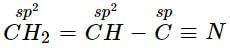All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Covalent Bond (GC) for MCAT Exam
Hybridisation of Acetylene is- a)sp
- b)sp2
- c)sp3
- d)dsp2
Correct answer is option 'A'. Can you explain this answer?
Hybridisation of Acetylene is
a)
sp
b)
sp2
c)
sp3
d)
dsp2
|
|
Riya Banerjee answered |
Since acetylene is made up of triple bond. So the hybridization of carbon in acetylene is sp.
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
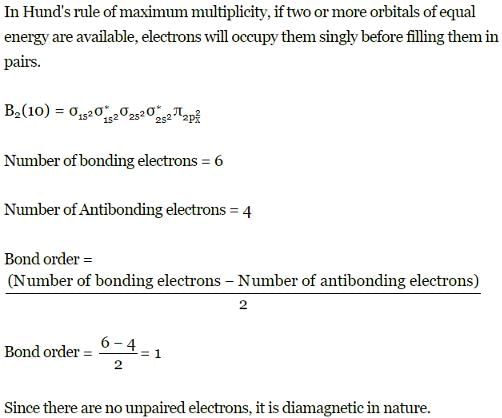
Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
- a)1 and diamagnetic
- b)0 and diamagnetic
- c)1 and paramagnetic
- d)0 and paramagnetic
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
a)
1 and diamagnetic
b)
0 and diamagnetic
c)
1 and paramagnetic
d)
0 and paramagnetic
|
|
Anjana Sharma answered |
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3 . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
- a)HF > H2O > NH3
- b)H2O > HF > NH3
- c)NH3 > HF > H2O
- d)NH3 > H2O > HF
Correct answer is option 'B'. Can you explain this answer?
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3 . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
a)
HF > H2O > NH3
b)
H2O > HF > NH3
c)
NH3 > HF > H2O
d)
NH3 > H2O > HF
|
|
Neha Joshi answered |
H2O>HF>NH3
Strength of hydrogen bonding depends on the size and electronegativity of the atom.
Smaller the size of the atom, greater is the electronegativity and hence stronger is the H−bonding. Thus, the order of strength of H-bonding is H...F>H...O>H...N.
But each HF molecule is linked only to two other HF molecules while each H2O molecule is linked to four other H2O molecules through H−bonding.
Hence, the decreasing order of boiling points is H2O>HF>NH3.
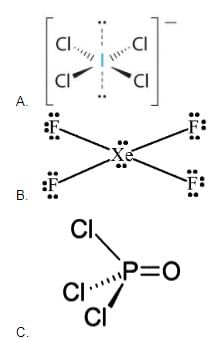
Select the correct statement(s) about IF7.- a)I atom is sp3d3-hybridised
- b)I atom is in highest oxidation state
- c)There are five I—F longest and two I—F shortest bonds
- d)It has pentagonal bipyramidal structure
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Select the correct statement(s) about IF7.
a)
I atom is sp3d3-hybridised
b)
I atom is in highest oxidation state
c)
There are five I—F longest and two I—F shortest bonds
d)
It has pentagonal bipyramidal structure
|
|
Rohit Shah answered |
Iodine heptafluoride, also known as iodine(VII) fluoride or iodine fluoride, is an interhalogen compound with the chemical formula IF7.[2][3] It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory.[4] The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a heptacoordinated system.[5] It forms colourless crystals, which melt at 4.5 degC: the liquid range is extremely narrow, with the boiling point at 4.77 degC. The dense vapor has a mouldy, acrid odour. The molecule has D5h symmetry. In IF7 out of 7 Flourine atoms 5 of them are placed on a plane in Pentagon shape .In remaining 2 flourines one is placed above the plane and other below the plane each at 90 degrees
Which is the correct order of the bond angle?- a)NH3 < NF3
- b)H2O > Cl2O
- c)PH3 < SbH3
- d)H2Te < H2S
Correct answer is option 'D'. Can you explain this answer?
Which is the correct order of the bond angle?
a)
NH3 < NF3
b)
H2O > Cl2O
c)
PH3 < SbH3
d)
H2Te < H2S

|
Nitin Patel answered |
The high electronegativity of F pulls the bonding electrons farther away from N than in NH3. Thus, repulsion between bond pairs is iess in NF3 than in NH3. Flence, the lone pair in N causes a greater distortion than NH3.
(b) as in (a)
Going down the group in periodic table, as size of central atom increases, repulsion increases.
Consider the two structures :
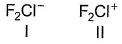
Select the correct statement(s).
a)I is bent, II is linearb)I is linear, II is bentc)Both is bentd)Both are linearCorrect answer is option 'B'. Can you explain this answer?
|
|
Geetika Shah answered |
1 has three lone pair and two bond pair, its representation will be AB2L3 and according to VSEPR theory it is linear in shape.
2 has two LP and two BP which representation will be AB2L2, so according to VSEPR theory it will be bent or it can also said to be 'V' shaped.
Hence B is correct.
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three? - a)SF4
- b)I3-
- c)SbCl52-
- d)PCl5
Correct answer is option 'C'. Can you explain this answer?
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three?
a)
SF4
b)
I3-
c)
SbCl52-
d)
PCl5
|
|
Geetika Shah answered |
Molecules having the same number of hybrid orbitals, have same hybridisation and number of hybrid oebitals.
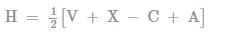
where,
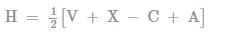
where,
V= number of valance electrons of central atom
X = number of monovalent atoms
C= charge on cation
A = charge on anion
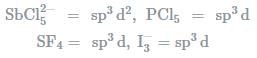
XeF2 is isostructural with- a)TeF2
- b)
- c)SbCI3
- d)BaCl2
Correct answer is option 'B'. Can you explain this answer?
XeF2 is isostructural with
a)
TeF2
b)
c)
SbCI3
d)
BaCl2
|
|
Varun Kapoor answered |
XeF2-sp3d hybridised Xe
Three lone pairs on equatorial positions to minimise repulsion; two F-atom at axial position. Thus, it is linear.
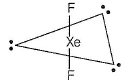
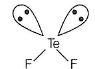
(a) TeF2,Te50 - Six electrons in valence shell
(b)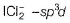 hybridised l- atom with three lone pairs at equatorial positions to minimise repulsion thus, linear.
hybridised l- atom with three lone pairs at equatorial positions to minimise repulsion thus, linear.
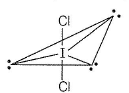
(c) SoCI3 - sp3-hybridised pyramidal Sb-one lone pair on Sb.
(d) BaCI2 - ionic .
Three lone pairs on equatorial positions to minimise repulsion; two F-atom at axial position. Thus, it is linear.
(a) TeF2,Te50 - Six electrons in valence shell
(b)
(c) SoCI3 - sp3-hybridised pyramidal Sb-one lone pair on Sb.
(d) BaCI2 - ionic .
Order of size of sp, sp2 and sp3 orbitals is- a)sp3 < sp2 < sp
- b)sp < sp2 < sp3
- c)sp2 < sp < sp3
- d)sp2 < sp3 < sp
Correct answer is option 'B'. Can you explain this answer?
Order of size of sp, sp2 and sp3 orbitals is
a)
sp3 < sp2 < sp
b)
sp < sp2 < sp3
c)
sp2 < sp < sp3
d)
sp2 < sp3 < sp
|
|
Raghav Bansal answered |
The percentage of s-character in sp3,sp2 and sp is 25%, 33% and 50% respectively. Order of size of orbitals is sp<sp2<sp3.
Which of the following statement is incorrect regarding NO2 molecule?- a)It has one unpaired electron is one of the sp2-hybridised orbital
- b)It is coloured due to unpaired electron
- c)It is deeply coloured in dimeric form
- d)Paramagnetic behaviour is lost in dimeric form
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is incorrect regarding NO2 molecule?
a)
It has one unpaired electron is one of the sp2-hybridised orbital
b)
It is coloured due to unpaired electron
c)
It is deeply coloured in dimeric form
d)
Paramagnetic behaviour is lost in dimeric form
|
|
Raghav Bansal answered |
NO2 molecule has unpaired electrons which are responsible for its brown colour and paramagnetic behavior.
NH4NO3 and NH4NO2 differ is
- a) hybridisation of N of the anion
- b) hybridisation of N of the cation and anion both
- c) decomposition product
- d) structure of the anion
Correct answer is option 'B,C,D'. Can you explain this answer?
NH4NO3 and NH4NO2 differ is
a)
hybridisation of N of the anionb)
hybridisation of N of the cation and anion bothc)
decomposition productd)
structure of the anion

|
Kritika Bajaj answered |
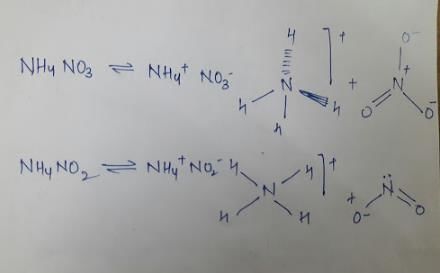
Hence we see that the two species differ in the hybridisation of Cation and anion, decomposition product and structure of anion.
Direction (Q. Nos. 12 and 14) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.Q. Statement I : BF3 molecule is planar but NF3 is pyramidal.Statement II : N atom is smaller than B.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 12 and 14) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : BF3 molecule is planar but NF3 is pyramidal.
Statement II : N atom is smaller than B.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Vikas Saini answered |
Nitrogen is smaller than boron.Wow what a chemistry
The orbitals formed after hybridization have equal energy.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
The orbitals formed after hybridization have equal energy.
a)
True
b)
False
|
|
Hema answered |
Hybridization: Mixing of atomic orbitals of different energy to get the hybrid orbitals of equivalent energy.
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. The shape [CIF4]- and [ClF2]- ions is respectively- a)see-saw and linear
- b)see-saw and bent
- c)tetrahedral and linear
- d)square planar and linear
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The shape [CIF4]- and [ClF2]- ions is respectively
a)
see-saw and linear
b)
see-saw and bent
c)
tetrahedral and linear
d)
square planar and linear
|
|
Raghav Bansal answered |
Direction (Q. Nos. 25 and 26) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).Q. i. Based on VSEPR theory, the number of 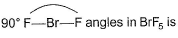
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......[JEE Advanced 2013]
Correct answer is '0'. Can you explain this answer?
Direction (Q. Nos. 25 and 26) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q.
i. Based on VSEPR theory, the number of 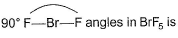
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......
[JEE Advanced 2013]
|
|
Geetika Shah answered |
(i) (0)
(Ip-bp) repulsion will cause distortion and thus, bond angle < 90° in each.
Thus, three lone pairs at equatorial position to minimise repulsion.
(iii) (6) Melamine is heterocyclic basic com pound (six N-atoms) each N having one lone pair.
Statement I : B atom is sp2-hybridised in B2H6.Statement II : There is no lone pair or unpaired electron in B2H6.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'D'. Can you explain this answer?
Statement I : B atom is sp2-hybridised in B2H6.
Statement II : There is no lone pair or unpaired electron in B2H6.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Neha Joshi answered |
(d) B2H6 has following types of bonding
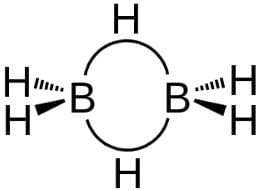
Bridging H-atoms and B-atoms are electron deficient. Each B-atom is however, joined to four H-atoms thus sp3-hybridised.
There is no lone pair or unpaired electron.
Thus, Statement I is incorrect but Statement II is correct.

Bridging H-atoms and B-atoms are electron deficient. Each B-atom is however, joined to four H-atoms thus sp3-hybridised.
There is no lone pair or unpaired electron.
Thus, Statement I is incorrect but Statement II is correct.
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?- a)trigonal pyramidal
- b)square pyramidal
- c)square planar
- d)tetrahedral
Correct answer is option 'C'. Can you explain this answer?
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?
a)
trigonal pyramidal
b)
square pyramidal
c)
square planar
d)
tetrahedral
|
|
Isaac Reed answered |
Explanation:
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
What is the systematic name of the following amino acid, which is commonly known as asparagine?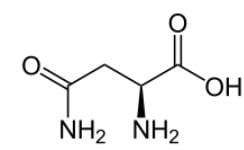
- a)(R)-2-amino-3-carboxylbutanamide
- b)(S)-2-amino-3-carbomylpropanoic acid
- c)(R)-3-amino-3-carboxylpropanamide
- d)(S)-2-amino-4-carbomylbutanoic acid
Correct answer is option 'B'. Can you explain this answer?
What is the systematic name of the following amino acid, which is commonly known as asparagine?

a)
(R)-2-amino-3-carboxylbutanamide
b)
(S)-2-amino-3-carbomylpropanoic acid
c)
(R)-3-amino-3-carboxylpropanamide
d)
(S)-2-amino-4-carbomylbutanoic acid

|
Orion Classes answered |
In naming any compound systemically, we must determine the primary substituent group which will become the suffix. Here it is the carboxylic acid group. We are looking for some kind of -oic acid.
We can determine the stereochemistry of the compound, which is (S), and that is shown below:
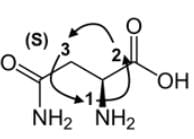
Technically, we should have the answer with those two pieces of information, but there are two answers with To be thorough, the next step in IUPAC nomenclature is to find the longest carbon chain starting at the carbon of the carboxylic acid. It would seem it would be 4 carbons, but it will be only 3 carbons because of the amide group as a prefix.
The amine group at C-2 will become 2-amino, and the amide group attached at C-3, the substituent including the carbon, will become 3-carbamoyl and not 4-carbamoy
The name of the compound is (S)-2-amino-3-carbomylpropanoic acid.
Nitrates are used to treat angina pectoris and acts to dilate the blood vessels to lower blood pressure. They exert their effects by their conversion into nitric oxide, a powerful vasodilator. What is the bond order and fractional charge on each oxygen of the nitrate ion?- a)Bond order of 1(1/3) fractional charge of −1/3
- b)Bond order of 2 fractional charge of −1/3
- c)Bond order of 1(1/3) fractional charge of −2/3
- d)Bond order of 2 fractional charge of −2/3
Correct answer is option 'C'. Can you explain this answer?
Nitrates are used to treat angina pectoris and acts to dilate the blood vessels to lower blood pressure. They exert their effects by their conversion into nitric oxide, a powerful vasodilator. What is the bond order and fractional charge on each oxygen of the nitrate ion?
a)
Bond order of 1(1/3) fractional charge of −1/3
b)
Bond order of 2 fractional charge of −1/3
c)
Bond order of 1(1/3) fractional charge of −2/3
d)
Bond order of 2 fractional charge of −2/3
|
|
Hannah Wright answered |
To determine the bond order and fractional charge on each oxygen of the nitrate ion, we first need to draw its Lewis structure:
O
||
N-O
|
O
In the nitrate ion (NO3-), there are three oxygen atoms bonded to a central nitrogen atom. Each oxygen atom forms a single bond with the nitrogen atom and carries a lone pair of electrons.
The bond order is defined as the number of shared electron pairs between two atoms. In the nitrate ion, each oxygen atom shares one electron pair with the nitrogen atom, resulting in a bond order of 1.
The fractional charge on each oxygen can be determined by considering the formal charge. The formal charge is calculated using the formula:
Formal charge = valence electrons - unshared electrons - 1/2(shared electrons)
For the oxygen atoms in the nitrate ion:
Formal charge = 6 (valence electrons) - 6 (unshared electrons) - 1/2(2 shared electrons)
Formal charge = 6 - 6 - 1
Formal charge = -1
Therefore, each oxygen atom in the nitrate ion has a bond order of 1 and a fractional charge of -1.
O
||
N-O
|
O
In the nitrate ion (NO3-), there are three oxygen atoms bonded to a central nitrogen atom. Each oxygen atom forms a single bond with the nitrogen atom and carries a lone pair of electrons.
The bond order is defined as the number of shared electron pairs between two atoms. In the nitrate ion, each oxygen atom shares one electron pair with the nitrogen atom, resulting in a bond order of 1.
The fractional charge on each oxygen can be determined by considering the formal charge. The formal charge is calculated using the formula:
Formal charge = valence electrons - unshared electrons - 1/2(shared electrons)
For the oxygen atoms in the nitrate ion:
Formal charge = 6 (valence electrons) - 6 (unshared electrons) - 1/2(2 shared electrons)
Formal charge = 6 - 6 - 1
Formal charge = -1
Therefore, each oxygen atom in the nitrate ion has a bond order of 1 and a fractional charge of -1.
Determining Lewis dot structures can be used to determine whether a compound has any bond dipoles and consequently any molecular dipole. Which of the following compounds does NOT have a molecular dipole?- a)NH3
- b)PO33-
- c)CIO3-
- d)SO3
Correct answer is option 'D'. Can you explain this answer?
Determining Lewis dot structures can be used to determine whether a compound has any bond dipoles and consequently any molecular dipole. Which of the following compounds does NOT have a molecular dipole?
a)
NH3
b)
PO33-
c)
CIO3-
d)
SO3
|
|
Ayesha Joshi answered |
The hypochlorite ion ClO3- has 2 double-bonded oxygens and 1 single-bonded oxygen to the chlorine central atom. Since chlorine can have 7 bonds, with 5 bonds to oxygen, there are 2 electrons remaining as an unbonded pair. Hypochlorite has the trigonal pyramidal molecular geometry.
The phosphite ion PO33- has a central phosphorus atom with 3 single-bonded oxygens each with a negative charge. Since phosphorus has 5 valence electrons, there are 2 electrons remaining for a lone pair. Phosphite has the trigonal pyramidal molecular geometry.
Ammonia NH3 , has a central nitrogen with 3 single-bonded hydrogens. Since nitrogen has 5 valence electrons, there are 2 electrons remaining for an unbonded pair.
Sulfur trioxide SO3 has a central sulfur atom attached to 3 oxygens with double bonds. The molecular geometry is trigonal planar, and it has no molecular dipole.
Carbon monoxide (CO) is a potent competitive inhibitor of hemoglobin, and it has an affinity for hemoglobin over 200 times greater than oxygen. What is the formal charge of the oxygen on the molecule?- a)+1
- b)0
- c)-1
- d)-2
Correct answer is option 'A'. Can you explain this answer?
Carbon monoxide (CO) is a potent competitive inhibitor of hemoglobin, and it has an affinity for hemoglobin over 200 times greater than oxygen. What is the formal charge of the oxygen on the molecule?
a)
+1
b)
0
c)
-1
d)
-2
|
|
Ayesha Joshi answered |
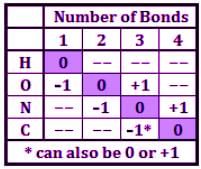
The HONC (pronounced hunk or honk) shortcut, which correspond to 1, 2, 3, or 4 bonds, will be employed for all questions in this set. When these four atoms have the corresponding bonds, then the atoms are neutral. One less bond to that atom makes its more negative by 1, and one more bond more positive by 1.
Here is a chart that summarizes the shortcut:
Here is a chart that summarizes the shortcut:
Start by drawing a skeleton of C−O and consider the double bond. Looking at the formal charges, carbon would have no formal charge, as well as the oxygen. This would seem correct, but remember the octet rule. It is not much of a rule since C, N, O, and F are the only four elements that must adhere to the rule. Unless there is a carbene (:CH2), carbon usually needs to fill its octet even though C=O seems to be a feasible Lewis dot structure.
n analyzing the triple bond C≡O, carbon has a formal charge of -1, and oxygen a formal charge of +1. Both have their octets filled, and so here is the Lewis dot structure:
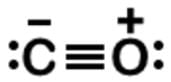
To find the formal charge of the oxygen, start with the number of valence electrons, which is 6. Subtract any unbonded electrons, which is 2, and then subtract one-half of any bonded electrons, which is 3.
Generally in determining structures, the more electronegative atom tends to have a negative formal charge, and the more electropositive a positive one. Despite being more electronegative, oxygen does not get assigned the automatic negative charge but has instead a formal charge of +1.
The parent compound, 2-butanol, undergoes a set of reactions to produce compounds A and B. Which of the following statements accurately describes the absolute and relative configurations of the compounds in the reaction series below?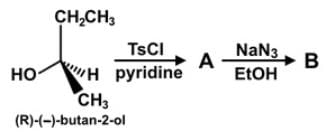
- a)The relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known
- b)The relative configurations of the products are (-) and (+), while the absolute configuration of 2-butanol has to be determined.
- c)The relative configurations of A and B can be determined to be (S) and (R), and the absolute configurations cannot be determined without further experimentation.
- d)The absolute configurations of A and B can be determined to be (R) and (S), but the relative configurations cannot be determined without further experimentation.
Correct answer is option 'A'. Can you explain this answer?
The parent compound, 2-butanol, undergoes a set of reactions to produce compounds A and B. Which of the following statements accurately describes the absolute and relative configurations of the compounds in the reaction series below?

a)
The relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known
b)
The relative configurations of the products are (-) and (+), while the absolute configuration of 2-butanol has to be determined.
c)
The relative configurations of A and B can be determined to be (S) and (R), and the absolute configurations cannot be determined without further experimentation.
d)
The absolute configurations of A and B can be determined to be (R) and (S), but the relative configurations cannot be determined without further experimentation.

|
Orion Classes answered |
When 2-butanol is placed in pyridine with 4-toluenesulfonyl chloride, the alcohol is converted into the tosylate, which makes it a better leaving group. The reaction is known to occur with retention of configuration,here is no change in the stereochemistry.
When the tosylate is then placed into a sodium azide and ethanol solution, the azide acts as a nucleophile in a SN2 fashion to attack from the backside and substitute the tosylate with an azide group. There is an inversion of stereochemistry due to the backside attack.
2-butanol has a configuration of (R), so then compound A will have an (R) configuration and compound B an (S) configuration due to the inversion. These are our relative configurations of A and B.
Since we know the sign of rotation and the designation of R or S for the parent, we have its absolute configuration.
While for compounds A and B, without further experimentation, we do not ascertain whether R corresponds to (+) or (-). But, because you know the configuration of the starting alcohol, then the absolute configuration of the product is also known.
The answer is that the relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known.
Which of the following statement is true regarding hybrid orbitals?- a)The amount of orbitals formed after the hybridization is not equal to the number of orbitals before hybridization
- b)The hybrid orbitals don’t have equal energy
- c)They can form more stable bonds than the pure orbitals
- d)Hybridization doesn’t indicate geometry
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is true regarding hybrid orbitals?
a)
The amount of orbitals formed after the hybridization is not equal to the number of orbitals before hybridization
b)
The hybrid orbitals don’t have equal energy
c)
They can form more stable bonds than the pure orbitals
d)
Hybridization doesn’t indicate geometry
|
|
Vivek Khatri answered |
The true statements of the incorrect ones are the number of orbitals formed after the hybridization is equal to the number of orbitals before hybridization, the hybrid orbitals have equal energy and the hybridization indicates geometry.
Match the column I with column II and mark the appropriate choice.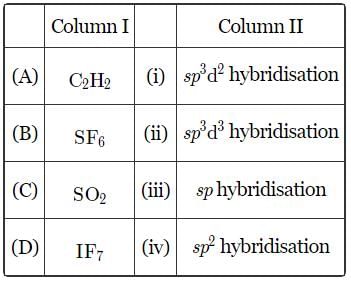
- a)(A)→(i),(B)→(iii),(C)→(ii),(D)→(iv)
- b)(A)→(iii),(B)→(i),(C)→(iv),(D)→(ii)
- c)(A)→(ii),(B)→(iii),(C)→(i),(D)→(iv)
- d)(A)→(iv),(B)→(i),(C)→(iii),(D)→(ii)
Correct answer is option 'B'. Can you explain this answer?
Match the column I with column II and mark the appropriate choice.

a)
(A)→(i),(B)→(iii),(C)→(ii),(D)→(iv)
b)
(A)→(iii),(B)→(i),(C)→(iv),(D)→(ii)
c)
(A)→(ii),(B)→(iii),(C)→(i),(D)→(iv)
d)
(A)→(iv),(B)→(i),(C)→(iii),(D)→(ii)
|
|
Jaspreet answered |
In C2H2 hybridisation is sp
In SF6 using formula of hybridisation
H= 1/2 (6+6 ) = 1/2 ×12 = 6 sp³d²
Same way for SO2 it is
H = 1/2 (6+0) = 1/2 × 6 =3 sp²
For IF7
H = 1/2 (7+7 ) =1/2 × 14 = 7 sp³d³
Hence correct option is B :)
In SF6 using formula of hybridisation
H= 1/2 (6+6 ) = 1/2 ×12 = 6 sp³d²
Same way for SO2 it is
H = 1/2 (6+0) = 1/2 × 6 =3 sp²
For IF7
H = 1/2 (7+7 ) =1/2 × 14 = 7 sp³d³
Hence correct option is B :)
Which of the following statements most accurately describes the difference between assigning formal charge and oxidation state?- a)In determining formal charge, treat the bonds as if they are ionic bonds, and generally do not assign a charge greater than 1.
- b)The atoms can be assigned any value from -4 to +7 in determining oxidation states.
- c)In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons.
- d)The assigned formal charges and oxidation states can separately add up to different values.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements most accurately describes the difference between assigning formal charge and oxidation state?
a)
In determining formal charge, treat the bonds as if they are ionic bonds, and generally do not assign a charge greater than 1.
b)
The atoms can be assigned any value from -4 to +7 in determining oxidation states.
c)
In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons.
d)
The assigned formal charges and oxidation states can separately add up to different values.
|
|
Olivia Johnson answered |
Understanding Formal Charge vs. Oxidation State
When differentiating between formal charge and oxidation state, it's essential to grasp how each is calculated and what they represent.
Formal Charge
- Formal charge is a bookkeeping tool used to help predict the stability of a molecule.
- It is calculated using the formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (Bonding Electrons/2).
- In this method, bonds are treated as if they are covalent, meaning that electrons are shared equally between atoms.
Oxidation State
- Oxidation state indicates the degree of oxidation (loss of electrons) of an atom in a compound.
- It is calculated by assigning electrons based on electronegativity; the more electronegative atom in a bond is assigned both electrons.
- This approach treats bonds as if they are ionic, reflecting the charge distribution more accurately.
Why Option C is Correct
- Option C states, "In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons."
- This is correct because oxidation states reflect the theoretical charge an atom would have if all bonds were ionic.
- It highlights the fundamental difference from formal charge, where bonds are considered covalent and shared.
Key Takeaways
- Formal charge helps evaluate molecular stability, while oxidation states indicate electron distribution.
- Understanding this distinction is crucial for analyzing chemical reactions and bonding scenarios effectively.
When differentiating between formal charge and oxidation state, it's essential to grasp how each is calculated and what they represent.
Formal Charge
- Formal charge is a bookkeeping tool used to help predict the stability of a molecule.
- It is calculated using the formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (Bonding Electrons/2).
- In this method, bonds are treated as if they are covalent, meaning that electrons are shared equally between atoms.
Oxidation State
- Oxidation state indicates the degree of oxidation (loss of electrons) of an atom in a compound.
- It is calculated by assigning electrons based on electronegativity; the more electronegative atom in a bond is assigned both electrons.
- This approach treats bonds as if they are ionic, reflecting the charge distribution more accurately.
Why Option C is Correct
- Option C states, "In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons."
- This is correct because oxidation states reflect the theoretical charge an atom would have if all bonds were ionic.
- It highlights the fundamental difference from formal charge, where bonds are considered covalent and shared.
Key Takeaways
- Formal charge helps evaluate molecular stability, while oxidation states indicate electron distribution.
- Understanding this distinction is crucial for analyzing chemical reactions and bonding scenarios effectively.
Which of the following statements most accurately describes the details of the hybridization of sulfur hexafluoride?- a)The sulfur atom undergoes excitation to promote two electrons from the 2s orbital to two empty 3d orbitals.
- b)Sulfur forms six σ hybridized bonds with fluorine atoms, and each fluorine atom makes use of a half-filled 3pz orbital for the bond formation.
- c)The orbitals are all oriented such that all bond angles are 90° and have an octahedral configuration.
- d)In the excited state, intermixing of one s, three p and three d orbitals gives 6 half-filled sp2d3 hybrid orbitals.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements most accurately describes the details of the hybridization of sulfur hexafluoride?
a)
The sulfur atom undergoes excitation to promote two electrons from the 2s orbital to two empty 3d orbitals.
b)
Sulfur forms six σ hybridized bonds with fluorine atoms, and each fluorine atom makes use of a half-filled 3pz orbital for the bond formation.
c)
The orbitals are all oriented such that all bond angles are 90° and have an octahedral configuration.
d)
In the excited state, intermixing of one s, three p and three d orbitals gives 6 half-filled sp2d3 hybrid orbitals.
|
|
Bella Perry answered |
Understanding Sulfur Hexafluoride Hybridization
Sulfur hexafluoride (SF6) is a molecular compound where sulfur is centrally located and bonded to six fluorine atoms. The hybridization and bonding details provide insight into its geometric structure.
Excitation and Hybridization
- In the formation of SF6, the sulfur atom undergoes excitation, where an electron from the 3s orbital is promoted to the 3d orbital. This is crucial for accommodating six bonding pairs.
- Sulfur utilizes one 3s orbital, three 3p orbitals, and two 3d orbitals to create six equivalent hybrid orbitals known as sp3d2 hybrid orbitals.
Bond Formation
- Each of the six sp3d2 hybrid orbitals forms a sigma bond with a fluorine atom. Each fluorine atom contributes a half-filled 2p orbital for bond formation.
Molecular Geometry
- The resulting molecular geometry of SF6 is octahedral. This configuration allows for optimal spatial arrangement of the bonded fluorine atoms.
- The bond angles in an octahedral structure are exactly 90 degrees, confirming that the statement regarding bond angles in option 'C' is accurate.
Conclusion
- The hybridization of sulfur in SF6 is a clear example of how electron promotion and orbital hybridization lead to specific molecular geometries, confirming that option 'C' accurately describes the details of sulfur hexafluoride hybridization.
Sulfur hexafluoride (SF6) is a molecular compound where sulfur is centrally located and bonded to six fluorine atoms. The hybridization and bonding details provide insight into its geometric structure.
Excitation and Hybridization
- In the formation of SF6, the sulfur atom undergoes excitation, where an electron from the 3s orbital is promoted to the 3d orbital. This is crucial for accommodating six bonding pairs.
- Sulfur utilizes one 3s orbital, three 3p orbitals, and two 3d orbitals to create six equivalent hybrid orbitals known as sp3d2 hybrid orbitals.
Bond Formation
- Each of the six sp3d2 hybrid orbitals forms a sigma bond with a fluorine atom. Each fluorine atom contributes a half-filled 2p orbital for bond formation.
Molecular Geometry
- The resulting molecular geometry of SF6 is octahedral. This configuration allows for optimal spatial arrangement of the bonded fluorine atoms.
- The bond angles in an octahedral structure are exactly 90 degrees, confirming that the statement regarding bond angles in option 'C' is accurate.
Conclusion
- The hybridization of sulfur in SF6 is a clear example of how electron promotion and orbital hybridization lead to specific molecular geometries, confirming that option 'C' accurately describes the details of sulfur hexafluoride hybridization.
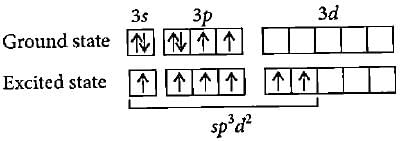
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?- a)Due to octahedral shape ofSatoms
- b)Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited state
- c)Due to sp3 hybridisation of S atom which provides 6 electrons to 6 F atoms
- d)Due to presence of 3 sigma and 3 pi bonds between S and F
Correct answer is option 'B'. Can you explain this answer?
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?
a)
Due to octahedral shape ofSatoms
b)
Due to presence of vacant 3d-orbitals which provide 6 unpaired electrons in excited state
c)
Due to sp3 hybridisation of S atom which provides 6 electrons to 6 F atoms
d)
Due to presence of 3 sigma and 3 pi bonds between S and F
|
|
Gaurav Kumar answered |
S can go into excited state with 6 unpaired electrons due to presence of vacant 3d-orbitals, which are overlapped by six F electrons.
If there are five electron pairs in outer shell, then structure and bond angle as predicted by Sidgwick-Powell theory is- a)octahedral, (90°)
- b)trigonal bipyramidal, (120° and 90°)
- c)pentagonal bipyramidal (72° and 90°)
- d)tetrahedral, (90°)
Correct answer is option 'B'. Can you explain this answer?
If there are five electron pairs in outer shell, then structure and bond angle as predicted by Sidgwick-Powell theory is
a)
octahedral, (90°)
b)
trigonal bipyramidal, (120° and 90°)
c)
pentagonal bipyramidal (72° and 90°)
d)
tetrahedral, (90°)

|
Moumita Chakraborty answered |
Three electron pairs are inclined at 120°.
Two electron-pairs are at 90° with the first three.
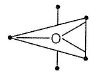
Structure is trigonal bipyramidal.
Two electron-pairs are at 90° with the first three.
Structure is trigonal bipyramidal.
Which of the following compounds does NOT have a bent molecular geometry?- a)H2O
- b)l3
- c)ClO2-
- d)SO2
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds does NOT have a bent molecular geometry?
a)
H2O
b)
l3
c)
ClO2-
d)
SO2
|
|
Ayesha Joshi answered |
Sulfur dioxide SO2 is a classic trap for linear molecular geometry due to its likeness to CO2, which is definitely linear. SO2 is bent due to the single lone pair:
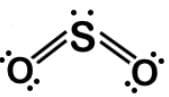
H2O is bent due to the two lone pairs on the oxygen. Here is the Lewis dot structure:
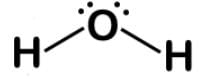
The chlorite ion ClO2- is also bent due to the two lone pairs on the chlorine. Here is the Lewis dot structure:
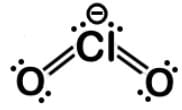
The triiodide ion I3- is not bent because it has 3 lone pairs, but it has a linear molecular geometry with a trigonal bipyramidal electronic geometry.
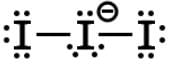
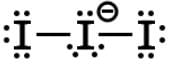
Which of the following molecules does NOT have an atom that is sp-hybridized?- a)NO2
- b)
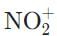
- c)HC≡C-
- d)CO2-
Correct answer is option 'A'. Can you explain this answer?
Which of the following molecules does NOT have an atom that is sp-hybridized?
a)
NO2
b)
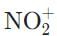
c)
HC≡C-
d)
CO2-
|
|
Ayesha Joshi answered |
The acetylide ion (HCC‾ ) has 2 sp-hybridized carbons due to the triple bond since two unhybridized p orbitals are needed to form the two π bonds. The C-H bond is a sigma bond formed from the overlap of a sp hybridized and 1s orbital.
The nitronium ion (NO2⁺) has an sp-hybridized nitrogen, and the cation is formed from removing the lone electron from paramagnetic NO. The nitrogen is double-bonded to each of the oxygens necessitating two unhybridized p orbitals to form the two π bonds.
Carbon dioxide should be familiar as a linear molecule, in which the carbon has no lone pairs. The central carbon is sp-hybridized since it is double-bonded to each of the oxygens.
The nitrite ion (NO2‾) will be sp2-hybridized since nitrogen forms a π bond with one of the oxygens through an unhybridized p orbital. The other orbitals become hybridized into sp2, and nitrogen forms a hybridized bond to each of the oxygens and has a lone pair in the other hybridized orbital. One oxygen is single-bonded and has a negative charge, and the other is double-bonded and neutral.
Statement I : BCI3 exists as monomer while AICI3 exists as dimer.Statement II : Boron atom is too small to form the stable 4-membered ring system- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement I : BCI3 exists as monomer while AICI3 exists as dimer.
Statement II : Boron atom is too small to form the stable 4-membered ring system
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Gowri Kulkarni answered |
Size of B is too small to form stable 4-membered ring system. (Also delocalised n-bonding is disturbed). Hence, dimerofBCI3is not formed.
Dimer AI2CI6 is stable due to stable ring
Thus, Statements I and II both are correct and Statement II is the correct explanation of Statement
The orbitals that are resulted from sp hybridization have _______% s-character and ________% p-character.- a)25, 75
- b)75, 25
- c)20, 80
- d)50, 50
Correct answer is option 'D'. Can you explain this answer?
The orbitals that are resulted from sp hybridization have _______% s-character and ________% p-character.
a)
25, 75
b)
75, 25
c)
20, 80
d)
50, 50

|
Anagha Bajaj answered |
Explanation:
- Sp hybridization is a type of hybridization that occurs when one s orbital and one p orbital combine to form two sp hybrid orbitals.
- The resulting orbitals have a specific percentage of s-character and p-character.
Calculation:
- In sp hybridization, one s orbital and one p orbital combine to form two sp hybrid orbitals.
- The s orbital contributes 50% s-character, while the p orbital contributes 50% p-character.
- Therefore, each sp hybrid orbital has 50% s-character and 50% p-character.
- Since there are two sp hybrid orbitals, the total s-character is 2 * 50% = 100% and the total p-character is 2 * 50% = 100%.
- To determine the percentage of s-character and p-character in the resulting orbitals, we divide the contribution of each orbital by the total contribution.
Answer:
The orbitals that result from sp hybridization have 50% s-character and 50% p-character. Therefore, the correct answer is option D, which states that the orbitals have 50% s-character and 50% p-character.
- Sp hybridization is a type of hybridization that occurs when one s orbital and one p orbital combine to form two sp hybrid orbitals.
- The resulting orbitals have a specific percentage of s-character and p-character.
Calculation:
- In sp hybridization, one s orbital and one p orbital combine to form two sp hybrid orbitals.
- The s orbital contributes 50% s-character, while the p orbital contributes 50% p-character.
- Therefore, each sp hybrid orbital has 50% s-character and 50% p-character.
- Since there are two sp hybrid orbitals, the total s-character is 2 * 50% = 100% and the total p-character is 2 * 50% = 100%.
- To determine the percentage of s-character and p-character in the resulting orbitals, we divide the contribution of each orbital by the total contribution.
Answer:
The orbitals that result from sp hybridization have 50% s-character and 50% p-character. Therefore, the correct answer is option D, which states that the orbitals have 50% s-character and 50% p-character.
Which of the following shows dsp2 hybridisation and a square planar geometry?- a)SF6
- b)BeF5
- c)PCl5
- d)[Ni(CN)4]2-
Correct answer is option 'D'. Can you explain this answer?
Which of the following shows dsp2 hybridisation and a square planar geometry?
a)
SF6
b)
BeF5
c)
PCl5
d)
[Ni(CN)4]2-
|
|
Uday Dasgupta answered |
Understanding dsp2 Hybridisation
d) [Ni(CN)4]2- is a classic example of dsp2 hybridisation leading to a square planar geometry. Let's break down the reasoning:
Coordination Number
- The coordination number of [Ni(CN)4]2- is 4, indicating that nickel is surrounded by four cyanide ligands.
- A square planar geometry is often observed in transition metal complexes with a coordination number of 4.
Hybridisation Process
- In [Ni(CN)4]2-, nickel has the electronic configuration of [Ar] 3d8 4s2.
- To accommodate four ligands, one 4s and three 3d orbitals hybridise to form four dsp2 hybrid orbitals.
- The d orbitals involved are from the 3d subshell, while s and p orbitals are from the 4th shell.
Geometry of the Complex
- The dsp2 hybrid orbitals arrange themselves in a square planar manner to minimize electron pair repulsion.
- The ligands (cyanide in this case) are positioned at the corners of a square, with the metal ion at the center.
Comparison with Other Options
- a) SF6 has sp3d2 hybridisation and octahedral geometry.
- b) BeF5 is trigonal bipyramidal and involves sp3d hybridisation.
- c) PCl5 features sp3d hybridisation and has a trigonal bipyramidal geometry.
Conclusion
- Hence, [Ni(CN)4]2- exemplifies dsp2 hybridisation and square planar geometry, making it the correct answer among the provided options.
d) [Ni(CN)4]2- is a classic example of dsp2 hybridisation leading to a square planar geometry. Let's break down the reasoning:
Coordination Number
- The coordination number of [Ni(CN)4]2- is 4, indicating that nickel is surrounded by four cyanide ligands.
- A square planar geometry is often observed in transition metal complexes with a coordination number of 4.
Hybridisation Process
- In [Ni(CN)4]2-, nickel has the electronic configuration of [Ar] 3d8 4s2.
- To accommodate four ligands, one 4s and three 3d orbitals hybridise to form four dsp2 hybrid orbitals.
- The d orbitals involved are from the 3d subshell, while s and p orbitals are from the 4th shell.
Geometry of the Complex
- The dsp2 hybrid orbitals arrange themselves in a square planar manner to minimize electron pair repulsion.
- The ligands (cyanide in this case) are positioned at the corners of a square, with the metal ion at the center.
Comparison with Other Options
- a) SF6 has sp3d2 hybridisation and octahedral geometry.
- b) BeF5 is trigonal bipyramidal and involves sp3d hybridisation.
- c) PCl5 features sp3d hybridisation and has a trigonal bipyramidal geometry.
Conclusion
- Hence, [Ni(CN)4]2- exemplifies dsp2 hybridisation and square planar geometry, making it the correct answer among the provided options.
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF6-)Q. Select the correct statement(s).- a)CIF3 changes its structure from pyramidal to angular in CIF+2
- b)AsF5 changes to structure from angular bipyramidal to octahedral in (AsF6)-
- c)Both (a) and (b) are correct
- d)None of the above is correct
Correct answer is option 'B'. Can you explain this answer?
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF6-)
Q.
Select the correct statement(s).
a)
CIF3 changes its structure from pyramidal to angular in CIF+2
b)
AsF5 changes to structure from angular bipyramidal to octahedral in (AsF6)-
c)
Both (a) and (b) are correct
d)
None of the above is correct
|
|
Muskaan Kumar answered |
Which of the following sets of molecules have different shape but same hybridisation of the central atoms?
- a)NH3, H2O
- b)H2S, SO2
- c)Ni(CO)4,[NiCI4]2-
- d)SF4, PCI5
Correct answer is option 'A,D'. Can you explain this answer?
Which of the following sets of molecules have different shape but same hybridisation of the central atoms?
a)
NH3, H2O
b)
H2S, SO2
c)
Ni(CO)4,[NiCI4]2-
d)
SF4, PCI5

|
Abhay Sen answered |
NH3 N atom sp3 -hybridised - Bipyramidal
H2O O a to m sp3 -hybridised - V -shaped
H2S S atom is sp3 -hybridised - Bent
SO2 S atom is sp2 -hybridised - Bent
Ni(CO)4 Ni atom is sp3-hybridised - Tetrahedral
[NiCI4]2- Ni atom is sp3-hybridised -Tetrahedral.
SF4 S atom is sp3d -hybridised - See-saw
PCI5 P atom is sp3 -hybridised - Bipyramidal
H2O O a to m sp3 -hybridised - V -shaped
H2S S atom is sp3 -hybridised - Bent
SO2 S atom is sp2 -hybridised - Bent
Ni(CO)4 Ni atom is sp3-hybridised - Tetrahedral
[NiCI4]2- Ni atom is sp3-hybridised -Tetrahedral.
SF4 S atom is sp3d -hybridised - See-saw
PCI5 P atom is sp3 -hybridised - Bipyramidal
Which of the following pairs are isostructural?- a)SO2-4 and BF-4
- b)NH3 and NH+4
- c)CO32- and CO2
- d)CH4 and BF3
Correct answer is option 'A'. Can you explain this answer?
Which of the following pairs are isostructural?
a)
SO2-4 and BF-4
b)
NH3 and NH+4
c)
CO32- and CO2
d)
CH4 and BF3
|
|
Gaurav Kumar answered |
SO2-4 and BF-4 have sp3 hybridisation and are tetrahedral in shape.
Who introduced the concept of hybridization?- a)Pauling
- b)London
- c)Sidgwick
- d)Alexander
Correct answer is option 'A'. Can you explain this answer?
Who introduced the concept of hybridization?
a)
Pauling
b)
London
c)
Sidgwick
d)
Alexander
|
|
Vivek Khatri answered |
In order to explain the bonding and shapes of every polyatomic atomic molecule like methane, carbon tetrachloride, water, boron trifluoride, etc, Pauling put-forth the concept of hybridization.
Sanglifehrin A (SFA) is a novel cyclophilin-binding compound showing immunosuppressive activity, but its role has not been entirely elucidated yet. How many stereogenic centers (R,S; E/Z) are there in the following compound sanglifehrin A?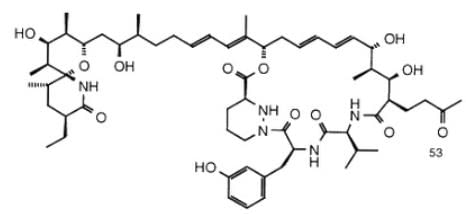
- a)21
- b)17
- c)26
- d)18
Correct answer is option 'A'. Can you explain this answer?
Sanglifehrin A (SFA) is a novel cyclophilin-binding compound showing immunosuppressive activity, but its role has not been entirely elucidated yet. How many stereogenic centers (R,S; E/Z) are there in the following compound sanglifehrin A?

a)
21
b)
17
c)
26
d)
18
|
|
Ayesha Joshi answered |
This is an exercise in counting the total number of stereogenic centers. Stereogenic centers include chiral carbons and double-bonded carbons in cis-trans alkenes.
On the left half of the molecule, there are 9 asymmetric carbons and 2 double bonds:
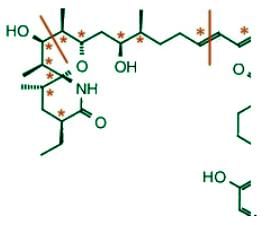
On the right half of the molecule, there are 8 asymmetric carbons and 2 double bonds:
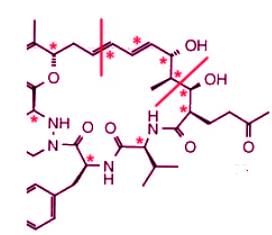
There are 21 stereogenic centers in total in this compound.
How many stereogenic centers are there in the following tetrasubstituted adamantane molecule?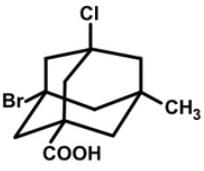
- a)0
- b)1
- c)4
- d)6
Correct answer is option 'B'. Can you explain this answer?
How many stereogenic centers are there in the following tetrasubstituted adamantane molecule?

a)
0
b)
1
c)
4
d)
6

|
Orion Classes answered |
The adamantane derivative features four asymmetric substituted carbon atoms, and it only exists as two stereoisomers (enantiomers) rather than the sixteen predicted by the 2n rule. However, the number of observed stereoisomers is only 2 since once the first stereocenter is defined, the remaining three are determined by their relative configuration.
The configurations of the asymmetric bridgehead carbon units in this structure are (R), (S), (R) and (R) respectively, for the Br, Cl, CO2H and CH3 substituents.
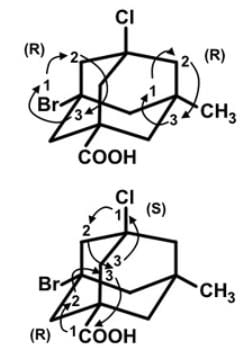
The central adamantyl residue may be regarded as an extended tetrahedron, and the stereogenic center is located in the center of the adamantyl cage, shown by the dotted lines below connecting at the center of the molecule, and the configuration shown here is (R).
Periodic acid (H5IO6) will selectively cleave vicinal diols into two aldehyde or ketone fragments, which can be used to label the 3’-termini of RNA instead of DNA. Periodic acid can be dehydrated twice to form metal periodic acid. What are the molecular geometries of the two compounds respectively?- a)octahedral and tetrahedral
- b)trigonal bipyramidal and see-saw
- c)trigonal bipyramidal and square planar
- d)hexagonal and tetrahedral
Correct answer is option 'A'. Can you explain this answer?
Periodic acid (H5IO6) will selectively cleave vicinal diols into two aldehyde or ketone fragments, which can be used to label the 3’-termini of RNA instead of DNA. Periodic acid can be dehydrated twice to form metal periodic acid. What are the molecular geometries of the two compounds respectively?
a)
octahedral and tetrahedral
b)
trigonal bipyramidal and see-saw
c)
trigonal bipyramidal and square planar
d)
hexagonal and tetrahedral
|
|
Ayesha Joshi answered |
Starting with periodic acid, iodine has to be the central atom since it is the only atom that can expand its octet.
Since there are 5 hydrogens and 6 oxygens, it seems likely there are 5 hydroxyl (-OH) groups and a lone bonded oxygen. Since iodine has 7 valence electrons, it can expand its octet to form 7 bonds.
since each hydroxyl group is single bonded, then the lone oxygen has to be double bonded to form this Lewis dot structure:
since each hydroxyl group is single bonded, then the lone oxygen has to be double bonded to form this Lewis dot structure:
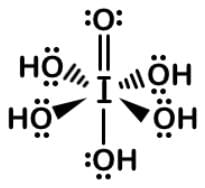
Since iodine could have 7 bonds, each of the 3 oxygens has to be double bonded and the 1 hydroxyl group single bonded to form this Lewis dot structure:
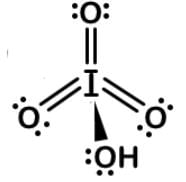
Periodic acid has an octahedral molecular geometry and metaperiodic acid has tetrahedral molecular geometry.
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3 or ClF5. What is the molecular geometry of chlorine trifluoride?- a)trigonal planar
- b)trigonal pyramidal
- c)T-shaped
- d)trigonal bipyramidal
Correct answer is option 'C'. Can you explain this answer?
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3 or ClF5. What is the molecular geometry of chlorine trifluoride?
a)
trigonal planar
b)
trigonal pyramidal
c)
T-shaped
d)
trigonal bipyramidal
|
|
Olivia Johnson answered |
Molecular Geometry of Chlorine Trifluoride
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Chapter doubts & questions for Covalent Bond (GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Covalent Bond (GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup