbon atom from the double bond.
- Isomer 1: This isomer cannot give 3-methyl hexane on catalytic hydrogenation because it does not have a methyl group attached to the third carbon atom from the double bond.
- Isomer 2: This isomer can give 3-methyl hexane on catalytic hydrogenation because it has a methyl group attached to the third carbon atom from the double bond.
- Isomer 3: This isomer cannot give 3-methyl hexane on catalytic hydrogenation because it does not have a methyl group attached to the third carbon atom from the double bond.
- Isomer 4: This isomer can give 3-methyl hexane on catalytic hydrogenation because it has a methyl group attached to the third carbon atom from the double bond.
- Isomer 5: This isomer cannot give 3-methyl hexane on catalytic hydrogenation because it does not have a methyl group attached to the third carbon atom from the double bond.
- Isomer 6: This isomer can give 3-methyl hexane on catalytic hydrogenation because it has a methyl group attached to the third carbon atom from the double bond.
- Isomer 7: This isomer cannot give 3-methyl hexane on catalytic hydrogenation because it does not have a methyl group attached to the third carbon atom from the double bond.
- Isomer 8: This isomer can give 3-methyl hexane on catalytic hydrogenation because it has a methyl group attached to the third carbon atom from the double bond.
- Isomer 9: This isomer can
Which of the following are not functional isomers of each other?
- a)CH3CH2NO2 and CH3CH2ON=O
- b)C2H5CHO and CH3COCH3
- c)CH3CH2NH2 and CH3NHCH3
- d)C3H7NH2 and (CH3)2CHNH2
Correct answer is option 'D'. Can you explain this answer?
Which of the following are not functional isomers of each other?
a)
CH3CH2NO2 and CH3CH2ON=O
b)
C2H5CHO and CH3COCH3
c)
CH3CH2NH2 and CH3NHCH3
d)
C3H7NH2 and (CH3)2CHNH2
|
|
Anjali Sharma answered |
Functional isomers are structural isomers that have the same molecular formula (that is, the same number of atoms of the same elements), but the atoms are connected in different ways so that the groupings are dissimilar or different functional groups.
10 amine to different 10 amines are not functional isomers.
Hence, option ′d′ is the answer.

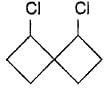
How many geometrical isomers exist for the molecule shown below ?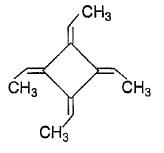
- a)2
- b)3
- c)5
- d)6
Correct answer is option 'B'. Can you explain this answer?
How many geometrical isomers exist for the molecule shown below ?

a)
2
b)
3
c)
5
d)
6

|
EduRev JEE answered |
The correct answer is option B
Threegeometrical isomers exits of the molecule given cis-cis, tran-trans and cis-trans.
Threegeometrical isomers exits of the molecule given cis-cis, tran-trans and cis-trans.
Organic compounds are broadly classified as
- a)alicyclic compounds and acyclic compounds
- b)Open chain compounds and linear chain compounds
- c)Cyclic compounds and alicyclic compounds
- d)Open chain compounds and closed compounds
Correct answer is option 'D'. Can you explain this answer?
Organic compounds are broadly classified as
a)
alicyclic compounds and acyclic compounds
b)
Open chain compounds and linear chain compounds
c)
Cyclic compounds and alicyclic compounds
d)
Open chain compounds and closed compounds

|
Arpita Nambiar answered |
The correct answer is option D
Organic compounds are broadly classified into open chain and closed chain compounds. Explanation: open chain compounds or acyclic compounds are otherwise called as aliphatic compounds.
Organic compounds are broadly classified into open chain and closed chain compounds. Explanation: open chain compounds or acyclic compounds are otherwise called as aliphatic compounds.
Which is not true regarding conformers of ethane?- a)Theoretically infinite conformers exist
- b)Staggered conformer has lower torsional strain than eclipsed one
- c)Increasing temperature increases the percentage of eclipsed conformer
- d)By precise experimental setup, staggered conformer can be separated out of system
Correct answer is option 'D'. Can you explain this answer?
Which is not true regarding conformers of ethane?
a)
Theoretically infinite conformers exist
b)
Staggered conformer has lower torsional strain than eclipsed one
c)
Increasing temperature increases the percentage of eclipsed conformer
d)
By precise experimental setup, staggered conformer can be separated out of system
|
|
Neha Joshi answered |
The correct answer is Option D.
Although conformers differ in potential energy and stability, the difference is so small that it does not allow their practical separation.
Although conformers differ in potential energy and stability, the difference is so small that it does not allow their practical separation.
The number of isomers of C6H14 is:- a)4
- b)5
- c)6
- d)7
Correct answer is option 'B'. Can you explain this answer?
The number of isomers of C6H14 is:
a)
4
b)
5
c)
6
d)
7
|
|
Anu Mukherjee answered |
**Explanation:**
To determine the number of isomers of C6H14, we need to consider the different ways in which carbon atoms can be arranged and the different ways in which hydrogen atoms can be distributed among these carbon atoms.
**1. Straight-chain isomers:**
The simplest isomer is the straight chain, where the carbon atoms are arranged in a linear sequence. For C6H14, there is only one straight-chain isomer.
**2. Branched-chain isomers:**
In branched-chain isomers, the carbon atoms are arranged in a branched manner. To determine the number of possible branched-chain isomers, we need to consider the different ways in which the branches can be arranged and the different positions at which the branches can be attached to the main chain.
In this case, one of the carbons in the straight chain can have three branches attached to it. We can have three different arrangements of these branches, which are:
- One branch attached to the first carbon and two branches attached to the second carbon.
- Two branches attached to the first carbon and one branch attached to the second carbon.
- Three branches attached to the first carbon and no branches attached to the second carbon.
For each of these arrangements, there are different possibilities for the positions of the branches along the main chain. Therefore, for the branched-chain isomers, there are a total of three possibilities.
**3. Cycloalkane isomers:**
Cycloalkanes are ring structures where the carbon atoms are arranged in a closed loop. For C6H14, we can have cycloalkane isomers with different ring sizes.
- Cyclohexane: In this case, all six carbon atoms form a single ring. There is only one possibility.
- Cyclopentane: In this case, five carbon atoms form a ring and the remaining carbon atom is attached to one of the carbons in the ring. There is only one possibility.
Therefore, the total number of isomers of C6H14 is the sum of the straight-chain isomer, branched-chain isomers, and cycloalkane isomers, which is 1 + 3 + 2 = 6.
Therefore, the correct answer is option **c) 6**.
To determine the number of isomers of C6H14, we need to consider the different ways in which carbon atoms can be arranged and the different ways in which hydrogen atoms can be distributed among these carbon atoms.
**1. Straight-chain isomers:**
The simplest isomer is the straight chain, where the carbon atoms are arranged in a linear sequence. For C6H14, there is only one straight-chain isomer.
**2. Branched-chain isomers:**
In branched-chain isomers, the carbon atoms are arranged in a branched manner. To determine the number of possible branched-chain isomers, we need to consider the different ways in which the branches can be arranged and the different positions at which the branches can be attached to the main chain.
In this case, one of the carbons in the straight chain can have three branches attached to it. We can have three different arrangements of these branches, which are:
- One branch attached to the first carbon and two branches attached to the second carbon.
- Two branches attached to the first carbon and one branch attached to the second carbon.
- Three branches attached to the first carbon and no branches attached to the second carbon.
For each of these arrangements, there are different possibilities for the positions of the branches along the main chain. Therefore, for the branched-chain isomers, there are a total of three possibilities.
**3. Cycloalkane isomers:**
Cycloalkanes are ring structures where the carbon atoms are arranged in a closed loop. For C6H14, we can have cycloalkane isomers with different ring sizes.
- Cyclohexane: In this case, all six carbon atoms form a single ring. There is only one possibility.
- Cyclopentane: In this case, five carbon atoms form a ring and the remaining carbon atom is attached to one of the carbons in the ring. There is only one possibility.
Therefore, the total number of isomers of C6H14 is the sum of the straight-chain isomer, branched-chain isomers, and cycloalkane isomers, which is 1 + 3 + 2 = 6.
Therefore, the correct answer is option **c) 6**.
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q.
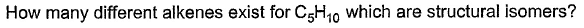
- a)3
- b)5
- c)6
- d)7
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
a)
3
b)
5
c)
6
d)
7
|
|
Naina Sharma answered |
C5 H10
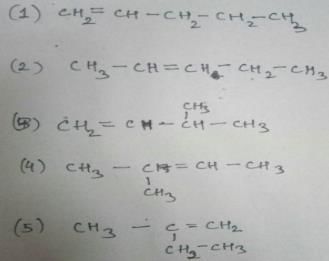
Two times double bond can’t appear as it has asked alkene and not alkadiene. So, these are the only types of alkenes that can be formed which are structural isomers.
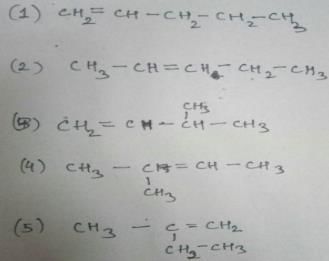
Two times double bond can’t appear as it has asked alkene and not alkadiene. So, these are the only types of alkenes that can be formed which are structural isomers.
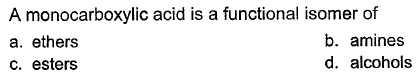 [JEE Main 2013 Online Exam]
[JEE Main 2013 Online Exam]- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
[JEE Main 2013 Online Exam]
a)
a
b)
b
c)
c
d)
d
|
|
Om Desai answered |
In ethers, alcohols and amines, there is no double bond. However, esters have one double bond as same as monocarboxylic acid. So monocarboxylic acid is a functional isomer of ester (only for no. of carbon greater than 2. As we need to have at least 3 carbon atoms to form ester.)
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
a)
a
b)
b
c)
c
d)
d
|
|
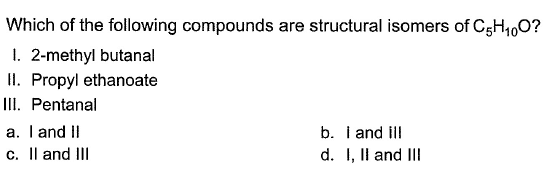
Om Desai answered |
Double bond equivalent of C5H10O is 1. So, it will have either a double bond or a ring.So, it may be an aldehyde, ketone, alcohol( carbon chain having double bond) or epoxide.
Propyl ethanoate has 2 oxygen atoms. So, it will never be its isomer. HOwever option a and c will be its isomer as they are aldehyde.
Propyl ethanoate has 2 oxygen atoms. So, it will never be its isomer. HOwever option a and c will be its isomer as they are aldehyde.
Match the systems in Column I with their corresponding characterstics from Column II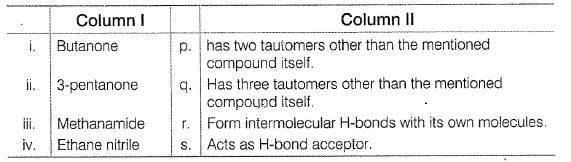 Codes
Codes 

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Match the systems in Column I with their corresponding characterstics from Column II

Codes


a)
a
b)
b
c)
c
d)
d
|
|
Lavanya Menon answered |
The correct answer is option A
i. Butanone- q) Has three tautomers other than the mentioned compound.
s) Acts as H-bonds acceptor.
ii. 3-pentanone- p) has two tautomer’s other than the mentioned compound itself
s) Acts as H-bonds acceptor.
iii. Methanamide - p) has two tautomer’s other than the mentioned compound itself
r) Form intermolecular H-bonds with its own molecules.
s) Acts as H-bonds acceptor.
Iv. Ethane nitrite - p) has two tautomer’s other than the mentioned compound itself
s) Acts as H-bonds acceptor.
i. Butanone- q) Has three tautomers other than the mentioned compound.
s) Acts as H-bonds acceptor.
ii. 3-pentanone- p) has two tautomer’s other than the mentioned compound itself
s) Acts as H-bonds acceptor.
iii. Methanamide - p) has two tautomer’s other than the mentioned compound itself
r) Form intermolecular H-bonds with its own molecules.
s) Acts as H-bonds acceptor.
Iv. Ethane nitrite - p) has two tautomer’s other than the mentioned compound itself
s) Acts as H-bonds acceptor.
Which of the following correctly ranks the cycloalkanes in order of increasing ring strain per methylene group? - a)Cyclopropane < Cyclobutane < Cyclopentane < Cyclohexane
- b)Cyclohexane < Cyclopentane < Cyclobutane < Cyclopropane
- c)Cyclohexane < Cyclobutane < Cyclopentane < Cyclopropane
- d)Cyclopropane < Cyclopentane < Cyclobutane < Cyclohexane
Correct answer is option 'B'. Can you explain this answer?
Which of the following correctly ranks the cycloalkanes in order of increasing ring strain per methylene group?
a)
Cyclopropane < Cyclobutane < Cyclopentane < Cyclohexane
b)
Cyclohexane < Cyclopentane < Cyclobutane < Cyclopropane
c)
Cyclohexane < Cyclobutane < Cyclopentane < Cyclopropane
d)
Cyclopropane < Cyclopentane < Cyclobutane < Cyclohexane
|
|
Rajesh Gupta answered |
The correct answer is Option B.
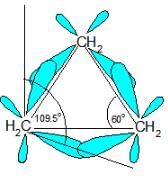
The C-C-C bond angles in cyclopropane (60o) and cyclobutane (90o) are much different than the ideal bond angle of 109.5o.This bond angle causes cyclopropane and cyclobutane to have a high ring strain. However, molecules, such as cyclohexane and cyclopentane, would have a much lower ring strain because the bond angle between the carbons is much closer to 109.5o.

The C-C-C bond angles in cyclopropane (60o) and cyclobutane (90o) are much different than the ideal bond angle of 109.5o.This bond angle causes cyclopropane and cyclobutane to have a high ring strain. However, molecules, such as cyclohexane and cyclopentane, would have a much lower ring strain because the bond angle between the carbons is much closer to 109.5o.
The organic reaction which proceeds through heterolytic bond cleavage are known as:- a)Covalent reactions
- b)Ionic reactions
- c)Free radical reaction
- d)Polar reactions
Correct answer is option 'B'. Can you explain this answer?
The organic reaction which proceeds through heterolytic bond cleavage are known as:
a)
Covalent reactions
b)
Ionic reactions
c)
Free radical reaction
d)
Polar reactions
|
|
Hansa Sharma answered |
In heterolytic bond cleavage the bond breaks unevenly and the shared pair of electrons is accommodated by one of the products, which produces one or more ions.As heterolytic bond cleavage gives ions. So the reaction which proceeds through heterolytic bond cleavage is an ionic reaction.
Hence B is the correct answer.
Which,of the following correctly lists the conformations of cyclohexane in order of increasing potential energies?- a)Chair < Boat < Twist boat < Half-chair
- b)Half-chair < Boat < Twist boat < Chair
- c)Chair < Twist boat < Half-chair < Boat
- d)Chair < Twist boat < Boat < Half-chair
Correct answer is option 'D'. Can you explain this answer?
Which,of the following correctly lists the conformations of cyclohexane in order of increasing potential energies?
a)
Chair < Boat < Twist boat < Half-chair
b)
Half-chair < Boat < Twist boat < Chair
c)
Chair < Twist boat < Half-chair < Boat
d)
Chair < Twist boat < Boat < Half-chair
|
|
Riya Banerjee answered |
Correct answer is option D
Chair >Twist boat > Boat > Half-chair
above is the stability order of
the conformed.
Stability is inversely proportional to potential energy.
the conformed.
Stability is inversely proportional to potential energy.
Aliphatic compound is the other name for- a)Acyclic compounds
- b)Alicyclic compounds
- c)Ring compounds
- d)Closed chain compounds
Correct answer is option 'A'. Can you explain this answer?
Aliphatic compound is the other name for
a)
Acyclic compounds
b)
Alicyclic compounds
c)
Ring compounds
d)
Closed chain compounds

|
Mansi Mukherjee answered |
Open chain compounds or acyclic compounds are otherwise called as aliphatic compounds.
A species having a carbon atom possessing a sextet of electrons and a positive charge is called as:- a)Chirality
- b)Metamerism
- c)Carbocation
- d)Carbaanion
Correct answer is option 'C'. Can you explain this answer?
A species having a carbon atom possessing a sextet of electrons and a positive charge is called as:
a)
Chirality
b)
Metamerism
c)
Carbocation
d)
Carbaanion

|
Rounak Desai answered |
Carbocation:
A carbocation is a species that contains a positively charged carbon atom. It is formed when a carbon atom loses a pair of electrons, leaving it with only six electrons in its valence shell. This electron deficiency makes the carbon atom highly reactive and prone to attracting electrons from nearby atoms or molecules.
Structure and Bonding:
In a carbocation, the carbon atom is attached to three other atoms or groups. It forms three sigma bonds by overlapping its atomic orbitals with the orbitals of the other atoms or groups. These sigma bonds provide stability to the positively charged carbon atom.
The carbon atom in a carbocation possesses only six electrons in its valence shell, which results in an incomplete octet. It is called a sextet because it has six valence electrons instead of the usual eight. The positive charge on the carbon atom indicates that it has lost an electron, resulting in a deficiency of negative charge.
Reactivity:
Carbocations are highly reactive species due to their electron deficiency. They are electron acceptors and can react with nucleophiles, which are electron-rich species. The nucleophile donates a pair of electrons to the positively charged carbon atom, neutralizing the charge and forming a new bond.
The reactivity of a carbocation depends on its stability. Carbocations can be classified into three types based on the number of alkyl groups attached to the positively charged carbon atom:
1. Primary carbocation: It has one alkyl group attached to the carbon atom.
2. Secondary carbocation: It has two alkyl groups attached to the carbon atom.
3. Tertiary carbocation: It has three alkyl groups attached to the carbon atom.
Tertiary carbocations are more stable than secondary carbocations, and secondary carbocations are more stable than primary carbocations. This stability is due to the electron-donating nature of the alkyl groups, which helps to disperse the positive charge on the carbon atom.
Applications:
Carbocations are intermediates in many organic reactions, including electrophilic additions, rearrangements, and substitution reactions. Understanding their reactivity and stability is crucial in organic chemistry for predicting reaction mechanisms and designing synthetic routes.
A carbocation is a species that contains a positively charged carbon atom. It is formed when a carbon atom loses a pair of electrons, leaving it with only six electrons in its valence shell. This electron deficiency makes the carbon atom highly reactive and prone to attracting electrons from nearby atoms or molecules.
Structure and Bonding:
In a carbocation, the carbon atom is attached to three other atoms or groups. It forms three sigma bonds by overlapping its atomic orbitals with the orbitals of the other atoms or groups. These sigma bonds provide stability to the positively charged carbon atom.
The carbon atom in a carbocation possesses only six electrons in its valence shell, which results in an incomplete octet. It is called a sextet because it has six valence electrons instead of the usual eight. The positive charge on the carbon atom indicates that it has lost an electron, resulting in a deficiency of negative charge.
Reactivity:
Carbocations are highly reactive species due to their electron deficiency. They are electron acceptors and can react with nucleophiles, which are electron-rich species. The nucleophile donates a pair of electrons to the positively charged carbon atom, neutralizing the charge and forming a new bond.
The reactivity of a carbocation depends on its stability. Carbocations can be classified into three types based on the number of alkyl groups attached to the positively charged carbon atom:
1. Primary carbocation: It has one alkyl group attached to the carbon atom.
2. Secondary carbocation: It has two alkyl groups attached to the carbon atom.
3. Tertiary carbocation: It has three alkyl groups attached to the carbon atom.
Tertiary carbocations are more stable than secondary carbocations, and secondary carbocations are more stable than primary carbocations. This stability is due to the electron-donating nature of the alkyl groups, which helps to disperse the positive charge on the carbon atom.
Applications:
Carbocations are intermediates in many organic reactions, including electrophilic additions, rearrangements, and substitution reactions. Understanding their reactivity and stability is crucial in organic chemistry for predicting reaction mechanisms and designing synthetic routes.
What is wrong about enantiomers of 2-chloropropanoic acid?- a)Have same solubility in water
- b)Have same pKa value
- c)Have same refractive indices
- d)Have same rate of reactions with (+)-2-butanol
Correct answer is option 'D'. Can you explain this answer?
What is wrong about enantiomers of 2-chloropropanoic acid?
a)
Have same solubility in water
b)
Have same pKa value
c)
Have same refractive indices
d)
Have same rate of reactions with (+)-2-butanol
|
|
Preeti Iyer answered |
The correct answer is option D.
Pair of enantiomers react differently with pure enantiomers of other compounds.
Pair of enantiomers react differently with pure enantiomers of other compounds.
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as:- a)Reaction mechanism
- b)Reaction kinetics
- c)Thermodynamics
- d)Equation
Correct answer is option 'A'. Can you explain this answer?
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as:
a)
Reaction mechanism
b)
Reaction kinetics
c)
Thermodynamics
d)
Equation

|
Rounak Desai answered |
Reaction Mechanism
The correct answer is option 'A', reaction mechanism. A reaction mechanism provides a detailed account of each step involved in a chemical reaction, including the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. It explains how reactant molecules rearrange and bond with each other to form products.
Electron Movement
In a chemical reaction, electrons play a crucial role in the formation and breaking of chemical bonds. The movement of electrons determines the reactivity and stability of molecules. The reaction mechanism describes how electrons are transferred or shared between atoms during a reaction. It explains the movement of electrons from high energy orbitals to low energy orbitals, leading to the formation of new bonds and the breaking of existing bonds.
Energetics during Bond Cleavage and Bond Formation
During a chemical reaction, bonds between atoms are broken and new bonds are formed. The reaction mechanism explains the energy changes associated with these processes. Bond cleavage requires an input of energy, known as bond dissociation energy, as bonds are broken. This energy is absorbed from the surroundings. On the other hand, bond formation releases energy, known as bond formation energy, as new bonds are created. The reaction mechanism describes the energy changes during these processes and how they influence the overall energetics of the reaction.
Rates of Transformation (Kinetics)
The reaction mechanism also provides information about the rates of transformation of reactants into products. It describes the individual steps involved in the reaction and the order in which they occur. Each step in the mechanism has a specific rate constant associated with it, which determines how fast that step proceeds. The overall rate of the reaction is determined by the slowest step, known as the rate-determining step. The reaction mechanism helps to identify this step and understand the factors that control the reaction rate.
Conclusion
In summary, a reaction mechanism is a detailed account of the steps involved in a chemical reaction. It describes the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. Understanding the reaction mechanism is crucial for predicting and controlling chemical reactions.
The correct answer is option 'A', reaction mechanism. A reaction mechanism provides a detailed account of each step involved in a chemical reaction, including the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. It explains how reactant molecules rearrange and bond with each other to form products.
Electron Movement
In a chemical reaction, electrons play a crucial role in the formation and breaking of chemical bonds. The movement of electrons determines the reactivity and stability of molecules. The reaction mechanism describes how electrons are transferred or shared between atoms during a reaction. It explains the movement of electrons from high energy orbitals to low energy orbitals, leading to the formation of new bonds and the breaking of existing bonds.
Energetics during Bond Cleavage and Bond Formation
During a chemical reaction, bonds between atoms are broken and new bonds are formed. The reaction mechanism explains the energy changes associated with these processes. Bond cleavage requires an input of energy, known as bond dissociation energy, as bonds are broken. This energy is absorbed from the surroundings. On the other hand, bond formation releases energy, known as bond formation energy, as new bonds are created. The reaction mechanism describes the energy changes during these processes and how they influence the overall energetics of the reaction.
Rates of Transformation (Kinetics)
The reaction mechanism also provides information about the rates of transformation of reactants into products. It describes the individual steps involved in the reaction and the order in which they occur. Each step in the mechanism has a specific rate constant associated with it, which determines how fast that step proceeds. The overall rate of the reaction is determined by the slowest step, known as the rate-determining step. The reaction mechanism helps to identify this step and understand the factors that control the reaction rate.
Conclusion
In summary, a reaction mechanism is a detailed account of the steps involved in a chemical reaction. It describes the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. Understanding the reaction mechanism is crucial for predicting and controlling chemical reactions.
Organic compounds can be classified even based upon the function groups. Identify the one which is not a functional group- a)Isocyanide
- b)Isocyano
- c)Carboxyl
- d)Carbonyl
Correct answer is option 'A'. Can you explain this answer?
Organic compounds can be classified even based upon the function groups. Identify the one which is not a functional group
a)
Isocyanide
b)
Isocyano
c)
Carboxyl
d)
Carbonyl

|
Mansi Mukherjee answered |
Isocyanide is a compound and it is not a functional group.
The minimum number of C atoms required for a hydrocarbon to exhibit optical isomerism:
- a)4
- b)5
- c)6
- d)7
Correct answer is option 'D'. Can you explain this answer?
The minimum number of C atoms required for a hydrocarbon to exhibit optical isomerism:
a)
4
b)
5
c)
6
d)
7
|
|
Varun Kapoor answered |
The minimum number of C atoms required for a hydrocarbon to exhibit optical isomerism = 7

Which among the following does not exhibit geometric isomerism- a)1-hexene
- b)2-hexene
- c)3-hexene
- d)4-hexene
Correct answer is option 'A'. Can you explain this answer?
Which among the following does not exhibit geometric isomerism
a)
1-hexene
b)
2-hexene
c)
3-hexene
d)
4-hexene

|
Abhiram Choudhary answered |
Alkenes like 1-hexene when flipped from top to bottom they have identical structures and also they have C=CH2 unit which does not exist as cis- trans isomers.
Direction (Q. Nos. 27-30) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q.
For hydrocarbon with molecular formula C5H8, how many acyclic isomers without consecutive pi-bonds are possible?
Correct answer is '3'. Can you explain this answer?
Direction (Q. Nos. 27-30) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q.
For hydrocarbon with molecular formula C5H8, how many acyclic isomers without consecutive pi-bonds are possible?
|
|
Preeti Iyer answered |
C5H8 has the following three isomers :
CH3CH2CH2C≡CH
CH3CH2CH2C≡CH
CH3CH2C≡CCH3
(CH3)2CH−C≡CH
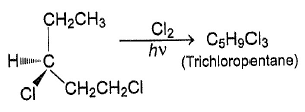
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer?
|
|
Om Desai answered |
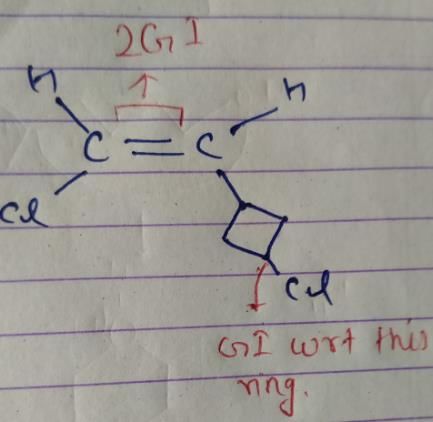
So, there are 4 isomers of given compound.
Which among the following is a very unstable and reactive species:- a)Carbaanion
- b)Polar ions
- c)Carbocation
- d)Free radical
Correct answer is option 'C'. Can you explain this answer?
Which among the following is a very unstable and reactive species:
a)
Carbaanion
b)
Polar ions
c)
Carbocation
d)
Free radical

|
Geethika Reddy answered |
Actually carbanions are filled with octet, so they are stable and less reactive, in case of polar ions already they are stable, and free radicle is heptet i.e, near to octet whereas carbocation is sextet in nature, so in order to gain to octet it is more reactive and less stable
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?- a)4-methyl-2-hexene and 3-methyl hexane
- b)3-methyl-1-pentene and 3-methyl pentane
- c) 4-methyl cyclopentene and methyl cyclopentane
- d)2-methyl-1-butene and 2-methyl butane
Correct answer is option 'B'. Can you explain this answer?
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
a)
4-methyl-2-hexene and 3-methyl hexane
b)
3-methyl-1-pentene and 3-methyl pentane
c)
4-methyl cyclopentene and methyl cyclopentane
d)
2-methyl-1-butene and 2-methyl butane
|
|
Pooja Shah answered |
The correct answer is Option B.
The optically active hydrocarbon X is 3-methyl-1-pentene CH2=CH−CH(CH3)CH2CH3. On catalytic hydrogenation, it forms 3-methyl pentane CH3CH2CH(CH3)CH2CH3, which is optically inactive.
The optically active hydrocarbon X is 3-methyl-1-pentene CH2=CH−CH(CH3)CH2CH3. On catalytic hydrogenation, it forms 3-methyl pentane CH3CH2CH(CH3)CH2CH3, which is optically inactive.
How many cyclic Isomers (structural and geometrical only) exist for C5H10? - a)8
- b)6
- c)5
- d)3
Correct answer is option 'C'. Can you explain this answer?
How many cyclic Isomers (structural and geometrical only) exist for C5H10?
a)
8
b)
6
c)
5
d)
3
|
|
Lavanya Menon answered |
5 isomers are possible. C5H10(CnH2n). Molecules having the CnH2n formula are most likely to be cyclic alkanes. The five isomers are:

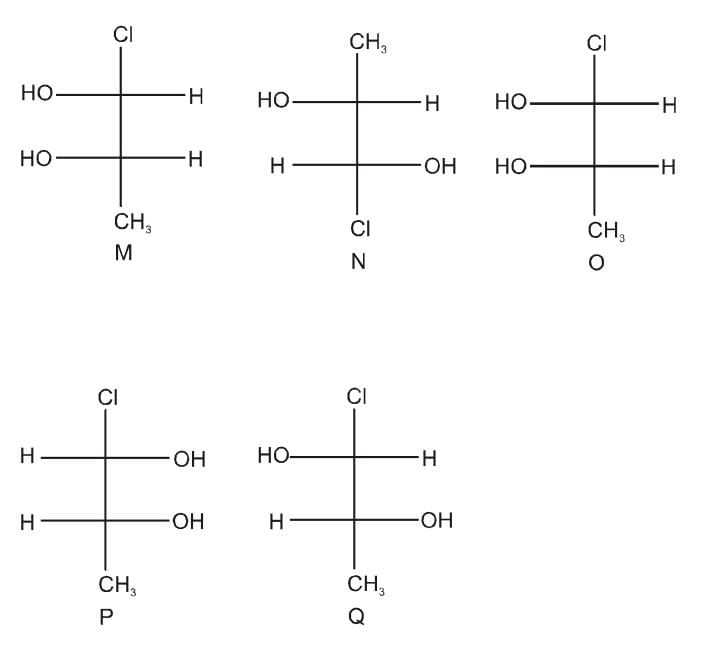
Which of the given statement (s) about N, O, P, and Q with respect to M is (are) correct?
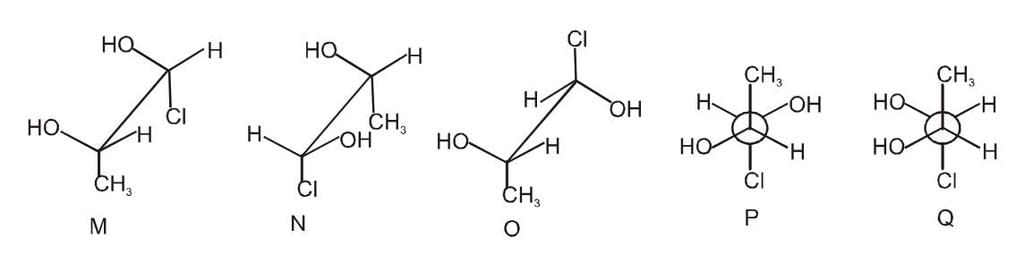
- a)M and N are non-mirror image stereoisomers
- b)M and O are identical
- c)M and P are enantiomers
- d)M and Q are identical
Correct answer is option 'A,B,C'. Can you explain this answer?
Which of the given statement (s) about N, O, P, and Q with respect to M is (are) correct?

a)
M and N are non-mirror image stereoisomers
b)
M and O are identical
c)
M and P are enantiomers
d)
M and Q are identical
|
|
Tejas Verma answered |
The Fischer Projections of the given compounds are below.
- M and N are non-mirror image stereoisomers
- M and O are identical
- M and P are enantiomers
The correct statement(s) about the compound given below is/are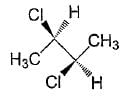
- a)The compound is optically active
- b)The compound possesses centre of symmentry
- c)The Compound possesses plane of symmetry
- d)the compound possessses axis of symmetry
Correct answer is option 'A,D'. Can you explain this answer?
The correct statement(s) about the compound given below is/are

a)
The compound is optically active
b)
The compound possesses centre of symmentry
c)
The Compound possesses plane of symmetry
d)
the compound possessses axis of symmetry
|
|
Pooja Shah answered |
The correct answer is option A & D
The given image is Self-Explanatory.
We know that following are the cases when a compound is Optically Inactive:
1. When it does not have a chiral Carbon Atom eg.: Methyl bromide
2. When it has a Plane or Axis of Symmetry eg.: Tartaric Acid (these compounds are called as Meso Compounds)
3. When it forms a Racemic Mixture eg.: Equimolar mixture of d-Lactic Acid and l-Lactic Acid.
The given compound in the question possesses an Axis of Symmetry (shown in the attached image). Hence, it is Optically Inactive.
The given image is Self-Explanatory.
We know that following are the cases when a compound is Optically Inactive:
1. When it does not have a chiral Carbon Atom eg.: Methyl bromide
2. When it has a Plane or Axis of Symmetry eg.: Tartaric Acid (these compounds are called as Meso Compounds)
3. When it forms a Racemic Mixture eg.: Equimolar mixture of d-Lactic Acid and l-Lactic Acid.
The given compound in the question possesses an Axis of Symmetry (shown in the attached image). Hence, it is Optically Inactive.
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
a)
a
b)
b
c)
c
d)
d
|
|
Preeti Iyer answered |
The correct answer is Option B.
CH3−CH=CH−CH=CH−CH3
2,4-hexadiene
Three different geometrical isomers of cis-cis, trans-trans and sic-trans are possible.
CH3−CH=CH−CH=CH−CH3
2,4-hexadiene
Three different geometrical isomers of cis-cis, trans-trans and sic-trans are possible.
Chapter doubts & questions for Hydrocarbon Isomers - Chemistry 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Hydrocarbon Isomers - Chemistry in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Chemistry
132 videos|251 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily