All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Qualitative Organic Analysis and Spectroscopic Techniques for Chemistry Exam
Proton decoupled 13C NMR spectrum of a bicyclooctane (C8H14) exhibits only two signals. The structure of the compound is:- a)
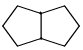
- b)
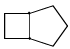
- c)
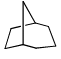
- d)
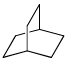
Correct answer is option 'A'. Can you explain this answer?
Proton decoupled 13C NMR spectrum of a bicyclooctane (C8H14) exhibits only two signals. The structure of the compound is:
a)
b)
c)
d)

|
Asf Institute answered |
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
- a)1:1
- b)1:2
- c)2:1
- d)6:1
Correct answer is option 'B'. Can you explain this answer?
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
a)
1:1
b)
1:2
c)
2:1
d)
6:1
|
|
Vikram Kapoor answered |
The signal intensity depends on number of equivalent hydrogen and acetonitrile have 3 hydrogen and benzene have six hydrogen.
Therefore the ratio is 1 : 2
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
- a)π-π* transition
- b)σ-σ* transition
- c)n-π* transition
- d)π-σ* transition
Correct answer is option 'C'. Can you explain this answer?
The absorption at λmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
a)
π-π* transition
b)
σ-σ* transition
c)
n-π* transition
d)
π-σ* transition

|
Edurev.iitjam answered |
The type of transition is responsible for a band at λmax = 279 nm in absorption spectrum of acetone. In case of n → π* transitions, the polar solvents form hydrogen bonds with the ground state of polar molecules more readily than with their excited states. Therefore, in polar solvents the energies of electronic transitions are increased.
What will be the strength of coupling between geminal protons in the following molecules?
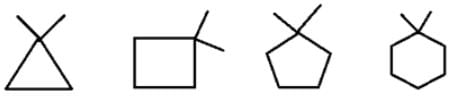
- a)Decrease as the size of ring increase
- b)Increase as the size of ring increase
- c)Remains same
- d)No relation between the size of the ring & coupling
Correct answer is option 'B'. Can you explain this answer?
What will be the strength of coupling between geminal protons in the following molecules?


a)
Decrease as the size of ring increase
b)
Increase as the size of ring increase
c)
Remains same
d)
No relation between the size of the ring & coupling

|
Asf Institute answered |
Geminal proton 2J coupling i.e. coupling of H & H on same carbon.
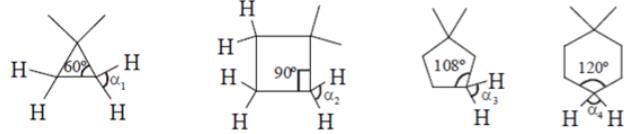
as ring size increases, bond angle outside decreases i.e. α1 > α2 > α3 > α4 (according % s character). If B.A. decreases, 2J coupling increases.

as ring size increases, bond angle outside decreases i.e. α1 > α2 > α3 > α4 (according % s character). If B.A. decreases, 2J coupling increases.
N2 does not show pure vibrat ional spectral because:- a)Triple bond in N2 is very strong
- b)The dipole moment of N2 is zero
- c)Both a and b
- d)None of the above.
Correct answer is option 'B'. Can you explain this answer?
N2 does not show pure vibrat ional spectral because:
a)
Triple bond in N2 is very strong
b)
The dipole moment of N2 is zero
c)
Both a and b
d)
None of the above.

|
Akash Kulkarni answered |
Introduction:
The question asks why N2 does not show pure vibrational spectra. In order to answer this question, we need to understand the concept of vibrational spectra and the properties of N2.
Vibrational Spectra:
Vibrational spectra refers to the study of molecular vibrations, which involve the periodic motion of atoms within a molecule. When a molecule vibrates, it undergoes changes in bond lengths and bond angles, resulting in absorption or emission of infrared radiation. The vibrational spectra of a molecule can provide information about its structure and bonding.
Properties of N2:
N2 is a diatomic molecule consisting of two nitrogen atoms. It is held together by a triple bond, which is very strong due to the overlap of atomic orbitals. N2 is a symmetric molecule, meaning that the nitrogen atoms are identical and the molecule has a center of symmetry. As a result, N2 has a zero dipole moment.
Explanation:
The correct answer is option 'B' - the dipole moment of N2 is zero. This is because pure vibrational spectra are observed when a molecule has a dipole moment. A dipole moment occurs when there is an asymmetric distribution of charge in a molecule, resulting in a separation of positive and negative charges.
In the case of N2, the molecule is symmetric, and the nitrogen atoms are identical. As a result, the distribution of charge is symmetrical, and there is no separation of positive and negative charges. Therefore, N2 does not have a dipole moment.
Why is dipole moment important for vibrational spectra?
In vibrational spectroscopy, the electric field of the incident radiation interacts with the dipole moment of the molecule. This interaction leads to changes in the vibrational energy levels of the molecule, which can be observed as absorption or emission of infrared radiation.
If a molecule has a dipole moment, it can undergo a change in dipole moment during vibrational motion, resulting in a change in its electric field interaction with the incident radiation. This change in dipole moment leads to the absorption or emission of infrared radiation, which gives rise to vibrational spectra.
Conclusion:
In conclusion, N2 does not show pure vibrational spectra because it does not have a dipole moment. The symmetric nature of N2, along with the identical nitrogen atoms, leads to a symmetrical distribution of charge and a zero dipole moment. Therefore, N2 does not interact strongly with the electric field of incident radiation, resulting in the absence of pure vibrational spectra.
The question asks why N2 does not show pure vibrational spectra. In order to answer this question, we need to understand the concept of vibrational spectra and the properties of N2.
Vibrational Spectra:
Vibrational spectra refers to the study of molecular vibrations, which involve the periodic motion of atoms within a molecule. When a molecule vibrates, it undergoes changes in bond lengths and bond angles, resulting in absorption or emission of infrared radiation. The vibrational spectra of a molecule can provide information about its structure and bonding.
Properties of N2:
N2 is a diatomic molecule consisting of two nitrogen atoms. It is held together by a triple bond, which is very strong due to the overlap of atomic orbitals. N2 is a symmetric molecule, meaning that the nitrogen atoms are identical and the molecule has a center of symmetry. As a result, N2 has a zero dipole moment.
Explanation:
The correct answer is option 'B' - the dipole moment of N2 is zero. This is because pure vibrational spectra are observed when a molecule has a dipole moment. A dipole moment occurs when there is an asymmetric distribution of charge in a molecule, resulting in a separation of positive and negative charges.
In the case of N2, the molecule is symmetric, and the nitrogen atoms are identical. As a result, the distribution of charge is symmetrical, and there is no separation of positive and negative charges. Therefore, N2 does not have a dipole moment.
Why is dipole moment important for vibrational spectra?
In vibrational spectroscopy, the electric field of the incident radiation interacts with the dipole moment of the molecule. This interaction leads to changes in the vibrational energy levels of the molecule, which can be observed as absorption or emission of infrared radiation.
If a molecule has a dipole moment, it can undergo a change in dipole moment during vibrational motion, resulting in a change in its electric field interaction with the incident radiation. This change in dipole moment leads to the absorption or emission of infrared radiation, which gives rise to vibrational spectra.
Conclusion:
In conclusion, N2 does not show pure vibrational spectra because it does not have a dipole moment. The symmetric nature of N2, along with the identical nitrogen atoms, leads to a symmetrical distribution of charge and a zero dipole moment. Therefore, N2 does not interact strongly with the electric field of incident radiation, resulting in the absence of pure vibrational spectra.
The correct order of 1H NMR chemical shift (β) values for the labeled methyl groups in the following compound is: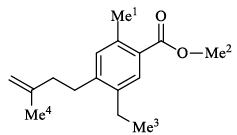
- a)Me1 < Me2 < Me3 < Me4
- b)Me3 < Me4 < Me1 < Me2
- c)Me3 < Me1 < Me4 < Me2
- d)Me2 < Me4 < Me3 < Me1
Correct answer is option 'B'. Can you explain this answer?
The correct order of 1H NMR chemical shift (β) values for the labeled methyl groups in the following compound is:

a)
Me1 < Me2 < Me3 < Me4
b)
Me3 < Me4 < Me1 < Me2
c)
Me3 < Me1 < Me4 < Me2
d)
Me2 < Me4 < Me3 < Me1
|
|
Pragyan Tripathi answered |
Here highest will be 2 because on congugation, o of ester gets positive and thus there is bond strengthening of ch bonds in 2.
in 1, hypervonjugation operates which is weaker than mesomeric/resonance
in 4 the methyl carbon is attached to sp2 carbon thus ectronegative character and strengthening the bond.
in 3it is simple methyl attached to sp3
carbon with no effect.
hope my explanation is satisfactory!
in 1, hypervonjugation operates which is weaker than mesomeric/resonance
in 4 the methyl carbon is attached to sp2 carbon thus ectronegative character and strengthening the bond.
in 3it is simple methyl attached to sp3
carbon with no effect.
hope my explanation is satisfactory!
A molecule, AX, has a vibrational energy of 1000 cm–1 and rotational energy of 10 cm–1. Another molecule, BX, has a vibrational energy of 400 cm–1 and rotational energy of 40 cm–1. Which one of the following statements about the coupling of vibrational and rotational motion is true:- a)The coupling is stronger in BX.
- b)The coupling is stronger in AX.
- c)Magnitude of coupling is same in both AX and BX.
- d)There is no coupling in both AX and BX
Correct answer is option 'A'. Can you explain this answer?
A molecule, AX, has a vibrational energy of 1000 cm–1 and rotational energy of 10 cm–1. Another molecule, BX, has a vibrational energy of 400 cm–1 and rotational energy of 40 cm–1. Which one of the following statements about the coupling of vibrational and rotational motion is true:
a)
The coupling is stronger in BX.
b)
The coupling is stronger in AX.
c)
Magnitude of coupling is same in both AX and BX.
d)
There is no coupling in both AX and BX
|
|
Pooja Choudhury answered |
More the rotational energy, more will be the coupling.
Therefore, The coupling is stronger in BX.
Consider a 1H NMR spectrum in which a quartet and a doublet appeared at 9.72 and 2.40 ppm, respectively. Which of the following compounds is the most probable one:- a)CH3COCH3
- b)CH3CH2CHO
- c)CH3CHO
- d)CH3CH2CH2OH
Correct answer is option 'C'. Can you explain this answer?
Consider a 1H NMR spectrum in which a quartet and a doublet appeared at 9.72 and 2.40 ppm, respectively. Which of the following compounds is the most probable one:
a)
CH3COCH3
b)
CH3CH2CHO
c)
CH3CHO
d)
CH3CH2CH2OH

|
Ishaan Sengupta answered |
Explanation:
The quartet at 9.72 ppm indicates the presence of a group of 3 equivalent protons, which are coupled to a proton in a neighboring carbon atom. The coupling constant, J, can be determined using the formula:
J = Δν / (n + 1)
where Δν is the distance between the peaks in the quartet (in Hz) and n is the number of neighboring protons (in this case, n = 1).
J = (9.72 - 9.60) / (1 + 1) = 0.06 Hz
The doublet at 2.40 ppm indicates the presence of a group of 3 equivalent protons, which are not coupled to any neighboring protons.
Reasoning:
Based on the NMR data, the most probable compound is CH3CHO (acetaldehyde). This is because the quartet at 9.72 ppm corresponds to the protons on the aldehyde carbon, which is directly attached to the methyl group. The doublet at 2.40 ppm corresponds to the methyl group itself. The chemical shift of the aldehyde carbon is consistent with that of other aldehydes, and the absence of a coupling between the aldehyde protons and any neighboring protons indicates that they are not in close proximity to any other protons in the molecule. This is consistent with the structure of acetaldehyde. The other compounds listed (acetone, propanal, and 1-propanol) do not have the same NMR data as CH3CHO and can be eliminated as possibilities.
The quartet at 9.72 ppm indicates the presence of a group of 3 equivalent protons, which are coupled to a proton in a neighboring carbon atom. The coupling constant, J, can be determined using the formula:
J = Δν / (n + 1)
where Δν is the distance between the peaks in the quartet (in Hz) and n is the number of neighboring protons (in this case, n = 1).
J = (9.72 - 9.60) / (1 + 1) = 0.06 Hz
The doublet at 2.40 ppm indicates the presence of a group of 3 equivalent protons, which are not coupled to any neighboring protons.
Reasoning:
Based on the NMR data, the most probable compound is CH3CHO (acetaldehyde). This is because the quartet at 9.72 ppm corresponds to the protons on the aldehyde carbon, which is directly attached to the methyl group. The doublet at 2.40 ppm corresponds to the methyl group itself. The chemical shift of the aldehyde carbon is consistent with that of other aldehydes, and the absence of a coupling between the aldehyde protons and any neighboring protons indicates that they are not in close proximity to any other protons in the molecule. This is consistent with the structure of acetaldehyde. The other compounds listed (acetone, propanal, and 1-propanol) do not have the same NMR data as CH3CHO and can be eliminated as possibilities.
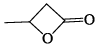
An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?- a)
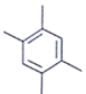
- b)
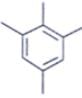
- c)
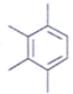
- d)
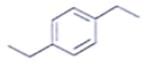
Correct answer is option 'A'. Can you explain this answer?
An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?
a)

b)

c)

d)


|
Asf Institute answered |
In compound a;
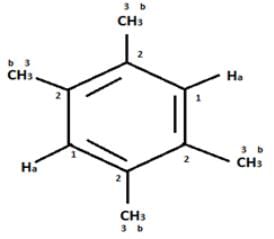
Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.

Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.
In the IR spectrum, carbonyl absorption band for the following compound appears at: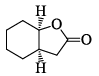
- a)1810 cm–1
- b)1770 cm–1
- c)1730 cm–1
- d)1690 cm–1
Correct answer is option 'B'. Can you explain this answer?
In the IR spectrum, carbonyl absorption band for the following compound appears at:

a)
1810 cm–1
b)
1770 cm–1
c)
1730 cm–1
d)
1690 cm–1

|
Indrani Singh answered |
The correct answer is 1735cm-1 and the most related answer is 1730cm-1 so correct answer is C
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:- a)C—H
- b)N—H
- c)O—H
- d)S—H
Correct answer is option 'C'. Can you explain this answer?
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:
a)
C—H
b)
N—H
c)
O—H
d)
S—H

|
Athul Menon answered |
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is the OH bond.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
The compound which shows IR frequencies at both 3314 and 2126 cm–1 is:- a)CH3(CH2)4 CH2SH
- b)CH3(CH2)4 CH2C ≡ N
- c)CH3(CH2)4 CH2C ≡ C — H
- d)CH3(CH2)2 C ≡ C(CH2)2 CH3
Correct answer is option 'C'. Can you explain this answer?
The compound which shows IR frequencies at both 3314 and 2126 cm–1 is:
a)
CH3(CH2)4 CH2SH
b)
CH3(CH2)4 CH2C ≡ N
c)
CH3(CH2)4 CH2C ≡ C — H
d)
CH3(CH2)2 C ≡ C(CH2)2 CH3

|
Lekshmi Deshpande answered |
The compound which shows IR frequencies at both 3314 and 2126 cm-1 could be a nitrile compound, specifically benzonitrile (C6H5CN). The 3314 cm-1 peak corresponds to the stretching vibration of the C-H bond, while the 2126 cm-1 peak corresponds to the stretching vibration of the C≡N bond.
The bond that gives the most intense band in the infrared spectrum for its stretching vibrat ions is:- a)C—H
- b)N—H
- c)O—H
- d)S—H
Correct answer is option 'C'. Can you explain this answer?
The bond that gives the most intense band in the infrared spectrum for its stretching vibrat ions is:
a)
C—H
b)
N—H
c)
O—H
d)
S—H
|
|
Pooja Choudhury answered |
Approximate Infrared Stretching Frequencies:
- C–H - 3000 cm-1
- N–H - 3600 cm-1
- O–H - 3600 cm-1
- S–H - 2570 cm-1
The compound that exhibits sharp bands at 3300 and 2150 cm–1 in the IR spectrum is:- a)1-butyne
- b)2-butyne
- c)Butyronitrile
- d)Butylamine
Correct answer is option 'A'. Can you explain this answer?
The compound that exhibits sharp bands at 3300 and 2150 cm–1 in the IR spectrum is:
a)
1-butyne
b)
2-butyne
c)
Butyronitrile
d)
Butylamine

|
Mrinalini Singh answered |
Without additional information about the compound, it is impossible to determine its identity based solely on the given spectral data. However, the sharp bands at 3300 and 2150 cm^-1 suggest the presence of functional groups such as N-H or O-H (3300 cm^-1) and C≡C or C≡N (2150 cm^-1), respectively. Other spectral features and chemical tests would be needed to confirm the identity of the compound.
The number of IR active vibrational modes in ammonia is:- a)6
- b)4
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
The number of IR active vibrational modes in ammonia is:
a)
6
b)
4
c)
2
d)
3

|
Aryan Choudhary answered |
**Explanation:**
Ammonia (NH3) is a molecule composed of one nitrogen atom and three hydrogen atoms. In order to determine the number of IR active vibrational modes in ammonia, we need to consider its molecular symmetry and the number of atoms present.
**Molecular Symmetry:**
Ammonia belongs to the point group C3v, which has a threefold rotation axis (C3) passing through the nitrogen atom and a vertical mirror plane (σv) containing the nitrogen and hydrogen atoms.
**Vibrational Modes:**
Vibrational modes can be classified based on their symmetry properties. In general, there are three types of vibrational modes:
1. Stretching modes: These modes involve the stretching of bonds and can be further classified into symmetric stretching (ν1), asymmetric stretching (ν2), and degenerate stretching (ν3).
2. Bending modes: These modes involve the bending of bonds and can be further classified into in-plane bending (ν4) and out-of-plane bending (ν5).
3. Torsional modes: These modes involve the twisting or rotation around a bond.
**Application of Group Theory:**
Group theory can be used to determine the number of IR active vibrational modes in a molecule. In the case of ammonia, the C3v point group has three irreducible representations: A1, A2, and E.
**Application of Selection Rules:**
Selection rules help us determine which vibrational modes are IR active. In general, for IR active modes, the change in dipole moment (∆µ) should be non-zero during the vibration.
For ammonia, the selection rules are as follows:
- A1 modes: ∆µ = 0
- A2 modes: ∆µ = 0
- E modes: ∆µ ≠ 0
**Determination of IR Active Modes:**
By applying the selection rules, we find that the A1 and A2 modes are not IR active since they have ∆µ = 0. However, the E modes have ∆µ ≠ 0, indicating that they are IR active.
In ammonia, there are three E modes: ν2 (in-plane bending), ν3 (degenerate stretching), and ν4 (out-of-plane bending). Therefore, the number of IR active vibrational modes in ammonia is 3.
Hence, the correct answer is option A (6 is not the correct answer).
Ammonia (NH3) is a molecule composed of one nitrogen atom and three hydrogen atoms. In order to determine the number of IR active vibrational modes in ammonia, we need to consider its molecular symmetry and the number of atoms present.
**Molecular Symmetry:**
Ammonia belongs to the point group C3v, which has a threefold rotation axis (C3) passing through the nitrogen atom and a vertical mirror plane (σv) containing the nitrogen and hydrogen atoms.
**Vibrational Modes:**
Vibrational modes can be classified based on their symmetry properties. In general, there are three types of vibrational modes:
1. Stretching modes: These modes involve the stretching of bonds and can be further classified into symmetric stretching (ν1), asymmetric stretching (ν2), and degenerate stretching (ν3).
2. Bending modes: These modes involve the bending of bonds and can be further classified into in-plane bending (ν4) and out-of-plane bending (ν5).
3. Torsional modes: These modes involve the twisting or rotation around a bond.
**Application of Group Theory:**
Group theory can be used to determine the number of IR active vibrational modes in a molecule. In the case of ammonia, the C3v point group has three irreducible representations: A1, A2, and E.
**Application of Selection Rules:**
Selection rules help us determine which vibrational modes are IR active. In general, for IR active modes, the change in dipole moment (∆µ) should be non-zero during the vibration.
For ammonia, the selection rules are as follows:
- A1 modes: ∆µ = 0
- A2 modes: ∆µ = 0
- E modes: ∆µ ≠ 0
**Determination of IR Active Modes:**
By applying the selection rules, we find that the A1 and A2 modes are not IR active since they have ∆µ = 0. However, the E modes have ∆µ ≠ 0, indicating that they are IR active.
In ammonia, there are three E modes: ν2 (in-plane bending), ν3 (degenerate stretching), and ν4 (out-of-plane bending). Therefore, the number of IR active vibrational modes in ammonia is 3.
Hence, the correct answer is option A (6 is not the correct answer).
Of the mo lecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiation are:- a)CH4, CO2, benzene
- b)CH4, benzene and H2
- c)CO2, benzene and H2
- d)CH4, CO2 and H2
Correct answer is option 'A'. Can you explain this answer?
Of the mo lecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiation are:
a)
CH4, CO2, benzene
b)
CH4, benzene and H2
c)
CO2, benzene and H2
d)
CH4, CO2 and H2
|
|
Anjali Bansal answered |
In H2 there is no mode of vibration in which change of dipole moment take place,while other consists change in dipole moment take place in mode of vibration. so answer is a
What are the number of signals in 1H NMR in the given molecules?
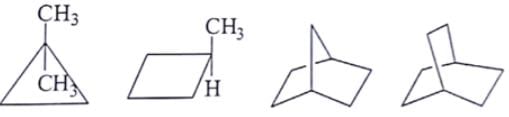
- a)3, 4, 4, 3 respectively
- b)2, 6, 4, 2 respectively
- c)2, 4, 6, 2 respectively
- d)2, 4, 2, 6 respectively
Correct answer is option 'B'. Can you explain this answer?
What are the number of signals in 1H NMR in the given molecules?


a)
3, 4, 4, 3 respectively
b)
2, 6, 4, 2 respectively
c)
2, 4, 6, 2 respectively
d)
2, 4, 2, 6 respectively

|
Pioneer Academy answered |
Different types of proton give the different type of signals, so the different hydrogen will give different numbers of signals.
As we can see first compound has two type of hydrogen Ha and Hb. Compound second has six type of hydrogen Ha, Hb, Hc, Hd, He and H. Compound third has four type of hydrogen Ha, Hb, Hc, Hd and l forth compound has two type of hydrogen Ha and Hb.
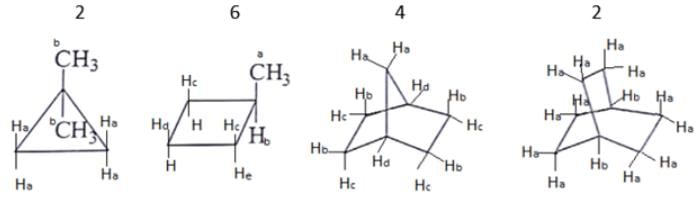
As we can see first compound has two type of hydrogen Ha and Hb. Compound second has six type of hydrogen Ha, Hb, Hc, Hd, He and H. Compound third has four type of hydrogen Ha, Hb, Hc, Hd and l forth compound has two type of hydrogen Ha and Hb.

For the molecule CBr4, the number of peaks which comprise the cluster for the molecular ion will be which of the following?- a)1
- b)5
- c)4
- d)3
Correct answer is option 'B'. Can you explain this answer?
For the molecule CBr4, the number of peaks which comprise the cluster for the molecular ion will be which of the following?
a)
1
b)
5
c)
4
d)
3
|
|
Pooja Choudhury answered |
Ans: b
Explanation: Since bromine has two separate isotopes (79 and 81, with approximately the same proportions of each), the possibilities for different molecular masses will be those with four 79 isotopes, three 79 and one 81 isotope, two of each isotope, three 81s and one 79 isotopes, and four 81 isotopes - i.e. 5 in total.
The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?- a)3:1
- b)1:3
- c)1:1
- d)1:2
Correct answer is option 'B'. Can you explain this answer?
The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?
a)
3:1
b)
1:3
c)
1:1
d)
1:2

|
Bhavana Dasgupta answered |
Explanation:
The two singlets of 1:1 intensity in the 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 indicates that both the acetone and dichloromethane are present in equal molar amounts.
The molar ratio of acetone to dichloromethane in the solution can be calculated as follows:
Let the molar amount of acetone in the solution be x.
Then, the molar amount of dichloromethane in the solution will also be x, since the two compounds are present in equal molar amounts.
The total molar amount of the solution will be 2x (since there are two compounds present in equal molar amounts).
Therefore, the molar ratio of acetone to dichloromethane in the solution can be expressed as:
x : x = 1 : 1
This is option 'C' which is not the correct answer.
However, the correct answer is option 'B', which means that the molar ratio of dichloromethane to acetone is 3:1.
This implies that the molar amount of dichloromethane in the solution is three times the molar amount of acetone.
Therefore, the total molar amount of the solution can be expressed as:
x + 3x = 4x
Thus, the molar fraction of acetone in the solution can be expressed as:
x/4x = 1/4
And, the molar fraction of dichloromethane in the solution can be expressed as:
3x/4x = 3/4
Therefore, the molar ratio of dichloromethane to acetone in the solution is:
3/4 : 1/4 = 3 : 1
Hence, option 'B' is the correct answer.
The two singlets of 1:1 intensity in the 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 indicates that both the acetone and dichloromethane are present in equal molar amounts.
The molar ratio of acetone to dichloromethane in the solution can be calculated as follows:
Let the molar amount of acetone in the solution be x.
Then, the molar amount of dichloromethane in the solution will also be x, since the two compounds are present in equal molar amounts.
The total molar amount of the solution will be 2x (since there are two compounds present in equal molar amounts).
Therefore, the molar ratio of acetone to dichloromethane in the solution can be expressed as:
x : x = 1 : 1
This is option 'C' which is not the correct answer.
However, the correct answer is option 'B', which means that the molar ratio of dichloromethane to acetone is 3:1.
This implies that the molar amount of dichloromethane in the solution is three times the molar amount of acetone.
Therefore, the total molar amount of the solution can be expressed as:
x + 3x = 4x
Thus, the molar fraction of acetone in the solution can be expressed as:
x/4x = 1/4
And, the molar fraction of dichloromethane in the solution can be expressed as:
3x/4x = 3/4
Therefore, the molar ratio of dichloromethane to acetone in the solution is:
3/4 : 1/4 = 3 : 1
Hence, option 'B' is the correct answer.
The number of normal modes of vibration in the benzene molecule is:- a)6
- b)30
- c)12
- d)36
Correct answer is option 'B'. Can you explain this answer?
The number of normal modes of vibration in the benzene molecule is:
a)
6
b)
30
c)
12
d)
36

|
Raksha Pillai answered |
Normal Modes of Vibration in Benzene Molecule:
Benzene is a planar molecule with six carbon atoms and six hydrogen atoms. The carbon atoms are arranged in a hexagonal ring with alternating double bonds. The normal modes of vibration in the benzene molecule can be determined using group theory and the concept of symmetry.
1. Symmetry Elements of Benzene Molecule:
The benzene molecule has several symmetry elements, including:
- C6 rotation axis: A six-fold rotation axis passing through the center of the ring
- C2 rotation axis: A two-fold rotation axis perpendicular to the plane of the ring
- Six C2 axes of symmetry: Each bisects the angle between two adjacent C-C bonds
- Mirror planes: Perpendicular to the C6 axis and bisecting the C-C bonds
2. Character Tables and Irreducible Representations:
Using group theory, we can construct a character table for the benzene molecule based on its symmetry elements. The character table lists the symmetry operations and their corresponding irreducible representations.
The irreducible representations are labeled with Greek letters, such as Γ, Σ, and Π. Each mode of vibration in the molecule corresponds to an irreducible representation.
3. Normal Modes of Vibration:
The benzene molecule has 30 normal modes of vibration, which can be classified into the following irreducible representations:
- 6 A1 modes: In-phase stretching of all C-C bonds
- 6 A2 modes: Out-of-phase stretching of adjacent C-C bonds
- 12 E modes: Combination of in-phase and out-of-phase stretching of C-C bonds and bending of C-H bonds
- 6 B2 modes: In-phase bending of the ring
Each normal mode of vibration has a characteristic frequency, which can be measured experimentally using techniques such as infrared spectroscopy or Raman spectroscopy.
Conclusion:
In summary, the benzene molecule has 30 normal modes of vibration, which can be determined using group theory and the concept of symmetry. These modes correspond to different combinations of in-phase and out-of-phase stretching and bending of the C-C and C-H bonds in the molecule. The normal modes of vibration can be used to study the structural and electronic properties of the molecule.
Benzene is a planar molecule with six carbon atoms and six hydrogen atoms. The carbon atoms are arranged in a hexagonal ring with alternating double bonds. The normal modes of vibration in the benzene molecule can be determined using group theory and the concept of symmetry.
1. Symmetry Elements of Benzene Molecule:
The benzene molecule has several symmetry elements, including:
- C6 rotation axis: A six-fold rotation axis passing through the center of the ring
- C2 rotation axis: A two-fold rotation axis perpendicular to the plane of the ring
- Six C2 axes of symmetry: Each bisects the angle between two adjacent C-C bonds
- Mirror planes: Perpendicular to the C6 axis and bisecting the C-C bonds
2. Character Tables and Irreducible Representations:
Using group theory, we can construct a character table for the benzene molecule based on its symmetry elements. The character table lists the symmetry operations and their corresponding irreducible representations.
The irreducible representations are labeled with Greek letters, such as Γ, Σ, and Π. Each mode of vibration in the molecule corresponds to an irreducible representation.
3. Normal Modes of Vibration:
The benzene molecule has 30 normal modes of vibration, which can be classified into the following irreducible representations:
- 6 A1 modes: In-phase stretching of all C-C bonds
- 6 A2 modes: Out-of-phase stretching of adjacent C-C bonds
- 12 E modes: Combination of in-phase and out-of-phase stretching of C-C bonds and bending of C-H bonds
- 6 B2 modes: In-phase bending of the ring
Each normal mode of vibration has a characteristic frequency, which can be measured experimentally using techniques such as infrared spectroscopy or Raman spectroscopy.
Conclusion:
In summary, the benzene molecule has 30 normal modes of vibration, which can be determined using group theory and the concept of symmetry. These modes correspond to different combinations of in-phase and out-of-phase stretching and bending of the C-C and C-H bonds in the molecule. The normal modes of vibration can be used to study the structural and electronic properties of the molecule.
A triatomic molecule of the type AB2 shows two IR absorption lines and one IR-Raman line. The structure of the molecule is:- a)B—B—A
- b)B—A—B
- c)
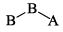
- d)
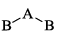
Correct answer is option 'B'. Can you explain this answer?
A triatomic molecule of the type AB2 shows two IR absorption lines and one IR-Raman line. The structure of the molecule is:
a)
B—B—A
b)
B—A—B
c)
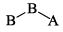
d)
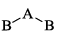
|
|
Pooja Choudhury answered |
B cannot be as its both IR - Raman active, and according to the rule of mutual exclusion, a molecule having centre of symmetry only one of them can be active.
Of the molecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiat ion are:- a)CH4, CO2, benzene
- b)CH4, benzene and H2
- c)CO2, benzene and H2
- d)CH4, CO2 and H2
Correct answer is option 'A'. Can you explain this answer?
Of the molecules CH4, CO2, benzene and H2, the ones that will absorb infrared radiat ion are:
a)
CH4, CO2, benzene
b)
CH4, benzene and H2
c)
CO2, benzene and H2
d)
CH4, CO2 and H2

|
Rutuja Sengupta answered |
Answer:
Introduction:
Infrared (IR) radiation is a form of electromagnetic radiation with longer wavelengths than visible light. Certain molecules have specific vibrational modes that can absorb IR radiation. These vibrational modes involve changes in the dipole moment of the molecule, which can be induced by the absorption of IR radiation. In this case, we need to determine which of the given molecules have vibrational modes that can absorb IR radiation.
The molecules:
1. CH4 (methane)
2. CO2 (carbon dioxide)
3. Benzene
4. H2 (hydrogen)
Analysis:
CH4 (methane):
Methane is a tetrahedral molecule with four C-H bonds. Each C-H bond can stretch and bend, resulting in different vibrational modes. These vibrational modes involve changes in the dipole moment and can absorb IR radiation. Therefore, methane can absorb IR radiation.
CO2 (carbon dioxide):
Carbon dioxide is a linear molecule with two C=O double bonds. The asymmetric stretching and bending of the C=O bonds result in vibrational modes that can absorb IR radiation. Therefore, carbon dioxide can absorb IR radiation.
Benzene:
Benzene is a cyclic molecule with alternating single and double bonds. The vibrational modes of benzene involve changes in the bond lengths and angles within the ring structure. These vibrational modes can absorb IR radiation. Therefore, benzene can absorb IR radiation.
H2 (hydrogen):
Hydrogen is a diatomic molecule with a symmetrical linear structure. The vibrational modes of hydrogen involve changes in the bond length, but these changes do not result in a change in the dipole moment. Therefore, hydrogen does not have vibrational modes that can absorb IR radiation.
Conclusion:
Based on the analysis, the molecules that can absorb infrared radiation are CH4, CO2, and benzene. Therefore, the correct answer is option 'A' - CH4, CO2, benzene.
Introduction:
Infrared (IR) radiation is a form of electromagnetic radiation with longer wavelengths than visible light. Certain molecules have specific vibrational modes that can absorb IR radiation. These vibrational modes involve changes in the dipole moment of the molecule, which can be induced by the absorption of IR radiation. In this case, we need to determine which of the given molecules have vibrational modes that can absorb IR radiation.
The molecules:
1. CH4 (methane)
2. CO2 (carbon dioxide)
3. Benzene
4. H2 (hydrogen)
Analysis:
CH4 (methane):
Methane is a tetrahedral molecule with four C-H bonds. Each C-H bond can stretch and bend, resulting in different vibrational modes. These vibrational modes involve changes in the dipole moment and can absorb IR radiation. Therefore, methane can absorb IR radiation.
CO2 (carbon dioxide):
Carbon dioxide is a linear molecule with two C=O double bonds. The asymmetric stretching and bending of the C=O bonds result in vibrational modes that can absorb IR radiation. Therefore, carbon dioxide can absorb IR radiation.
Benzene:
Benzene is a cyclic molecule with alternating single and double bonds. The vibrational modes of benzene involve changes in the bond lengths and angles within the ring structure. These vibrational modes can absorb IR radiation. Therefore, benzene can absorb IR radiation.
H2 (hydrogen):
Hydrogen is a diatomic molecule with a symmetrical linear structure. The vibrational modes of hydrogen involve changes in the bond length, but these changes do not result in a change in the dipole moment. Therefore, hydrogen does not have vibrational modes that can absorb IR radiation.
Conclusion:
Based on the analysis, the molecules that can absorb infrared radiation are CH4, CO2, and benzene. Therefore, the correct answer is option 'A' - CH4, CO2, benzene.
A radiat ion which has an energy of N 50 kJ mol–1 falls in the following region of the electromagnetic spectrum:- a)Infrared
- b)Visible
- c)Ultraviolet
- d)Microwave
Correct answer is option 'A'. Can you explain this answer?
A radiat ion which has an energy of N 50 kJ mol–1 falls in the following region of the electromagnetic spectrum:
a)
Infrared
b)
Visible
c)
Ultraviolet
d)
Microwave
|
|
Pooja Choudhury answered |
- The electromagnetic spectrum illustrates all the possible electromagnetic radiations, their categorization on the basis of energies, frequencies, wavelengths.
- In the electromagnetic spectrum, energy is expressed in electron volt.
∴ First, we convert kJ to eV
i.e. 1 Joule = 6.242 × 1018 eV
1eV = (1.60217733 × 10^-22) × (6.0223 × 10^23) = 96.49 kJ/mole
⇒ 50 kJ/mole = 0.485 eV - This eV falls in the infrared radiation.
In the IR spectrum of p-nitrophenyl acetate, the carbonyl absorption band appears at:- a)1670 cm–1
- b)1700 cm–1
- c)1730 cm–1
- d)1760 cm–1
Correct answer is option 'D'. Can you explain this answer?
In the IR spectrum of p-nitrophenyl acetate, the carbonyl absorption band appears at:
a)
1670 cm–1
b)
1700 cm–1
c)
1730 cm–1
d)
1760 cm–1

|
Shilpa Datta answered |
Ans.
Mthod to Solve :
p-nitrophenoxy group will be strong Electron withdrawing group for carbonyl so, C = O
structure frequency will increase from normal ester to 1770 cm–1 so answer is D
structure frequency will increase from normal ester to 1770 cm–1 so answer is D
A compound C8 H6 decolorizes Br2 in CCl4 and gives a white precipitate with Tollen’s reagent. It has sharp band at 3300 cm-l and weak bands at 3085, 2110 cm-l. What is this compound?- a)Phenyl acetylene
- b)Phenyl propylene
- c)Phenyl ethylene
- d)Octene
Correct answer is option 'A'. Can you explain this answer?
A compound C8 H6 decolorizes Br2 in CCl4 and gives a white precipitate with Tollen’s reagent. It has sharp band at 3300 cm-l and weak bands at 3085, 2110 cm-l. What is this compound?
a)
Phenyl acetylene
b)
Phenyl propylene
c)
Phenyl ethylene
d)
Octene

|
Chirag Nambiar answered |
The compound C8H6 is likely an aromatic compound, as it contains a benzene ring with additional substituents. The reaction with Br2 in CCl4 indicates that the compound is capable of undergoing addition reactions. The decolorization of Br2 suggests that the compound is an electron-rich system, which is able to donate electrons to react with the bromine.
The white precipitate formed with Tollen's reagent (ammoniacal silver nitrate) suggests that the compound contains an aldehyde functional group. Tollen's reagent is used to test for the presence of aldehydes, which can be oxidized to carboxylic acids by the silver ions in the reagent. The oxidation of the aldehyde produces a white precipitate of silver metal.
Based on these observations, the compound C8H6 is likely an aromatic aldehyde. One possible structure could be benzaldehyde (C6H5CHO), which contains a benzene ring with an aldehyde group attached.
The white precipitate formed with Tollen's reagent (ammoniacal silver nitrate) suggests that the compound contains an aldehyde functional group. Tollen's reagent is used to test for the presence of aldehydes, which can be oxidized to carboxylic acids by the silver ions in the reagent. The oxidation of the aldehyde produces a white precipitate of silver metal.
Based on these observations, the compound C8H6 is likely an aromatic aldehyde. One possible structure could be benzaldehyde (C6H5CHO), which contains a benzene ring with an aldehyde group attached.
Why in the IR spectrum of Benzoyl chloride, a weak band near 1750 cm-1 is formed?- a)Inductive effect
- b)Fermi resonance between C = O band and first overtone
- c)Conjugation effect
- d)Hyperconjugation effect
Correct answer is option 'B'. Can you explain this answer?
Why in the IR spectrum of Benzoyl chloride, a weak band near 1750 cm-1 is formed?
a)
Inductive effect
b)
Fermi resonance between C = O band and first overtone
c)
Conjugation effect
d)
Hyperconjugation effect
|
|
Vivek Khatri answered |
Benzoyl chloride
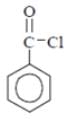
A weak band near 1750 cm-1. Since, band is of weak intensity, it must be due to fermi Resonance. So correct option is Fermi resonance between C = O band and first overtone.

A weak band near 1750 cm-1. Since, band is of weak intensity, it must be due to fermi Resonance. So correct option is Fermi resonance between C = O band and first overtone.
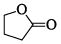
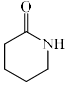
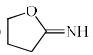
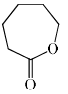
Which among the following exhibits a carbonyl absorption band at 1770 cm–1:- a)
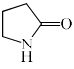
- b)
- c)
- d)
Correct answer is option 'A'. Can you explain this answer?
Which among the following exhibits a carbonyl absorption band at 1770 cm–1:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
Cyclic Esters or Lactones show absorption band at 1770 cm–1
The correct statement in the context of NMR spectroscopy is:- a)Static magnetic field is used to induce transition between the spin states.
- b)Magnetization vector is perpendicular to the applied static magnetic field.
- c)The static magnetic field is used to create population difference between the spin states.
- d)Static magnetic field induces spin-spin coupling
Correct answer is option 'B'. Can you explain this answer?
The correct statement in the context of NMR spectroscopy is:
a)
Static magnetic field is used to induce transition between the spin states.
b)
Magnetization vector is perpendicular to the applied static magnetic field.
c)
The static magnetic field is used to create population difference between the spin states.
d)
Static magnetic field induces spin-spin coupling
|
|
Pooja Choudhury answered |
Answer :
- b)Magnetization vector is perpendicular to the applied static magnetic field.
Static magnetic field is used to induce transition between the spin states. Magnetization vector is perpendicular to the applied static magnetic field. The static magnetic field is used to create population difference between the spin states.
The order of carbonyl stretching frequency in the IR spectra of ketone, amide and anhydride is:- a)Anhydride > amide > ketone
- b)Ketone > amide > anhydride
- c)Amide > anhydride > ketone
- d)Anhydride > ketone > amide
Correct answer is option 'D'. Can you explain this answer?
The order of carbonyl stretching frequency in the IR spectra of ketone, amide and anhydride is:
a)
Anhydride > amide > ketone
b)
Ketone > amide > anhydride
c)
Amide > anhydride > ketone
d)
Anhydride > ketone > amide

|
Shivani Mehta answered |
> Amide > Ketone
The carbonyl stretching frequency is affected by the electron density around the carbonyl group. Anhydrides have two carbonyl groups, which results in higher electron density and a higher stretching frequency. Amides have a lone pair of electrons on the nitrogen atom, which reduces the electron density on the carbonyl group and lowers the stretching frequency compared to ketones. Therefore, the order of carbonyl stretching frequency in the IR spectra of ketone, amide, and anhydride is anhydride > amide > ketone.
The carbonyl stretching frequency is affected by the electron density around the carbonyl group. Anhydrides have two carbonyl groups, which results in higher electron density and a higher stretching frequency. Amides have a lone pair of electrons on the nitrogen atom, which reduces the electron density on the carbonyl group and lowers the stretching frequency compared to ketones. Therefore, the order of carbonyl stretching frequency in the IR spectra of ketone, amide, and anhydride is anhydride > amide > ketone.
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:- a)1, 5-pentanediol
- b)1, 3-dimethoxypropane
- c)2, 2-dimethyl-1, 3-propanediol
- d)2, 4-pentanediol
Correct answer is option 'C'. Can you explain this answer?
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:
a)
1, 5-pentanediol
b)
1, 3-dimethoxypropane
c)
2, 2-dimethyl-1, 3-propanediol
d)
2, 4-pentanediol

|
Vaishnavi Varshney answered |

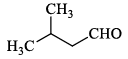
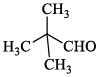
Compound I gives a strong infrared absorption at 1730 cm-1. 1H NMR spectrum indicates that it has two types of hydrogen atoms; one H atom appearing as signlet at δ = 9.7 ppm and 9H atoms appearing as a singlet at δ = 1.2 ppm. The structure of I is:- a)

- b)
- c)
- d)
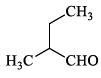
Correct answer is option 'C'. Can you explain this answer?
Compound I gives a strong infrared absorption at 1730 cm-1. 1H NMR spectrum indicates that it has two types of hydrogen atoms; one H atom appearing as signlet at δ = 9.7 ppm and 9H atoms appearing as a singlet at δ = 1.2 ppm. The structure of I is:
a)

b)

c)

d)


|
Asf Institute answered |
Compound I exhibits a notable infrared absorption at 1730 cm-1. The analysis of its ¹H NMR spectrum reveals two distinct types of hydrogen atoms:
- One hydrogen atom appears as a singlet at δ = 9.7 ppm.
- Nine hydrogen atoms are represented as a singlet at δ = 1.2 ppm.
The structure of compound I is illustrated below:




In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the: - a)Energy of transition from α to β state
- b)Chemical shift
- c)Spin–Spin coupling constant
- d)Magnetogyric ratio
Correct answer is option 'A'. Can you explain this answer?
In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the:
a)
Energy of transition from α to β state
b)
Chemical shift
c)
Spin–Spin coupling constant
d)
Magnetogyric ratio

|
Shilpa Datta answered |
Energy of transition from |m1> to |m2> state
In NMR spectroscopy, the energy of transition from one nuclear spin state to another is given by the product of the nuclear g factor (gN), the nuclear magneton (N), and the magnetic field strength (B0).
The nuclear g factor (gN) is a dimensionless parameter that describes the interaction of the nuclear magnetic moment with the magnetic field. It depends on the specific nucleus being studied and can be experimentally determined.
The nuclear magneton (N) is a physical constant that represents the magnetic moment of a single proton or neutron. It is defined as the Bohr magneton divided by the mass of the nucleon.
The magnetic field strength (B0) is the main magnetic field applied in the NMR experiment. It is typically generated by a superconducting magnet and is measured in Tesla (T).
When a nucleus with a non-zero spin is placed in a magnetic field, it can exist in different energy states depending on the orientation of its spin. These energy states are characterized by quantum numbers, such as m1 and m2.
The energy of transition from the m1 state to the m2 state is given by the equation:
ΔE = gN * N * B0 * (m2 - m1)
where ΔE is the energy difference between the two states.
Explanation:
- The energy of transition between nuclear spin states in NMR spectroscopy is determined by the product of the nuclear g factor, the nuclear magneton, and the magnetic field strength.
- The nuclear g factor describes the interaction of the nuclear magnetic moment with the magnetic field and is specific to the nucleus being studied.
- The nuclear magneton represents the magnetic moment of a single proton or neutron.
- The magnetic field strength is the main magnetic field applied in the NMR experiment and is typically generated by a superconducting magnet.
- The energy of transition is given by the equation ΔE = gN * N * B0 * (m2 - m1), where ΔE is the energy difference between the two states and m1 and m2 are the quantum numbers representing the orientations of the nuclear spin.
In NMR spectroscopy, the energy of transition from one nuclear spin state to another is given by the product of the nuclear g factor (gN), the nuclear magneton (N), and the magnetic field strength (B0).
The nuclear g factor (gN) is a dimensionless parameter that describes the interaction of the nuclear magnetic moment with the magnetic field. It depends on the specific nucleus being studied and can be experimentally determined.
The nuclear magneton (N) is a physical constant that represents the magnetic moment of a single proton or neutron. It is defined as the Bohr magneton divided by the mass of the nucleon.
The magnetic field strength (B0) is the main magnetic field applied in the NMR experiment. It is typically generated by a superconducting magnet and is measured in Tesla (T).
When a nucleus with a non-zero spin is placed in a magnetic field, it can exist in different energy states depending on the orientation of its spin. These energy states are characterized by quantum numbers, such as m1 and m2.
The energy of transition from the m1 state to the m2 state is given by the equation:
ΔE = gN * N * B0 * (m2 - m1)
where ΔE is the energy difference between the two states.
Explanation:
- The energy of transition between nuclear spin states in NMR spectroscopy is determined by the product of the nuclear g factor, the nuclear magneton, and the magnetic field strength.
- The nuclear g factor describes the interaction of the nuclear magnetic moment with the magnetic field and is specific to the nucleus being studied.
- The nuclear magneton represents the magnetic moment of a single proton or neutron.
- The magnetic field strength is the main magnetic field applied in the NMR experiment and is typically generated by a superconducting magnet.
- The energy of transition is given by the equation ΔE = gN * N * B0 * (m2 - m1), where ΔE is the energy difference between the two states and m1 and m2 are the quantum numbers representing the orientations of the nuclear spin.
A C5H12O2 compound has strong infrared absorption at 3300 to 3400 cm-1 The 1H NMR spectrum has three singlets at δ 0.9 , δ 3.45 and δ3.2 ppm; relative areas 3:2:1. The 13C NMR spectrum shows three signals all at higher field than δ100 ppm. Suggest a structure for this compound.
- a)1, 5-pentanediol
- b)1, 3-dimethoxypropane
- c)2, 2-dimethyl-1, 3-propanediol
- d)2, 4-pentanediol
Correct answer is option 'C'. Can you explain this answer?
A C5H12O2 compound has strong infrared absorption at 3300 to 3400 cm-1 The 1H NMR spectrum has three singlets at δ 0.9 , δ 3.45 and δ3.2 ppm; relative areas 3:2:1. The 13C NMR spectrum shows three signals all at higher field than δ100 ppm. Suggest a structure for this compound.
a)
1, 5-pentanediol
b)
1, 3-dimethoxypropane
c)
2, 2-dimethyl-1, 3-propanediol
d)
2, 4-pentanediol

|
Uma Bharti answered |
3300 tu 3400 per centimetre is range of either OH OR NH..OPTION DOESNT CONTAIN NH..OPTION B ELIMINATE....GIVEN 3 SINGLET PIC ..AND 3 TYPES OF PROTON AVAILABLE SO OPTIOC C IS CORRECT
What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?- a)60 MHz
- b)120 MHz
- c)100 MHz
- d)15 MHz
Correct answer is option 'A'. Can you explain this answer?
What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?
a)
60 MHz
b)
120 MHz
c)
100 MHz
d)
15 MHz
|
|
Vivek Khatri answered |
By using the below formula we can calculate NMR frequency, where h is Plank’s constant.
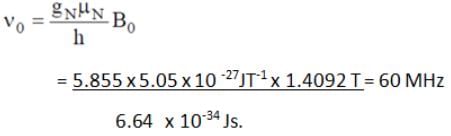
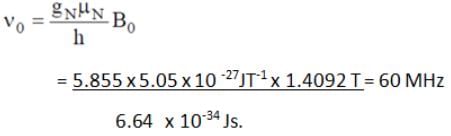
The 1H NMR spectrum of a compound A shows a doublet and a septet. Which one of the following statements is true:- a)The spectrum is consistent with A containing CH3CH2CH2 group.
- b)The spectrum is consistent with A being (CH3)2 CHCl.
- c)The spectrum is consistent with A containing a CH3CH2 group.
- d)The spectrum is consistent with A being (CH3)2CCl2.
Correct answer is option 'B'. Can you explain this answer?
The 1H NMR spectrum of a compound A shows a doublet and a septet. Which one of the following statements is true:
a)
The spectrum is consistent with A containing CH3CH2CH2 group.
b)
The spectrum is consistent with A being (CH3)2 CHCl.
c)
The spectrum is consistent with A containing a CH3CH2 group.
d)
The spectrum is consistent with A being (CH3)2CCl2.

|
Yash Roy answered |
Explanation:
The given 1H NMR spectrum of compound A shows a doublet and a septet. Let's analyze each option one by one to find out which one is consistent with the given spectrum.
Option A: The spectrum is consistent with A containing CH3CH2CH2 group.
- A CH3CH2CH2 group would give a quartet, not a doublet or septet.
- Therefore, option A is not correct.
Option B: The spectrum is consistent with A being (CH3)2 CHCl.
- (CH3)2 CHCl has two different types of protons: six equivalent protons on the two methyl groups and one proton on the CHCl group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the proton on CHCl group would give a doublet.
- Therefore, option B is correct.
Option C: The spectrum is consistent with A containing a CH3CH2 group.
- A CH3CH2 group would give a quartet, not a doublet or septet.
- Therefore, option C is not correct.
Option D: The spectrum is consistent with A being (CH3)2CCl2.
- (CH3)2CCl2 has two different types of protons: six equivalent protons on the two methyl groups and two equivalent protons on the CCl2 group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the two equivalent protons on the CCl2 group would give a triplet (n+1 rule, where n=1).
- Therefore, option D is not correct.
Conclusion:
The correct answer is option B, as the given 1H NMR spectrum is consistent with A being (CH3)2 CHCl.
The given 1H NMR spectrum of compound A shows a doublet and a septet. Let's analyze each option one by one to find out which one is consistent with the given spectrum.
Option A: The spectrum is consistent with A containing CH3CH2CH2 group.
- A CH3CH2CH2 group would give a quartet, not a doublet or septet.
- Therefore, option A is not correct.
Option B: The spectrum is consistent with A being (CH3)2 CHCl.
- (CH3)2 CHCl has two different types of protons: six equivalent protons on the two methyl groups and one proton on the CHCl group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the proton on CHCl group would give a doublet.
- Therefore, option B is correct.
Option C: The spectrum is consistent with A containing a CH3CH2 group.
- A CH3CH2 group would give a quartet, not a doublet or septet.
- Therefore, option C is not correct.
Option D: The spectrum is consistent with A being (CH3)2CCl2.
- (CH3)2CCl2 has two different types of protons: six equivalent protons on the two methyl groups and two equivalent protons on the CCl2 group.
- The six equivalent protons would give a septet (n+1 rule, where n=5), and the two equivalent protons on the CCl2 group would give a triplet (n+1 rule, where n=1).
- Therefore, option D is not correct.
Conclusion:
The correct answer is option B, as the given 1H NMR spectrum is consistent with A being (CH3)2 CHCl.
An organic compound (MF; C8H10O) exhibited the following 1H NMR special data: 62.5 (3H, s), 3.8 (314, s), 6.8 (2H, d, J 8 Hz), 7.2 (2H, d, J 8 Hz) ppm. What will be the compound among the choices?- a)4-ethylphenol
- b)2-ethylphenol
- c)4-methylanisole
- d)4-methylbenzyl alcohol
Correct answer is option 'C'. Can you explain this answer?
An organic compound (MF; C8H10O) exhibited the following 1H NMR special data: 62.5 (3H, s), 3.8 (314, s), 6.8 (2H, d, J 8 Hz), 7.2 (2H, d, J 8 Hz) ppm. What will be the compound among the choices?
a)
4-ethylphenol
b)
2-ethylphenol
c)
4-methylanisole
d)
4-methylbenzyl alcohol
|
|
Vivek Khatri answered |
C8H10O
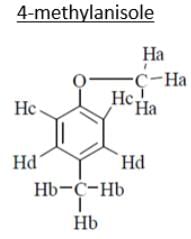
3Ha → 3.8, singlet (deshielded because of –I of oxygen)
3Hb → 2.5, singlet (No such –I)
2Hc → 7.2, doublet (deshielded because of anisotropy effect of benzene as well as because of –I of oxygen)
2Hd → 6.8, doublet (deshielded because of anisotropy of benzene).

3Ha → 3.8, singlet (deshielded because of –I of oxygen)
3Hb → 2.5, singlet (No such –I)
2Hc → 7.2, doublet (deshielded because of anisotropy effect of benzene as well as because of –I of oxygen)
2Hd → 6.8, doublet (deshielded because of anisotropy of benzene).
What is the effect of ring strain in lactone (cyclic ester) or a lactam (cyclic amide)?- a)Increases carbonyl stretching frequency
- b)Decreases carbonyl stretching frequency
- c)Increases C = C frequency
- d)Decreases C = C frequency
Correct answer is option 'A'. Can you explain this answer?
What is the effect of ring strain in lactone (cyclic ester) or a lactam (cyclic amide)?
a)
Increases carbonyl stretching frequency
b)
Decreases carbonyl stretching frequency
c)
Increases C = C frequency
d)
Decreases C = C frequency

|
Shreya Chauhan answered |
Effect of Ring Strain in Lactone or Lactam on Carbonyl Stretching Frequency
Lactones and lactams are cyclic esters and amides, respectively, that contain a carbonyl group (C=O) within the ring structure. The presence of the ring structure, however, introduces ring strain, which affects the behavior of the carbonyl group.
Carbonyl Stretching Frequency
The carbonyl stretching frequency is a vibrational mode that is sensitive to changes in the electronic environment of the carbonyl group, such as changes in bond length, bond angle, and bond strength. The carbonyl stretching frequency is typically observed in the infrared (IR) region of the electromagnetic spectrum, and is commonly used to identify the presence of carbonyl groups in organic molecules.
Effect of Ring Strain on Carbonyl Stretching Frequency
Ring strain affects the carbonyl stretching frequency in lactones and lactams by altering the bond length and bond angle of the carbonyl group. The ring strain increases the bond length and decreases the bond angle of the carbonyl group, which results in an increase in the carbonyl stretching frequency.
The increase in carbonyl stretching frequency is due to the increased bond strength and bond order of the carbonyl group. The increased bond strength is a result of the increased bond length, which causes the electrons in the carbonyl bond to be held more tightly by the nuclei of the atoms involved in the bond. The increased bond order is a result of the decreased bond angle, which increases the degree of overlap between the orbitals involved in the bond.
In summary, ring strain in lactones and lactams increases the carbonyl stretching frequency by increasing the bond strength and bond order of the carbonyl group. This effect can be observed in the IR spectrum and is useful for identifying the presence of lactones and lactams in organic molecules.
Lactones and lactams are cyclic esters and amides, respectively, that contain a carbonyl group (C=O) within the ring structure. The presence of the ring structure, however, introduces ring strain, which affects the behavior of the carbonyl group.
Carbonyl Stretching Frequency
The carbonyl stretching frequency is a vibrational mode that is sensitive to changes in the electronic environment of the carbonyl group, such as changes in bond length, bond angle, and bond strength. The carbonyl stretching frequency is typically observed in the infrared (IR) region of the electromagnetic spectrum, and is commonly used to identify the presence of carbonyl groups in organic molecules.
Effect of Ring Strain on Carbonyl Stretching Frequency
Ring strain affects the carbonyl stretching frequency in lactones and lactams by altering the bond length and bond angle of the carbonyl group. The ring strain increases the bond length and decreases the bond angle of the carbonyl group, which results in an increase in the carbonyl stretching frequency.
The increase in carbonyl stretching frequency is due to the increased bond strength and bond order of the carbonyl group. The increased bond strength is a result of the increased bond length, which causes the electrons in the carbonyl bond to be held more tightly by the nuclei of the atoms involved in the bond. The increased bond order is a result of the decreased bond angle, which increases the degree of overlap between the orbitals involved in the bond.
In summary, ring strain in lactones and lactams increases the carbonyl stretching frequency by increasing the bond strength and bond order of the carbonyl group. This effect can be observed in the IR spectrum and is useful for identifying the presence of lactones and lactams in organic molecules.
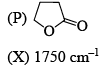
Correctly matched structure and carbonyl stretching frequency set is:
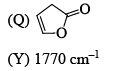
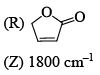
- a)P-Y, Q-Z, R-X
- b)P-Y, Q-X, R-Z
- c)P-Z, Q-Y, R-X
- d)P-X, Q-Z, R-Y
Correct answer is option 'A'. Can you explain this answer?
Correctly matched structure and carbonyl stretching frequency set is:



a)
P-Y, Q-Z, R-X
b)
P-Y, Q-X, R-Z
c)
P-Z, Q-Y, R-X
d)
P-X, Q-Z, R-Y

|
Ritwayan Mondal answered |
Here we can only noticed the position of this double bond in five members cyclic ring. As far the double bond with the carbonyl group and as close to the 'o' this stretching frequency will be greater (due to resonance with this 'o' lone pair stabilises this system).
So for (P) no double bond so normal frequency (1770cm-¹)
for (Q) Double bond closer to the 'o' so by resonance it stabilises this system.(1800cm-¹)
But (R) Double bond closer to the carbonyl carbon disstabilies this system by conjunction.(1750cm-¹)
So for (P) no double bond so normal frequency (1770cm-¹)
for (Q) Double bond closer to the 'o' so by resonance it stabilises this system.(1800cm-¹)
But (R) Double bond closer to the carbonyl carbon disstabilies this system by conjunction.(1750cm-¹)
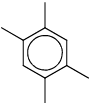
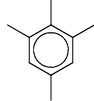
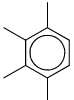
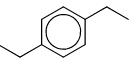
An organic compound having the molecular formula C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR spectrum. The compound is:- a)
- b)
- c)
- d)
Correct answer is option 'A'. Can you explain this answer?
An organic compound having the molecular formula C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR spectrum. The compound is:
a)
b)
c)
d)

|
Prakash Taur answered |
In option A 1H NMR show two singlet, one from 4 methyl group due to symmetry, and second is due to CH.
In 13C NMR due to symmetry shows three singlet.
In 13C NMR due to symmetry shows three singlet.
Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1?- a)Mesomeric (M) effect is dominant in acids over the inductive (I) effect
- b)I effect is dominant in carboxylic acids over the mesomeric effect
- c)I effect on ketones is dominant over the M effect
- d)M effect in ketones is dominant
Correct answer is option 'B'. Can you explain this answer?
Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1?
a)
Mesomeric (M) effect is dominant in acids over the inductive (I) effect
b)
I effect is dominant in carboxylic acids over the mesomeric effect
c)
I effect on ketones is dominant over the M effect
d)
M effect in ketones is dominant
|
|
Vivek Khatri answered |
Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1 because I effect is dominant in carboxylic acids over the mesomeric effect.
How many normal modes does the CO2 molecule have; What if the C and the O atoms were constrained to move in one dimensional:- a)4 normal modes for free CO2 and 4 for constrained CO2
- b)3 normal modes for free CO2 and 2 for constrained CO2
- c)3 normal modes for free CO2 and 3 for constrained CO2
- d)4 normal modes for free CO2 and 2 for constrained CO2
Correct answer is option 'D'. Can you explain this answer?
How many normal modes does the CO2 molecule have; What if the C and the O atoms were constrained to move in one dimensional:
a)
4 normal modes for free CO2 and 4 for constrained CO2
b)
3 normal modes for free CO2 and 2 for constrained CO2
c)
3 normal modes for free CO2 and 3 for constrained CO2
d)
4 normal modes for free CO2 and 2 for constrained CO2

|
Jaya Sen answered |
**Answer:**
The CO2 molecule consists of one carbon atom (C) double-bonded to two oxygen atoms (O). Each atom has three degrees of freedom (DOF) - three dimensions in which it can move.
**Free CO2:**
In a free CO2 molecule, all atoms are free to move in three dimensions. However, there are certain constraints due to the nature of the molecule.
1. **Translation (3 DOF):** The molecule can move as a whole in three dimensions - x, y, and z. This is the movement of the center of mass of the molecule.
2. **Rotation (3 DOF):** The molecule can also rotate around three axes - x, y, and z. This involves the spinning motion of the molecule.
3. **Vibration (4 DOF):** The CO2 molecule has two O-C-O bending vibrations and one symmetric O-C-O stretching vibration. These vibrations involve the stretching and bending of the bonds between the atoms.
Therefore, a free CO2 molecule has a total of 3 + 3 + 4 = 10 normal modes.
**Constrained CO2:**
If the C and O atoms were constrained to move in only one dimension, certain degrees of freedom would be lost.
1. **Translation (1 DOF):** The molecule can only move along the constrained dimension.
2. **Rotation (1 DOF):** The molecule can still rotate around the unconstrained axes (y and z).
3. **Vibration (2 DOF):** The CO2 molecule still retains its two O-C-O bending vibrations, but loses the symmetric O-C-O stretching vibration since the atoms cannot move in the constrained direction.
Therefore, a constrained CO2 molecule has a total of 1 + 1 + 2 = 4 normal modes.
Hence, the correct answer is option 'D' - 4 normal modes for free CO2 and 2 normal modes for constrained CO2.
The CO2 molecule consists of one carbon atom (C) double-bonded to two oxygen atoms (O). Each atom has three degrees of freedom (DOF) - three dimensions in which it can move.
**Free CO2:**
In a free CO2 molecule, all atoms are free to move in three dimensions. However, there are certain constraints due to the nature of the molecule.
1. **Translation (3 DOF):** The molecule can move as a whole in three dimensions - x, y, and z. This is the movement of the center of mass of the molecule.
2. **Rotation (3 DOF):** The molecule can also rotate around three axes - x, y, and z. This involves the spinning motion of the molecule.
3. **Vibration (4 DOF):** The CO2 molecule has two O-C-O bending vibrations and one symmetric O-C-O stretching vibration. These vibrations involve the stretching and bending of the bonds between the atoms.
Therefore, a free CO2 molecule has a total of 3 + 3 + 4 = 10 normal modes.
**Constrained CO2:**
If the C and O atoms were constrained to move in only one dimension, certain degrees of freedom would be lost.
1. **Translation (1 DOF):** The molecule can only move along the constrained dimension.
2. **Rotation (1 DOF):** The molecule can still rotate around the unconstrained axes (y and z).
3. **Vibration (2 DOF):** The CO2 molecule still retains its two O-C-O bending vibrations, but loses the symmetric O-C-O stretching vibration since the atoms cannot move in the constrained direction.
Therefore, a constrained CO2 molecule has a total of 1 + 1 + 2 = 4 normal modes.
Hence, the correct answer is option 'D' - 4 normal modes for free CO2 and 2 normal modes for constrained CO2.
A compound of formula C5H12 gives one signal in the 1H NMR and two signals in the 13C NMR spectra. The compound is:- a)Pentane
- b)2-methylbutane
- c)2, 2-dimethylpropane
- d)Cannot tell without more information
Correct answer is option 'C'. Can you explain this answer?
A compound of formula C5H12 gives one signal in the 1H NMR and two signals in the 13C NMR spectra. The compound is:
a)
Pentane
b)
2-methylbutane
c)
2, 2-dimethylpropane
d)
Cannot tell without more information

|
Sparsh Menon answered |
Explanation:
The given compound has the formula C5H12, which suggests it is an alkane.
1H NMR:
The compound gives one signal in the 1H NMR. This indicates that there are no chemically distinct hydrogen atoms in the molecule.
13C NMR:
The compound gives two signals in the 13C NMR. This indicates that there are two different types of carbon atoms in the molecule.
Possible structures:
The compound can have the following structures:
a) Pentane: This compound has five carbon atoms and no branching. It would give only one signal in both 1H and 13C NMR spectra. Therefore, it is not the correct option.
b) 2-Methylbutane: This compound has a branched structure with four carbon atoms in the main chain and one methyl group attached to the second carbon atom. It would give one signal in the 1H NMR and two signals in the 13C NMR spectra. However, the signals in the 13C NMR spectra would be very close to each other, indicating that the carbon atoms are not very different in chemical environment. Therefore, this option is not the correct one.
c) 2,2-Dimethylpropane: This compound has a branched structure with three carbon atoms in the main chain and two methyl groups attached to the second and third carbon atoms. It would give one signal in the 1H NMR and two signals in the 13C NMR spectra. The signals in the 13C NMR spectra would be well separated, indicating that the carbon atoms are very different in chemical environment. Therefore, this option is the correct one.
d) Cannot tell without more information: This option can be eliminated based on the information provided in the question.
Conclusion:
The correct option is c) 2,2-Dimethylpropane, which has a branched structure with three carbon atoms in the main chain and two methyl groups attached to the second and third carbon atoms.
The given compound has the formula C5H12, which suggests it is an alkane.
1H NMR:
The compound gives one signal in the 1H NMR. This indicates that there are no chemically distinct hydrogen atoms in the molecule.
13C NMR:
The compound gives two signals in the 13C NMR. This indicates that there are two different types of carbon atoms in the molecule.
Possible structures:
The compound can have the following structures:
a) Pentane: This compound has five carbon atoms and no branching. It would give only one signal in both 1H and 13C NMR spectra. Therefore, it is not the correct option.
b) 2-Methylbutane: This compound has a branched structure with four carbon atoms in the main chain and one methyl group attached to the second carbon atom. It would give one signal in the 1H NMR and two signals in the 13C NMR spectra. However, the signals in the 13C NMR spectra would be very close to each other, indicating that the carbon atoms are not very different in chemical environment. Therefore, this option is not the correct one.
c) 2,2-Dimethylpropane: This compound has a branched structure with three carbon atoms in the main chain and two methyl groups attached to the second and third carbon atoms. It would give one signal in the 1H NMR and two signals in the 13C NMR spectra. The signals in the 13C NMR spectra would be well separated, indicating that the carbon atoms are very different in chemical environment. Therefore, this option is the correct one.
d) Cannot tell without more information: This option can be eliminated based on the information provided in the question.
Conclusion:
The correct option is c) 2,2-Dimethylpropane, which has a branched structure with three carbon atoms in the main chain and two methyl groups attached to the second and third carbon atoms.
Which of the following molecules has the lowest vibrat ional stretching frequency:- a)4H 35Cl
- b)2H 35Cl
- c)4H 36Cl
- d)4H 37Cl
Correct answer is option 'C'. Can you explain this answer?
Which of the following molecules has the lowest vibrat ional stretching frequency:
a)
4H 35Cl
b)
2H 35Cl
c)
4H 36Cl
d)
4H 37Cl
|
|
Pooja Choudhury answered |
C is correct, as more the reduced mass, less the vibrational stretching frequency.
At room temperature, what is the number of singlet resonances observed in the 1H NMR spectrum of Me3CC(O)NMe2 (N, N-Dimethylpivalamide)?- a)3
- b)4
- c)5
- d)2
Correct answer is option 'A'. Can you explain this answer?
At room temperature, what is the number of singlet resonances observed in the 1H NMR spectrum of Me3CC(O)NMe2 (N, N-Dimethylpivalamide)?
a)
3
b)
4
c)
5
d)
2
|
|
Vivek Khatri answered |
As shown below; three signals are observed. 3Ha will experience shielding due to O–, so They are different from 3Hb.

In NMR spectroscopy, what is the product of Nuclear ‘g’ factor (gN), the nuclear magneton and the magnetic field strength (B0)?- a)energy of transition from alpha to beta state
- b)chemical shift
- c)spin-spin coupling constant
- d)magnetogyric ratio
Correct answer is option 'A'. Can you explain this answer?
In NMR spectroscopy, what is the product of Nuclear ‘g’ factor (gN), the nuclear magneton and the magnetic field strength (B0)?
a)
energy of transition from alpha to beta state
b)
chemical shift
c)
spin-spin coupling constant
d)
magnetogyric ratio

|
Jay Nambiar answered |
Magnetic Resonance (NMR) spectroscopy is a technique used to analyze the structure and composition of molecules. The product of Nuclear Magnetic Resonance spectroscopy is a spectrum, which is a graph showing the intensity of different signals as a function of the frequency or chemical shift.
The spectrum obtained from NMR spectroscopy provides information about the number and type of different nuclei present in a molecule, their chemical environment, and their connectivity to neighboring atoms. This information can be used to determine the structure of the molecule, as well as other properties such as molecular dynamics and interactions.
Overall, the product of NMR spectroscopy is the spectrum, which is analyzed to obtain valuable information about the molecular structure and composition.
The spectrum obtained from NMR spectroscopy provides information about the number and type of different nuclei present in a molecule, their chemical environment, and their connectivity to neighboring atoms. This information can be used to determine the structure of the molecule, as well as other properties such as molecular dynamics and interactions.
Overall, the product of NMR spectroscopy is the spectrum, which is analyzed to obtain valuable information about the molecular structure and composition.
Chapter doubts & questions for Qualitative Organic Analysis and Spectroscopic Techniques - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Qualitative Organic Analysis and Spectroscopic Techniques - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup