All Exams >
NEET >
NCERTs at Fingertips: Textbooks, Tests & Solutions >
All Questions
All questions of Organic Chemistry : Some Basic Principles & Techniques for NEET Exam
The most stable free radical among the following Is- a)
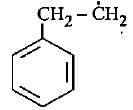
- b)

- c)
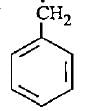
- d)
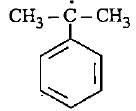
Correct answer is option 'D'. Can you explain this answer?
The most stable free radical among the following Is
a)

b)

c)

d)


|
Muskan Sharma answered |
In option D there is 6 alpha - H is present where as in option A only 2 alpha - H, option B 3 alpha - H and option C no alpha - H is present. This is because of hyperconjugation, more alpha - H more stability therefore option D is more stable.
What is the minimum number of carbon atoms of an alkane must have to form an isomer?- a)4
- b)3
- c)2
- d)1
Correct answer is option 'A'. Can you explain this answer?
What is the minimum number of carbon atoms of an alkane must have to form an isomer?
a)
4
b)
3
c)
2
d)
1
|
|
Ananya Das answered |
An alkane with minimum four carbon atoms can show isomerism.
The increasing order of electron donating inductive effect of alkyl groups is- a)- H < - CH3 < - C2H5 < - C3H7
- b)- H > - CH3 > - C2H5 > - C3H7
- c)- H < - C2H5 < - CH3 < - C3H7
- d)-H>-C2H5>-CH3>-C3H7
Correct answer is option 'A'. Can you explain this answer?
The increasing order of electron donating inductive effect of alkyl groups is
a)
- H < - CH3 < - C2H5 < - C3H7
b)
- H > - CH3 > - C2H5 > - C3H7
c)
- H < - C2H5 < - CH3 < - C3H7
d)
-H>-C2H5>-CH3>-C3H7
|
|
Mira Joshi answered |
The order of +I effect of alkyl group is given as
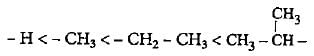
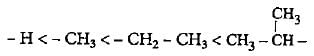
Which of the following is a false statement?- a)Free radicals, carbonium ions or carbanions are reaction intermediates
- b)Reaction between methane and chlorine in presence of sunlight proceeds via free radical
- c)The electronegative atom in the carbon chain produces +I effect
- d)Homolytlc fission of C - C bonds gives free radicals
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a false statement?
a)
Free radicals, carbonium ions or carbanions are reaction intermediates
b)
Reaction between methane and chlorine in presence of sunlight proceeds via free radical
c)
The electronegative atom in the carbon chain produces +I effect
d)
Homolytlc fission of C - C bonds gives free radicals
|
|
Raghav Bansal answered |
The electronegative atom in the carbon chain produces -I effect.
The carbocation 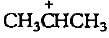 is less stable than
is less stable than- a)
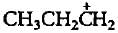
- b)
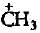
- c)
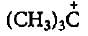
- d)
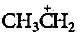
Correct answer is option 'C'. Can you explain this answer?
The carbocation 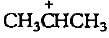 is less stable than
is less stable than
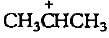 is less stable than
is less stable thana)
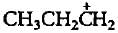
b)
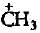
c)
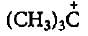
d)
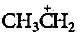

|
Shivkumar Tandale. answered |
That carbocation is always more stable which has large number of R group ।।
given carbocation has 2 R groups which are 2 methyl groups in the given options there is one option which has 3 R groups that is 3 methyl groups so option C) is correct one।।
Which of the following is an isomer of ethanol?- a)Methanol
- b)Acetone
- c)Diethylether
- d)Dimethylether
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an isomer of ethanol?
a)
Methanol
b)
Acetone
c)
Diethylether
d)
Dimethylether
|
|
Suresh Iyer answered |
CH3CH2OH and CH3-O-CH3 are functional isomers.
Which method can be applied to separate a mixture of camphor and benzole add?,- a)Sublimation
- b)Chemical methods
- c)Crystallisation
- d)Extractioh with solvent
Correct answer is option 'B'. Can you explain this answer?
Which method can be applied to separate a mixture of camphor and benzole add?,
a)
Sublimation
b)
Chemical methods
c)
Crystallisation
d)
Extractioh with solvent
|
|
Pankaj Dasgupta answered |
Separation of Camphor and Benzene
The method that can be applied to separate a mixture of camphor and benzene is chemical methods.
Explanation:
Chemical methods involve the use of chemical reactions to separate the components of a mixture. In this case, we can use a chemical reaction to selectively react with one of the components, thereby converting it into a different compound that can be separated from the mixture.
Here is a step-by-step explanation of how chemical methods can be applied to separate camphor and benzene:
1. Chemical Reaction: One possible method is to react camphor with a suitable reagent that can convert it into a different compound. For example, we can react camphor with bromine water (Br2) to form a solid compound called camphor bromide.
Camphor + Br2 -> Camphor bromide
2. Formation of Precipitate: Camphor bromide is a solid compound that is insoluble in the mixture. Therefore, it will precipitate out of the mixture as a solid.
3. Separation: The precipitated camphor bromide can be separated from the mixture by filtration. Filtration is a method that uses a porous barrier (filter paper) to separate the solid from the liquid.
4. Recovery: After filtration, the camphor bromide can be recovered by washing it with a suitable solvent, such as water, to remove any impurities.
5. Conversion back to Camphor: Finally, the camphor bromide can be converted back to camphor by reacting it with a reducing agent, such as zinc dust or sodium sulfite.
Camphor bromide + Reducing agent -> Camphor + By-products
By following these steps, we can successfully separate camphor from the mixture.
Advantages of Chemical Methods:
- Chemical methods are often highly selective and can target specific components in a mixture.
- They can be used to separate components that have similar physical properties.
- Chemical reactions can be controlled to ensure the desired separation is achieved.
Conclusion:
In conclusion, chemical methods can be applied to separate a mixture of camphor and benzene. By reacting camphor with a suitable reagent, we can convert it into a different compound that can be separated from the mixture. This method offers a selective and effective way to separate the components.
The method that can be applied to separate a mixture of camphor and benzene is chemical methods.
Explanation:
Chemical methods involve the use of chemical reactions to separate the components of a mixture. In this case, we can use a chemical reaction to selectively react with one of the components, thereby converting it into a different compound that can be separated from the mixture.
Here is a step-by-step explanation of how chemical methods can be applied to separate camphor and benzene:
1. Chemical Reaction: One possible method is to react camphor with a suitable reagent that can convert it into a different compound. For example, we can react camphor with bromine water (Br2) to form a solid compound called camphor bromide.
Camphor + Br2 -> Camphor bromide
2. Formation of Precipitate: Camphor bromide is a solid compound that is insoluble in the mixture. Therefore, it will precipitate out of the mixture as a solid.
3. Separation: The precipitated camphor bromide can be separated from the mixture by filtration. Filtration is a method that uses a porous barrier (filter paper) to separate the solid from the liquid.
4. Recovery: After filtration, the camphor bromide can be recovered by washing it with a suitable solvent, such as water, to remove any impurities.
5. Conversion back to Camphor: Finally, the camphor bromide can be converted back to camphor by reacting it with a reducing agent, such as zinc dust or sodium sulfite.
Camphor bromide + Reducing agent -> Camphor + By-products
By following these steps, we can successfully separate camphor from the mixture.
Advantages of Chemical Methods:
- Chemical methods are often highly selective and can target specific components in a mixture.
- They can be used to separate components that have similar physical properties.
- Chemical reactions can be controlled to ensure the desired separation is achieved.
Conclusion:
In conclusion, chemical methods can be applied to separate a mixture of camphor and benzene. By reacting camphor with a suitable reagent, we can convert it into a different compound that can be separated from the mixture. This method offers a selective and effective way to separate the components.
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N- a)(i) sp2, trigonal planar; (ii) sp3, tetrahedral
- b)(i) sp3, tetrahedral; (ii) sp, linear
- c)(i) sp, linear; (ii) sp2, trigonal planar
- d)(i) sp2, trigonal planar, (ii) sp2, trigonal planar
Correct answer is option 'B'. Can you explain this answer?
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N
(i) CH3F
(ii) HC ≡ N
a)
(i) sp2, trigonal planar; (ii) sp3, tetrahedral
b)
(i) sp3, tetrahedral; (ii) sp, linear
c)
(i) sp, linear; (ii) sp2, trigonal planar
d)
(i) sp2, trigonal planar, (ii) sp2, trigonal planar
|
|
Ankit Patel answered |
(i) CH3F:
The central atom in CH3F is carbon (C).
Hybridization:
Carbon is sp3 hybridized in CH3F. This is because carbon forms four sigma bonds. One sigma bond is formed with each of the three hydrogen atoms (H), and one sigma bond is formed with the fluorine atom (F).
Shape:
The shape of CH3F is tetrahedral. This is because carbon has four regions of electron density (three sigma bonds and one lone pair of electrons), resulting in a tetrahedral arrangement of these regions.
(ii) HC:
The central atom in HC is carbon (C).
Hybridization:
Carbon is sp hybridized in HC. This is because carbon forms one sigma bond with hydrogen (H) and one sigma bond with chlorine (Cl).
Shape:
The shape of HC is linear. This is because carbon has two regions of electron density (one sigma bond with hydrogen and one sigma bond with chlorine), resulting in a linear arrangement of these regions.
The central atom in CH3F is carbon (C).
Hybridization:
Carbon is sp3 hybridized in CH3F. This is because carbon forms four sigma bonds. One sigma bond is formed with each of the three hydrogen atoms (H), and one sigma bond is formed with the fluorine atom (F).
Shape:
The shape of CH3F is tetrahedral. This is because carbon has four regions of electron density (three sigma bonds and one lone pair of electrons), resulting in a tetrahedral arrangement of these regions.
(ii) HC:
The central atom in HC is carbon (C).
Hybridization:
Carbon is sp hybridized in HC. This is because carbon forms one sigma bond with hydrogen (H) and one sigma bond with chlorine (Cl).
Shape:
The shape of HC is linear. This is because carbon has two regions of electron density (one sigma bond with hydrogen and one sigma bond with chlorine), resulting in a linear arrangement of these regions.
Which one of the following acids would you expect to be the strongest?- a)I-CH2COOH
- b)CI-CH2COOH
- c)Br-CH2COOH
- d)F-CH2COOH
Correct answer is option 'D'. Can you explain this answer?
Which one of the following acids would you expect to be the strongest?
a)
I-CH2COOH
b)
CI-CH2COOH
c)
Br-CH2COOH
d)
F-CH2COOH
|
|
Mira Joshi answered |
Fluorine is most electronegative atom and exerts maximum -I effect Hence F - CH2COOH is the strongest acid.
Which of the following contains three pairs of electrons in valence shell?- a)Carbocations
- b)Carbanions
- c)Nucleophiles
- d)Carbenes
Correct answer is option 'A'. Can you explain this answer?
Which of the following contains three pairs of electrons in valence shell?
a)
Carbocations
b)
Carbanions
c)
Nucleophiles
d)
Carbenes
|
|
Anjali Sharma answered |
In carbocations, the carbon atom with positive charge has only 6 electrons in its valence shell.
Which of the following is the correctorder of acidity of carboxylic acids?
(i) CI3CCOOH > CI2CHCOOH > CICH2COOH
(ii) CH3CH2COOH > (CH3)2CHCOOH > CCH3)3CCOOH
(iii) F2CHCOOH > FCH2COOH > CICH2COOH- a)(i) and (ii)
- b)(ii) and (iii)
- c)(i) and (iii)
- d)(i), (ii) and (iii)
Correct answer is option 'D'. Can you explain this answer?
Which of the following is the correctorder of acidity of carboxylic acids?
(i) CI3CCOOH > CI2CHCOOH > CICH2COOH
(ii) CH3CH2COOH > (CH3)2CHCOOH > CCH3)3CCOOH
(iii) F2CHCOOH > FCH2COOH > CICH2COOH
(i) CI3CCOOH > CI2CHCOOH > CICH2COOH
(ii) CH3CH2COOH > (CH3)2CHCOOH > CCH3)3CCOOH
(iii) F2CHCOOH > FCH2COOH > CICH2COOH
a)
(i) and (ii)
b)
(ii) and (iii)
c)
(i) and (iii)
d)
(i), (ii) and (iii)
|
|
Pankaj Dasgupta answered |
The order of acidity of carboxylic acids can be determined by analyzing the electron-withdrawing or electron-donating groups present in the molecule. In this case, we are given three carboxylic acids: CI3CCOOH, CH3COOH, and CF3COOH.
The presence of electron-withdrawing groups such as halogens (Cl, F) increases the acidity of carboxylic acids. Therefore, the order of acidity from most acidic to least acidic would be:
(i) CI3CCOOH > CF3COOH > CH3COOH
The presence of electron-withdrawing groups such as halogens (Cl, F) increases the acidity of carboxylic acids. Therefore, the order of acidity from most acidic to least acidic would be:
(i) CI3CCOOH > CF3COOH > CH3COOH
The correct representation of 4-hydroxy-2-methylpent-2-en-1 -al Is- a)
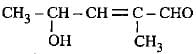
- b)
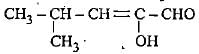
- c)
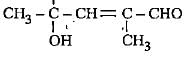
- d)
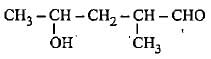
Correct answer is option 'A'. Can you explain this answer?
The correct representation of 4-hydroxy-2-methylpent-2-en-1 -al Is
a)
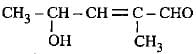
b)
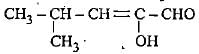
c)
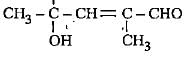
d)
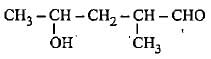
|
|
Hansa Sharma answered |
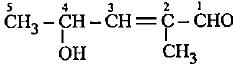
4-Hydroxy-2-methylpent-2-en-l-al
Which of the following ions is the most resonance stabilised?- a)Ethoxide
- b)Phenoxide
- c)Butoxide
- d)Isopropoxide
Correct answer is option 'B'. Can you explain this answer?
Which of the following ions is the most resonance stabilised?
a)
Ethoxide
b)
Phenoxide
c)
Butoxide
d)
Isopropoxide
|
|
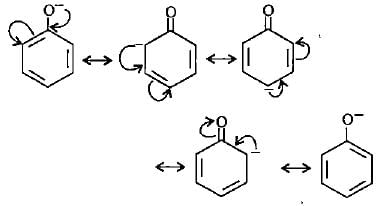
Raghav Bansal answered |
Phenoxide ion shows maximum resonating structures.

In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. What is the percentage of bromine in the compound?- a)68.08%
- b)34.04%
- c)42.1%
- d)50%
Correct answer is option 'B'. Can you explain this answer?
In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. What is the percentage of bromine in the compound?
a)
68.08%
b)
34.04%
c)
42.1%
d)
50%
|
|
Ajay Yadav answered |
Molar mass of AgBr = 108 + 80 = 188 g mol-1
Percentage of Br2 =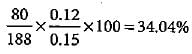
Percentage of Br2 =
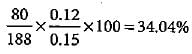
The percentage of oxygen in heavy water is- a)60
- b)50
- c)80
- d)5.9
Correct answer is option 'C'. Can you explain this answer?
The percentage of oxygen in heavy water is
a)
60
b)
50
c)
80
d)
5.9
|
|
Pooja Mukherjee answered |
The correct answer is option C, 80.
Explanation:
Heavy water, also known as deuterium oxide (D2O), is a form of water in which the hydrogen atoms are replaced by the isotope deuterium. Deuterium is an isotope of hydrogen that contains one proton and one neutron in its nucleus, making it twice as heavy as the common hydrogen isotope, protium.
The chemical formula for heavy water is D2O, indicating that it contains two deuterium atoms and one oxygen atom. In regular water (H2O), the oxygen atom is attached to two hydrogen atoms.
To determine the percentage of oxygen in heavy water, we need to calculate the molar mass of heavy water and the molar mass of oxygen.
The molar mass of heavy water (D2O) can be calculated as follows:
2 (molar mass of deuterium) + 1 (molar mass of oxygen)
= (2 x 2.014) + 15.999
= 4.028 + 15.999
= 20.027 g/mol
The molar mass of oxygen (O) is 15.999 g/mol.
To find the percentage of oxygen in heavy water, we can use the following formula:
Percentage of oxygen = (molar mass of oxygen / molar mass of heavy water) x 100
Plugging in the values, we get:
Percentage of oxygen = (15.999 / 20.027) x 100
= 0.799 x 100
= 79.9%
Therefore, the percentage of oxygen in heavy water is approximately 80% (option C).
Explanation:
Heavy water, also known as deuterium oxide (D2O), is a form of water in which the hydrogen atoms are replaced by the isotope deuterium. Deuterium is an isotope of hydrogen that contains one proton and one neutron in its nucleus, making it twice as heavy as the common hydrogen isotope, protium.
The chemical formula for heavy water is D2O, indicating that it contains two deuterium atoms and one oxygen atom. In regular water (H2O), the oxygen atom is attached to two hydrogen atoms.
To determine the percentage of oxygen in heavy water, we need to calculate the molar mass of heavy water and the molar mass of oxygen.
The molar mass of heavy water (D2O) can be calculated as follows:
2 (molar mass of deuterium) + 1 (molar mass of oxygen)
= (2 x 2.014) + 15.999
= 4.028 + 15.999
= 20.027 g/mol
The molar mass of oxygen (O) is 15.999 g/mol.
To find the percentage of oxygen in heavy water, we can use the following formula:
Percentage of oxygen = (molar mass of oxygen / molar mass of heavy water) x 100
Plugging in the values, we get:
Percentage of oxygen = (15.999 / 20.027) x 100
= 0.799 x 100
= 79.9%
Therefore, the percentage of oxygen in heavy water is approximately 80% (option C).
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N- a)(i) sp2, trigonal planar; (ii) sp3, tetrahedral
- b)(i) sp3, tetrahedral; (ii) sp, linear
- c)(i) sp, linear; (ii) sp2, trigonal planar
- d)(i) sp2, trigonal planar, (ii) sp2, trigonal planar
Correct answer is option 'B'. Can you explain this answer?
What are the hybridization and shapes of the following molecules?
(i) CH3F
(ii) HC ≡ N
(i) CH3F
(ii) HC ≡ N
a)
(i) sp2, trigonal planar; (ii) sp3, tetrahedral
b)
(i) sp3, tetrahedral; (ii) sp, linear
c)
(i) sp, linear; (ii) sp2, trigonal planar
d)
(i) sp2, trigonal planar, (ii) sp2, trigonal planar
|
|
Pankaj Dasgupta answered |
(i) CH3F:
The central atom in CH3F is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms four covalent bonds in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to each hydrogen atom, one single bond to the fluorine atom, and one lone pair on carbon. This gives us a total of four regions of electron density.
The hybridization of an atom with four regions of electron density is sp3. Therefore, the carbon atom in CH3F is sp3 hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, the lone pair and the three bond pairs are arranged in a tetrahedral geometry. However, the presence of a lone pair causes a distortion in the shape.
The lone pair occupies more space than the bond pairs and exerts a stronger repulsion. As a result, the bond angle between the three hydrogen atoms and the fluorine atom is slightly less than 109.5 degrees.
Therefore, the shape of CH3F is trigonal pyramidal.
(ii) HC:
The central atom in HC is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms one covalent bond in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to the hydrogen atom. This gives us a total of one region of electron density.
The hybridization of an atom with one region of electron density is sp. Therefore, the carbon atom in HC is sp hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, there is only one bond pair, so there is no specific shape associated with HC. However, the bond angle between the carbon and hydrogen atoms is approximately 180 degrees.
Therefore, the shape of HC is linear.
The central atom in CH3F is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms four covalent bonds in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to each hydrogen atom, one single bond to the fluorine atom, and one lone pair on carbon. This gives us a total of four regions of electron density.
The hybridization of an atom with four regions of electron density is sp3. Therefore, the carbon atom in CH3F is sp3 hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, the lone pair and the three bond pairs are arranged in a tetrahedral geometry. However, the presence of a lone pair causes a distortion in the shape.
The lone pair occupies more space than the bond pairs and exerts a stronger repulsion. As a result, the bond angle between the three hydrogen atoms and the fluorine atom is slightly less than 109.5 degrees.
Therefore, the shape of CH3F is trigonal pyramidal.
(ii) HC:
The central atom in HC is carbon, which has the electron configuration 1s2 2s2 2p2. Carbon forms one covalent bond in this molecule.
To determine the hybridization, we count the number of regions of electron density around the central atom. In this case, we have one single bond to the hydrogen atom. This gives us a total of one region of electron density.
The hybridization of an atom with one region of electron density is sp. Therefore, the carbon atom in HC is sp hybridized.
The shape of the molecule can be determined by looking at the arrangement of the regions of electron density. In this case, there is only one bond pair, so there is no specific shape associated with HC. However, the bond angle between the carbon and hydrogen atoms is approximately 180 degrees.
Therefore, the shape of HC is linear.
Which of the following Is a characteristic feature of a free radical?- a)It has a positive charge
- b)It has a negative charge
- c)It has all paired electrons
- d)It has an unpaired electrons
Correct answer is option 'D'. Can you explain this answer?
Which of the following Is a characteristic feature of a free radical?
a)
It has a positive charge
b)
It has a negative charge
c)
It has all paired electrons
d)
It has an unpaired electrons
|
|
Pankaj Dasgupta answered |
Free radicals are highly reactive chemical species that contain unpaired electrons. They are characterized by their high reactivity due to the presence of the unpaired electron. This unpaired electron makes free radicals unstable and highly reactive, as they seek to pair up with another electron to achieve stability.
Here are the key points explaining why a free radical has an unpaired electron:
1. Definition of a free radical:
- A free radical is a chemical species that contains one or more unpaired electrons in its outermost electron shell.
2. Unpaired electron:
- An unpaired electron is an electron that exists alone in an orbital, without a partner electron to pair with.
- In a stable atom or molecule, electrons are usually found in pairs, occupying the same orbital with opposite spins.
- However, in a free radical, one or more electrons are unpaired and do not have a partner to pair with.
3. Stability and reactivity:
- Free radicals are highly reactive due to the presence of the unpaired electron.
- The unpaired electron makes the free radical unstable and creates a strong tendency to react with other molecules in order to acquire stability.
- Free radicals can initiate chain reactions by reacting with other molecules and generating new free radicals in the process.
4. Electron configuration and stability:
- Atoms strive to achieve a stable electron configuration, usually by having a full outermost electron shell.
- Stable atoms have all their electrons paired, which provides a more energetically favorable state.
- Free radicals, with their unpaired electron, have an incomplete outermost electron shell, making them energetically unfavorable and highly reactive.
In conclusion, a characteristic feature of a free radical is the presence of an unpaired electron. This unpaired electron makes free radicals highly reactive and unstable, leading them to participate in various chemical reactions.
Here are the key points explaining why a free radical has an unpaired electron:
1. Definition of a free radical:
- A free radical is a chemical species that contains one or more unpaired electrons in its outermost electron shell.
2. Unpaired electron:
- An unpaired electron is an electron that exists alone in an orbital, without a partner electron to pair with.
- In a stable atom or molecule, electrons are usually found in pairs, occupying the same orbital with opposite spins.
- However, in a free radical, one or more electrons are unpaired and do not have a partner to pair with.
3. Stability and reactivity:
- Free radicals are highly reactive due to the presence of the unpaired electron.
- The unpaired electron makes the free radical unstable and creates a strong tendency to react with other molecules in order to acquire stability.
- Free radicals can initiate chain reactions by reacting with other molecules and generating new free radicals in the process.
4. Electron configuration and stability:
- Atoms strive to achieve a stable electron configuration, usually by having a full outermost electron shell.
- Stable atoms have all their electrons paired, which provides a more energetically favorable state.
- Free radicals, with their unpaired electron, have an incomplete outermost electron shell, making them energetically unfavorable and highly reactive.
In conclusion, a characteristic feature of a free radical is the presence of an unpaired electron. This unpaired electron makes free radicals highly reactive and unstable, leading them to participate in various chemical reactions.
Which of the following compounds Is not correctly matched with Its lUPAC name?- a)CH3CH2CH2COOCH2CH3 - Ethyl butanoate
- b)
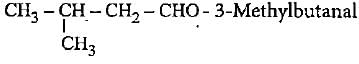
- c)
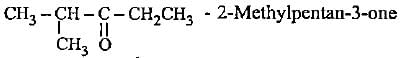
- d)
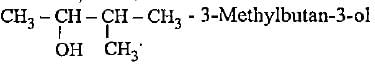
Correct answer is option 'D'. Can you explain this answer?
Which of the following compounds Is not correctly matched with Its lUPAC name?
a)
CH3CH2CH2COOCH2CH3 - Ethyl butanoate
b)
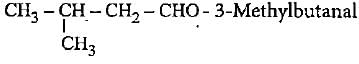
c)
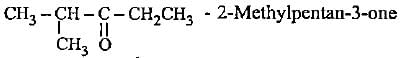
d)
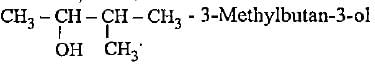
|
|
Gaurav Kumar answered |
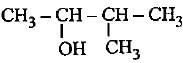
The correct name is 3-methylbutan-2-ol since functional group should get lowest number.
The order of decreasing stability of the following carbanions is
(i) (CH3)3C-
(ii) (CH3)2CH-
(iii) CH3CH-2
(iv) C6H5CH-2- a)(i) > (ii) > (iii) > (Iv)
- b)(iv) > (iii) > (ii) > (i)
- c)(iv) > (i) > (ii) > (iii)
- d)(iii) > (ii) > (i) > (iv)
Correct answer is option 'B'. Can you explain this answer?
The order of decreasing stability of the following carbanions is
(i) (CH3)3C-
(ii) (CH3)2CH-
(iii) CH3CH-2
(iv) C6H5CH-2
(i) (CH3)3C-
(ii) (CH3)2CH-
(iii) CH3CH-2
(iv) C6H5CH-2
a)
(i) > (ii) > (iii) > (Iv)
b)
(iv) > (iii) > (ii) > (i)
c)
(iv) > (i) > (ii) > (iii)
d)
(iii) > (ii) > (i) > (iv)
|
|
Bhargavi Choudhary answered |
(i) (CH3)3C-
(ii) (CH3)2CH-
(iii) CH3CH-2
(iv) C6H5CH-2
(ii) (CH3)2CH-
(iii) CH3CH-2
(iv) C6H5CH-2
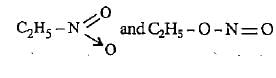 are examples of
are examples of- a)Functional isomers
- b)Tautomers
- c)Position isomers
- d)Metamers
Correct answer is option 'A'. Can you explain this answer?
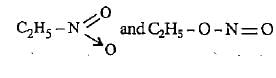 are examples of
are examples ofa)
Functional isomers
b)
Tautomers
c)
Position isomers
d)
Metamers
|
|
Suresh Iyer answered |
Compounds having same molecular formula but different functional groups are called functional isomers. Both the given compounds are functional isomers.
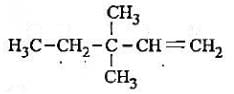
The lUPAC name of the compound having formula
- a)3,3,3-trimethylprop-l-ene
- b)1,1,1 - trlmethylprop-2-ene
- c)3,3-dimethylpent-l-ene
- d)2,2-dlmethylbut-3-ene
Correct answer is option 'C'. Can you explain this answer?
The lUPAC name of the compound having formula


a)
3,3,3-trimethylprop-l-ene
b)
1,1,1 - trlmethylprop-2-ene
c)
3,3-dimethylpent-l-ene
d)
2,2-dlmethylbut-3-ene
|
|
Spidey Man answered |
1st - should always choose longest chain where 2 methyl are excluded
2nd- double bond has more priority than single bond so numbering starts from there
2nd- double bond has more priority than single bond so numbering starts from there
Few pairs of molecules are given below. Which bond of the molecule of the pairs is more polar?
(i) H3C - H, H3C - Br
(ii) H3C-NH2,H3C-0H
(iii) H3C - OH, H3C - SH
(iv) H3C - Cl, H3C - Br- a)C-Br,C-N,C-O,C-Br
- b)C-Br,C-O,C-O,C-Cl
- c)C-Br,C-N,C-S,C-Cl
- d)C-Br,C-O,C-S,C-Br
Correct answer is option 'B'. Can you explain this answer?
Few pairs of molecules are given below. Which bond of the molecule of the pairs is more polar?
(i) H3C - H, H3C - Br
(ii) H3C-NH2,H3C-0H
(iii) H3C - OH, H3C - SH
(iv) H3C - Cl, H3C - Br
(i) H3C - H, H3C - Br
(ii) H3C-NH2,H3C-0H
(iii) H3C - OH, H3C - SH
(iv) H3C - Cl, H3C - Br
a)
C-Br,C-N,C-O,C-Br
b)
C-Br,C-O,C-O,C-Cl
c)
C-Br,C-N,C-S,C-Cl
d)
C-Br,C-O,C-S,C-Br
|
|
Ajay Yadav answered |
(i) Br is more electronegative than H.
(ii) O is more electronegative than N.
(iii) O is more electronegative than S.
(iv) C1 is more electronegative than Br.
(ii) O is more electronegative than N.
(iii) O is more electronegative than S.
(iv) C1 is more electronegative than Br.
Which of the following carbanion expected to be most stable?- a)P-NO2C6H4CH2
- b)0-NO2C6H4CH2
- c)o-CHOC6H4CH2
- d)P-CHOC6H4CH2
Correct answer is option 'B'. Can you explain this answer?
Which of the following carbanion expected to be most stable?
a)
P-NO2C6H4CH2
b)
0-NO2C6H4CH2
c)
o-CHOC6H4CH2
d)
P-CHOC6H4CH2
|
|
Yashvi Malik answered |
The most stable carbanion among the given options is option B, 0-NO2C6H4CH2. Let's analyze the reasons for its stability:
Resonance stabilization:
- The carbanion in option B can undergo resonance stabilization due to the presence of the nitro group (-NO2) and the aromatic ring.
- The lone pair of electrons on the carbon atom can delocalize into the adjacent nitro group and the aromatic ring through resonance.
- This delocalization of electrons spreads the negative charge over a larger area, making the carbanion more stable.
Inductive effect:
- The presence of the nitro group in option B also contributes to the stability of the carbanion through the inductive effect.
- The electronegative oxygen atoms in the nitro group withdraw electron density from the carbon atom, reducing the electron density on the carbanion.
- This reduction in electron density decreases the reactivity of the carbanion and increases its stability.
Steric hindrance:
- The presence of bulky groups near the carbanion can lead to steric hindrance, destabilizing the carbanion.
- In option B, the para position of the aromatic ring is substituted with a nitro group (-NO2), which is a relatively large group.
- This bulky nitro group can provide steric hindrance to the carbanion, making it less stable compared to other options.
Based on these factors, it can be concluded that option B, 0-NO2C6H4CH2, is the most stable carbanion among the given options.
Resonance stabilization:
- The carbanion in option B can undergo resonance stabilization due to the presence of the nitro group (-NO2) and the aromatic ring.
- The lone pair of electrons on the carbon atom can delocalize into the adjacent nitro group and the aromatic ring through resonance.
- This delocalization of electrons spreads the negative charge over a larger area, making the carbanion more stable.
Inductive effect:
- The presence of the nitro group in option B also contributes to the stability of the carbanion through the inductive effect.
- The electronegative oxygen atoms in the nitro group withdraw electron density from the carbon atom, reducing the electron density on the carbanion.
- This reduction in electron density decreases the reactivity of the carbanion and increases its stability.
Steric hindrance:
- The presence of bulky groups near the carbanion can lead to steric hindrance, destabilizing the carbanion.
- In option B, the para position of the aromatic ring is substituted with a nitro group (-NO2), which is a relatively large group.
- This bulky nitro group can provide steric hindrance to the carbanion, making it less stable compared to other options.
Based on these factors, it can be concluded that option B, 0-NO2C6H4CH2, is the most stable carbanion among the given options.
In Duma'smethod 0.52 g of an organic compound on combustion gave 68.6 mL N2 at 27oC and 76 mm pressure. What is the percentage of nitrogen in the compound?- a)12.22%
- b)14.93%
- c)15.84%
- d)16.23%
Correct answer is option 'B'. Can you explain this answer?
In Duma'smethod 0.52 g of an organic compound on combustion gave 68.6 mL N2 at 27oC and 76 mm pressure. What is the percentage of nitrogen in the compound?
a)
12.22%
b)
14.93%
c)
15.84%
d)
16.23%
|
|
Meera Singh answered |
V1 = 68.6 mL, P1 =. 756 mm, T1 = 300 K V2 = ?, P2 = 760 mm, T2 = 273 K
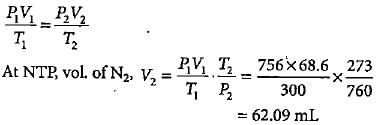
Percentage of nitrogen in organic compound
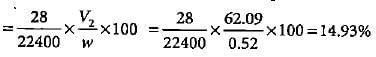
Percentage of nitrogen in organic compound
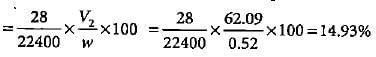
Which of the following sets of groups contains only electrophiles?- a)NH-2, NO+2, H2O, NH3
- b)F-,OH-,NH3,SO3
- c)NO+2, AlCl3> SO3,CH3C+=O
- d)NH3, BF3> AlCI3, H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following sets of groups contains only electrophiles?
a)
NH-2, NO+2, H2O, NH3
b)
F-,OH-,NH3,SO3
c)
NO+2, AlCl3> SO3,CH3C+=O
d)
NH3, BF3> AlCI3, H2O
|
|
Lavanya Menon answered |
NO2+,AlCl3,SO3 and CH3C+ = O are electrophiles.
1.6 g of an organic compound gave 2.6 g of magnesium pyrophosphate. The percentage of phosphorus in the compound is- a)45.38%
- b)54.38%
- c)37.76%
- d)19.02%
Correct answer is option 'A'. Can you explain this answer?
1.6 g of an organic compound gave 2.6 g of magnesium pyrophosphate. The percentage of phosphorus in the compound is
a)
45.38%
b)
54.38%
c)
37.76%
d)
19.02%
|
|
Sounak Shah answered |
Understanding the Problem
To find the percentage of phosphorus in the organic compound, we need to analyze the formation of magnesium pyrophosphate (Mg2P2O7) and the data provided.
Step 1: Calculate moles of magnesium pyrophosphate
- Molecular weight of Mg2P2O7:
- Mg: 24.31 g/mol (2 Mg = 48.62 g)
- P: 30.97 g/mol (2 P = 61.94 g)
- O: 16.00 g/mol (7 O = 112.00 g)
- Total: 48.62 + 61.94 + 112.00 = 222.56 g/mol
- Moles of Mg2P2O7 produced from 2.6 g:
- Moles = mass / molar mass = 2.6 g / 222.56 g/mol ≈ 0.0117 moles
Step 2: Calculate moles of phosphorus
- Each mole of Mg2P2O7 contains 2 moles of phosphorus (P).
- Moles of phosphorus = 2 × 0.0117 ≈ 0.0234 moles
Step 3: Calculate the mass of phosphorus
- Mass of phosphorus = moles × atomic weight
- Atomic weight of P = 30.97 g/mol
- Mass of phosphorus = 0.0234 moles × 30.97 g/mol ≈ 0.726 g
Step 4: Calculate percentage of phosphorus in the organic compound
- Percentage = (mass of phosphorus / mass of compound) × 100
- Percentage = (0.726 g / 1.6 g) × 100 ≈ 45.38%
Conclusion
The percentage of phosphorus in the organic compound is approximately 45.38%, which corresponds to option 'A'.
To find the percentage of phosphorus in the organic compound, we need to analyze the formation of magnesium pyrophosphate (Mg2P2O7) and the data provided.
Step 1: Calculate moles of magnesium pyrophosphate
- Molecular weight of Mg2P2O7:
- Mg: 24.31 g/mol (2 Mg = 48.62 g)
- P: 30.97 g/mol (2 P = 61.94 g)
- O: 16.00 g/mol (7 O = 112.00 g)
- Total: 48.62 + 61.94 + 112.00 = 222.56 g/mol
- Moles of Mg2P2O7 produced from 2.6 g:
- Moles = mass / molar mass = 2.6 g / 222.56 g/mol ≈ 0.0117 moles
Step 2: Calculate moles of phosphorus
- Each mole of Mg2P2O7 contains 2 moles of phosphorus (P).
- Moles of phosphorus = 2 × 0.0117 ≈ 0.0234 moles
Step 3: Calculate the mass of phosphorus
- Mass of phosphorus = moles × atomic weight
- Atomic weight of P = 30.97 g/mol
- Mass of phosphorus = 0.0234 moles × 30.97 g/mol ≈ 0.726 g
Step 4: Calculate percentage of phosphorus in the organic compound
- Percentage = (mass of phosphorus / mass of compound) × 100
- Percentage = (0.726 g / 1.6 g) × 100 ≈ 45.38%
Conclusion
The percentage of phosphorus in the organic compound is approximately 45.38%, which corresponds to option 'A'.
Separation of two substances by crystallisation depends upon their differences In- a)Densities
- b)Solubility
- c)Melting points
- d)Boiling points
Correct answer is option 'B'. Can you explain this answer?
Separation of two substances by crystallisation depends upon their differences In
a)
Densities
b)
Solubility
c)
Melting points
d)
Boiling points
|
|
Raghav Bansal answered |
Crystallisation is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
Those substances can be separated by steam distillation which are- a)Steam volatile and insoluble in water
- b)Steam volatile and soluble In water
- c)Steam volatile and sparingly soluble in water
- d)In liquid form In steam and solid form In water
Correct answer is option 'A'. Can you explain this answer?
Those substances can be separated by steam distillation which are
a)
Steam volatile and insoluble in water
b)
Steam volatile and soluble In water
c)
Steam volatile and sparingly soluble in water
d)
In liquid form In steam and solid form In water
|
|
Raghav Bansal answered |
Steam distillation is applied to separate substances which are steam volatile and are immiscible in water.
The correct decreasing order of priority for the functional groups of organic compounds In the lUPAC system of nomenclature Is- a)- CONH2, - CHO, - SO3H, - COOH
- b)- COOH, - SO3H, - CONH2, - CHO
- c)- SO3H, -COOH, -CONH2, - CHO
- d)- CHO, -COOH, - SO3H. -CONH2
Correct answer is option 'B'. Can you explain this answer?
The correct decreasing order of priority for the functional groups of organic compounds In the lUPAC system of nomenclature Is
a)
- CONH2, - CHO, - SO3H, - COOH
b)
- COOH, - SO3H, - CONH2, - CHO
c)
- SO3H, -COOH, -CONH2, - CHO
d)
- CHO, -COOH, - SO3H. -CONH2
|
|
Anjali Sharma answered |
The order of preference of functional groups is as follows;
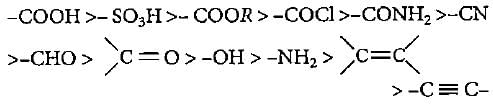
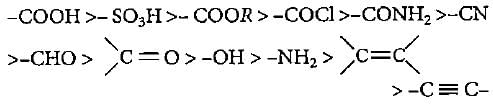
An organic compound gave 0.4655 g of CO2 on complete combustion. If the mass of the compound taken was 0.2115 g, what is the percentage of C in it?- a)13.30%
- b)26.67%
- c)60.03%
- d)28.80%
Correct answer is option 'C'. Can you explain this answer?
An organic compound gave 0.4655 g of CO2 on complete combustion. If the mass of the compound taken was 0.2115 g, what is the percentage of C in it?
a)
13.30%
b)
26.67%
c)
60.03%
d)
28.80%
|
|
Anjali Sharma answered |
Mass of CO2 formed = 0.4655 g
Mass of organic compound taken = 0.2115 g
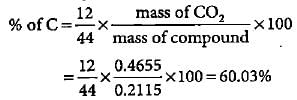
Mass of organic compound taken = 0.2115 g
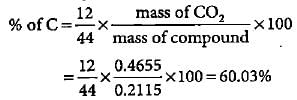
In which of the following species hyperconjugation is possible?- a)
- b)C6H5-CH3
- c)CH2 = CH2
- d)
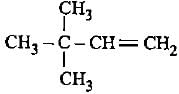
Correct answer is option 'B'. Can you explain this answer?
In which of the following species hyperconjugation is possible?
a)
b)
C6H5-CH3
c)
CH2 = CH2
d)

|
|
Jyoti Sengupta answered |
For hyperconjugation α-carbon with respect to sp2 hybridised carbon should have at least one hydrogen.
Which type of hybridisation of each carbon is there in the compound?
CH3 - CH = CH - CN- a)sp3, sp2, sp2, sp
- b)sp3, sp2, sp2, sp3
- c)sp3,sp2,sp3,sp3
- d)sp3,sp2,sp,sp3
Correct answer is option 'A'. Can you explain this answer?
Which type of hybridisation of each carbon is there in the compound?
CH3 - CH = CH - CN
CH3 - CH = CH - CN
a)
sp3, sp2, sp2, sp
b)
sp3, sp2, sp2, sp3
c)
sp3,sp2,sp3,sp3
d)
sp3,sp2,sp,sp3
|
|
Yash Majumdar answered |
Understanding Hybridization in CH3 - CH = CH - CN
To determine the hybridization of each carbon in the compound CH3 - CH = CH - CN, we analyze the bonding and geometry around each carbon atom.
1. Carbon 1 (CH3)
- This carbon is bonded to three hydrogen atoms and one other carbon atom.
- The geometry is tetrahedral.
- Hybridization: sp3
2. Carbon 2 (CH)
- This carbon is bonded to one hydrogen atom, one carbon atom (C1), and one carbon atom (C3) through a double bond.
- The geometry is trigonal planar due to the double bond.
- Hybridization: sp2
3. Carbon 3 (CH)
- This carbon is bonded to one hydrogen atom and one carbon atom (C2) through a double bond, and a cyanide group (CN).
- The geometry is also trigonal planar.
- Hybridization: sp2
4. Carbon 4 (CN)
- The carbon in the cyanide group is triple-bonded to nitrogen (N) and is also connected to carbon (C3).
- The geometry is linear due to the triple bond.
- Hybridization: sp
Summary of Hybridization
- Carbon 1: sp3
- Carbon 2: sp2
- Carbon 3: sp2
- Carbon 4: sp
Conclusion
The correct hybridization scheme for the compound CH3 - CH = CH - CN is sp3, sp2, sp2, sp. Thus, the correct answer is option 'A'.
To determine the hybridization of each carbon in the compound CH3 - CH = CH - CN, we analyze the bonding and geometry around each carbon atom.
1. Carbon 1 (CH3)
- This carbon is bonded to three hydrogen atoms and one other carbon atom.
- The geometry is tetrahedral.
- Hybridization: sp3
2. Carbon 2 (CH)
- This carbon is bonded to one hydrogen atom, one carbon atom (C1), and one carbon atom (C3) through a double bond.
- The geometry is trigonal planar due to the double bond.
- Hybridization: sp2
3. Carbon 3 (CH)
- This carbon is bonded to one hydrogen atom and one carbon atom (C2) through a double bond, and a cyanide group (CN).
- The geometry is also trigonal planar.
- Hybridization: sp2
4. Carbon 4 (CN)
- The carbon in the cyanide group is triple-bonded to nitrogen (N) and is also connected to carbon (C3).
- The geometry is linear due to the triple bond.
- Hybridization: sp
Summary of Hybridization
- Carbon 1: sp3
- Carbon 2: sp2
- Carbon 3: sp2
- Carbon 4: sp
Conclusion
The correct hybridization scheme for the compound CH3 - CH = CH - CN is sp3, sp2, sp2, sp. Thus, the correct answer is option 'A'.
The presence of carbon in an organic compound can be shown by- a)Heating the compound with sodium
- b)Heating the compound with cupric oxide
- c)Heating the compound on bunsen flame
- d)Heating the compound with magnesium.
Correct answer is option 'B'. Can you explain this answer?
The presence of carbon in an organic compound can be shown by
a)
Heating the compound with sodium
b)
Heating the compound with cupric oxide
c)
Heating the compound on bunsen flame
d)
Heating the compound with magnesium.
|
|
Ananya Das answered |
Compound when heated with CuO reduces CuO to Cu and oxidises C to CO2 which turns lime water milky.
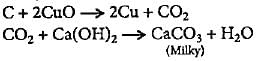
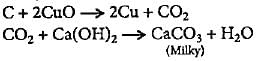
Inductive effect of which atom or group is taken as zero to compare inductive effect of other atoms?- a)Hydrogen
- b)Chlorine
- c)Carbon
- d)Oxygen
Correct answer is option 'A'. Can you explain this answer?
Inductive effect of which atom or group is taken as zero to compare inductive effect of other atoms?
a)
Hydrogen
b)
Chlorine
c)
Carbon
d)
Oxygen
|
|
Riya Banerjee answered |
Hydrogen does not exert I-effect. Its inductive effect is taken as zero. Electron releasing or electron withdrawing capability of other atoms are compared by hydrogen.
Glycerin can be purified by:- a)Vacuum distillation
- b)Simple distillation
- c)Steam distillation
- d)Fractional distillation
Correct answer is option 'A'. Can you explain this answer?
Glycerin can be purified by:
a)
Vacuum distillation
b)
Simple distillation
c)
Steam distillation
d)
Fractional distillation
|
|
Anoushka Dasgupta answered |
Purification of Glycerin by Vacuum Distillation
Introduction
Glycerin is a colorless, odorless, viscous liquid that is widely used in various industries, including pharmaceuticals, cosmetics, and food processing. However, the crude glycerin obtained from the transesterification process contains impurities such as water, salts, fatty acids, and other organic compounds. Therefore, it is necessary to purify glycerin to meet the required quality standards. Vacuum distillation is one of the methods commonly employed for the purification of glycerin.
Process of Vacuum Distillation
Vacuum distillation is a technique used to separate substances based on their boiling points under reduced pressure. It is performed in a vacuum distillation apparatus, which consists of a round-bottom flask, distillation column, condenser, and receiver.
1. Heating: The crude glycerin is placed in the round-bottom flask and heated. The heating is typically done using a heating mantle or a hot plate. As the temperature rises, the impurities in the glycerin vaporize and rise into the distillation column.
2. Reduced Pressure: In vacuum distillation, the pressure inside the apparatus is reduced to lower the boiling points of the substances. This allows for the separation of the components at lower temperatures, minimizing the chances of thermal degradation of glycerin.
3. Condensation: As the vapors rise in the distillation column, they come into contact with the condenser, which cools them down. The cooling causes the vapors to condense back into a liquid form.
4. Collection: The condensed liquid, which now contains purified glycerin, is collected in the receiver. This glycerin is now free from impurities and can be used for various applications.
Advantages of Vacuum Distillation for Glycerin Purification
- Reduced Thermal Degradation: Vacuum distillation allows for the purification of glycerin at lower temperatures, reducing the chances of thermal degradation that can occur at higher temperatures.
- Efficient Separation: The use of reduced pressure in vacuum distillation enables the separation of impurities from glycerin more effectively, as the lower boiling points of the impurities facilitate their removal.
- Energy Conservation: Vacuum distillation requires less energy compared to other distillation techniques, as the reduced pressure lowers the boiling points of the substances, resulting in lower energy consumption.
Conclusion
In summary, glycerin can be purified by vacuum distillation. This technique allows for the separation of impurities from glycerin by reducing the pressure and lowering the boiling points of the substances. Vacuum distillation is an efficient and energy-saving method for the purification of glycerin, resulting in a high-quality product that meets the required standards for various applications.
Introduction
Glycerin is a colorless, odorless, viscous liquid that is widely used in various industries, including pharmaceuticals, cosmetics, and food processing. However, the crude glycerin obtained from the transesterification process contains impurities such as water, salts, fatty acids, and other organic compounds. Therefore, it is necessary to purify glycerin to meet the required quality standards. Vacuum distillation is one of the methods commonly employed for the purification of glycerin.
Process of Vacuum Distillation
Vacuum distillation is a technique used to separate substances based on their boiling points under reduced pressure. It is performed in a vacuum distillation apparatus, which consists of a round-bottom flask, distillation column, condenser, and receiver.
1. Heating: The crude glycerin is placed in the round-bottom flask and heated. The heating is typically done using a heating mantle or a hot plate. As the temperature rises, the impurities in the glycerin vaporize and rise into the distillation column.
2. Reduced Pressure: In vacuum distillation, the pressure inside the apparatus is reduced to lower the boiling points of the substances. This allows for the separation of the components at lower temperatures, minimizing the chances of thermal degradation of glycerin.
3. Condensation: As the vapors rise in the distillation column, they come into contact with the condenser, which cools them down. The cooling causes the vapors to condense back into a liquid form.
4. Collection: The condensed liquid, which now contains purified glycerin, is collected in the receiver. This glycerin is now free from impurities and can be used for various applications.
Advantages of Vacuum Distillation for Glycerin Purification
- Reduced Thermal Degradation: Vacuum distillation allows for the purification of glycerin at lower temperatures, reducing the chances of thermal degradation that can occur at higher temperatures.
- Efficient Separation: The use of reduced pressure in vacuum distillation enables the separation of impurities from glycerin more effectively, as the lower boiling points of the impurities facilitate their removal.
- Energy Conservation: Vacuum distillation requires less energy compared to other distillation techniques, as the reduced pressure lowers the boiling points of the substances, resulting in lower energy consumption.
Conclusion
In summary, glycerin can be purified by vacuum distillation. This technique allows for the separation of impurities from glycerin by reducing the pressure and lowering the boiling points of the substances. Vacuum distillation is an efficient and energy-saving method for the purification of glycerin, resulting in a high-quality product that meets the required standards for various applications.
0.2 g of an organic compound contains C, H and O.On combustion, it yields 0.15 g CO2 and 0.12 g H2O.Ihe percentage of C, H and O respectively is- a)C = 15%, H = 20%, O = 65%
- b)C = 10%, H = 8.2%, O = 81.8%
- c)C = 12.2%, H = 8.8%, O = 79%
- d)C = 20%, H = 6.66%, O = 73.34%
Correct answer is option 'D'. Can you explain this answer?
0.2 g of an organic compound contains C, H and O.On combustion, it yields 0.15 g CO2 and 0.12 g H2O.Ihe percentage of C, H and O respectively is
a)
C = 15%, H = 20%, O = 65%
b)
C = 10%, H = 8.2%, O = 81.8%
c)
C = 12.2%, H = 8.8%, O = 79%
d)
C = 20%, H = 6.66%, O = 73.34%
|
|
Dhruba Chakraborty answered |
Given:
Mass of organic compound = 0.2 g
Mass of CO2 produced = 0.15 g
Mass of H2O produced = 0.12 g
To find:
Percentage of C, H, and O in the compound
Let's assume that the organic compound is composed of carbon, hydrogen, and oxygen only.
Step 1: Calculate the moles of CO2 produced
Molar mass of CO2 = 12.01 g/mol (C) + 2 * 16.00 g/mol (O) = 44.01 g/mol
Moles of CO2 = Mass of CO2 / Molar mass of CO2 = 0.15 g / 44.01 g/mol = 0.0034 mol
Step 2: Calculate the moles of H2O produced
Molar mass of H2O = 2 * 1.01 g/mol (H) + 16.00 g/mol (O) = 18.02 g/mol
Moles of H2O = Mass of H2O / Molar mass of H2O = 0.12 g / 18.02 g/mol = 0.0067 mol
Step 3: Calculate the moles of carbon
Since 1 mole of CO2 contains 1 mole of carbon, moles of carbon = moles of CO2 = 0.0034 mol
Step 4: Calculate the moles of hydrogen
Since 1 mole of H2O contains 2 moles of hydrogen, moles of hydrogen = 2 * moles of H2O = 2 * 0.0067 mol = 0.0134 mol
Step 5: Calculate the moles of oxygen
Moles of oxygen = (moles of CO2 * 2) + moles of H2O = (0.0034 mol * 2) + 0.0067 mol = 0.0135 mol
Step 6: Calculate the percentage of each element
Percentage of carbon = (moles of carbon * molar mass of carbon) / mass of compound * 100
Percentage of carbon = (0.0034 mol * 12.01 g/mol) / 0.2 g * 100 = 20%
Percentage of hydrogen = (moles of hydrogen * molar mass of hydrogen) / mass of compound * 100
Percentage of hydrogen = (0.0134 mol * 1.01 g/mol) / 0.2 g * 100 = 6.66%
Percentage of oxygen = (moles of oxygen * molar mass of oxygen) / mass of compound * 100
Percentage of oxygen = (0.0135 mol * 16.00 g/mol) / 0.2 g * 100 = 73.34%
Therefore, the percentage of C, H, and O in the compound is:
C = 20%, H = 6.66%, O = 73.34%
Hence, the correct answer is option D.
Mass of organic compound = 0.2 g
Mass of CO2 produced = 0.15 g
Mass of H2O produced = 0.12 g
To find:
Percentage of C, H, and O in the compound
Let's assume that the organic compound is composed of carbon, hydrogen, and oxygen only.
Step 1: Calculate the moles of CO2 produced
Molar mass of CO2 = 12.01 g/mol (C) + 2 * 16.00 g/mol (O) = 44.01 g/mol
Moles of CO2 = Mass of CO2 / Molar mass of CO2 = 0.15 g / 44.01 g/mol = 0.0034 mol
Step 2: Calculate the moles of H2O produced
Molar mass of H2O = 2 * 1.01 g/mol (H) + 16.00 g/mol (O) = 18.02 g/mol
Moles of H2O = Mass of H2O / Molar mass of H2O = 0.12 g / 18.02 g/mol = 0.0067 mol
Step 3: Calculate the moles of carbon
Since 1 mole of CO2 contains 1 mole of carbon, moles of carbon = moles of CO2 = 0.0034 mol
Step 4: Calculate the moles of hydrogen
Since 1 mole of H2O contains 2 moles of hydrogen, moles of hydrogen = 2 * moles of H2O = 2 * 0.0067 mol = 0.0134 mol
Step 5: Calculate the moles of oxygen
Moles of oxygen = (moles of CO2 * 2) + moles of H2O = (0.0034 mol * 2) + 0.0067 mol = 0.0135 mol
Step 6: Calculate the percentage of each element
Percentage of carbon = (moles of carbon * molar mass of carbon) / mass of compound * 100
Percentage of carbon = (0.0034 mol * 12.01 g/mol) / 0.2 g * 100 = 20%
Percentage of hydrogen = (moles of hydrogen * molar mass of hydrogen) / mass of compound * 100
Percentage of hydrogen = (0.0134 mol * 1.01 g/mol) / 0.2 g * 100 = 6.66%
Percentage of oxygen = (moles of oxygen * molar mass of oxygen) / mass of compound * 100
Percentage of oxygen = (0.0135 mol * 16.00 g/mol) / 0.2 g * 100 = 73.34%
Therefore, the percentage of C, H, and O in the compound is:
C = 20%, H = 6.66%, O = 73.34%
Hence, the correct answer is option D.
0.92 g of an organic compound was analysed by combustion method. The mass of the U-tube increased by 1.08 g. What is the percentage of hydrogen in the compound?- a)13.04%
- b)52.17%
- c)65.21%
- d)11.30%
Correct answer is option 'A'. Can you explain this answer?
0.92 g of an organic compound was analysed by combustion method. The mass of the U-tube increased by 1.08 g. What is the percentage of hydrogen in the compound?
a)
13.04%
b)
52.17%
c)
65.21%
d)
11.30%
|
|
Raghav Bansal answered |
Increase in mass of U-tube = 1.08 g
Mass of water formed = 1.08 g
18gH20 = 2gH
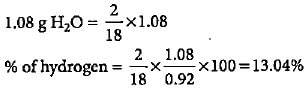
Mass of water formed = 1.08 g
18gH20 = 2gH
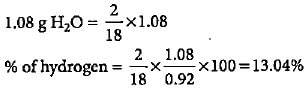
Free radicals can undergo- a)Rearrangement to a more stable free radical
- b)Decomposition to give another free radical
- c)Combination with other free radical
- d)All are correct
Correct answer is option 'D'. Can you explain this answer?
Free radicals can undergo
a)
Rearrangement to a more stable free radical
b)
Decomposition to give another free radical
c)
Combination with other free radical
d)
All are correct
|
|
Ananya Das answered |
Free radicals can undergo all given types of processes.
Which of the following statements is not true about the stability of carbanions?- a)Stability of carbanions increases with increase in s-character of orbital
- b)The electron withdrawing groups like -NO2, - CN, > c = o increases the stability ofcarbanions
- c)Order of stability of carbanions is 3º > 2º > 1º
- d)The negatively charged carbon is sp3 hybridised and tetrahedral
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is not true about the stability of carbanions?
a)
Stability of carbanions increases with increase in s-character of orbital
b)
The electron withdrawing groups like -NO2, - CN, > c = o increases the stability ofcarbanions
c)
Order of stability of carbanions is 3º > 2º > 1º
d)
The negatively charged carbon is sp3 hybridised and tetrahedral
|
|
Pankaj Dasgupta answered |
And -COOH stabilize carbanionsc)The presence of resonance structures increases the stability of carbanionsd)Carbanions are more stable when they are more electronegative than the atom to which they are attached.
In kjeldahl's method of estimation of nitrogen, nitrogen is quantitatively converted to ammonium sulphate. It is the treated with the standard solution of alkali. The nitrogen which is present is estimated as:- a)N2 gas
- b)NO2 gas
- c)NH3 gas
- d)(NH4)2SO4
Correct answer is option 'C'. Can you explain this answer?
In kjeldahl's method of estimation of nitrogen, nitrogen is quantitatively converted to ammonium sulphate. It is the treated with the standard solution of alkali. The nitrogen which is present is estimated as:
a)
N2 gas
b)
NO2 gas
c)
NH3 gas
d)
(NH4)2SO4
|
|
Jyoti Sengupta answered |
When treated with standard solution of alkali nitrogen is liberated as NH3 which is absorbed in H2SO4 to get percentage of nitrogen.
The substance which can be used as adsorbent in column chromatography is- a)Na2O
- b)Na2SO4
- c)AI2O3
- d)NaCl
Correct answer is option 'C'. Can you explain this answer?
The substance which can be used as adsorbent in column chromatography is
a)
Na2O
b)
Na2SO4
c)
AI2O3
d)
NaCl
|
|
Riya Banerjee answered |
Alumina or silica gel are generally used as adsorbent in column chromatography.
Stability of alkyl carbocations can be explained by- a)Inductive effect only
- b)Hyperconjugation only
- c)Both inductive effect and hyperconjugation
- d)Electromeric effect only.
Correct answer is option 'C'. Can you explain this answer?
Stability of alkyl carbocations can be explained by
a)
Inductive effect only
b)
Hyperconjugation only
c)
Both inductive effect and hyperconjugation
d)
Electromeric effect only.
|
|
Jyoti Sengupta answered |
Stability of alkyl carbocations can be explained by both inductive effect and hyperconjugation.
Chapter doubts & questions for Organic Chemistry : Some Basic Principles & Techniques - NCERTs at Fingertips: Textbooks, Tests & Solutions 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Organic Chemistry : Some Basic Principles & Techniques - NCERTs at Fingertips: Textbooks, Tests & Solutions in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup


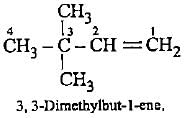
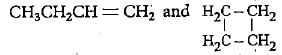 both show ring-chain isomerism.
both show ring-chain isomerism.