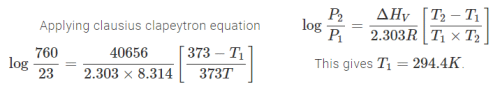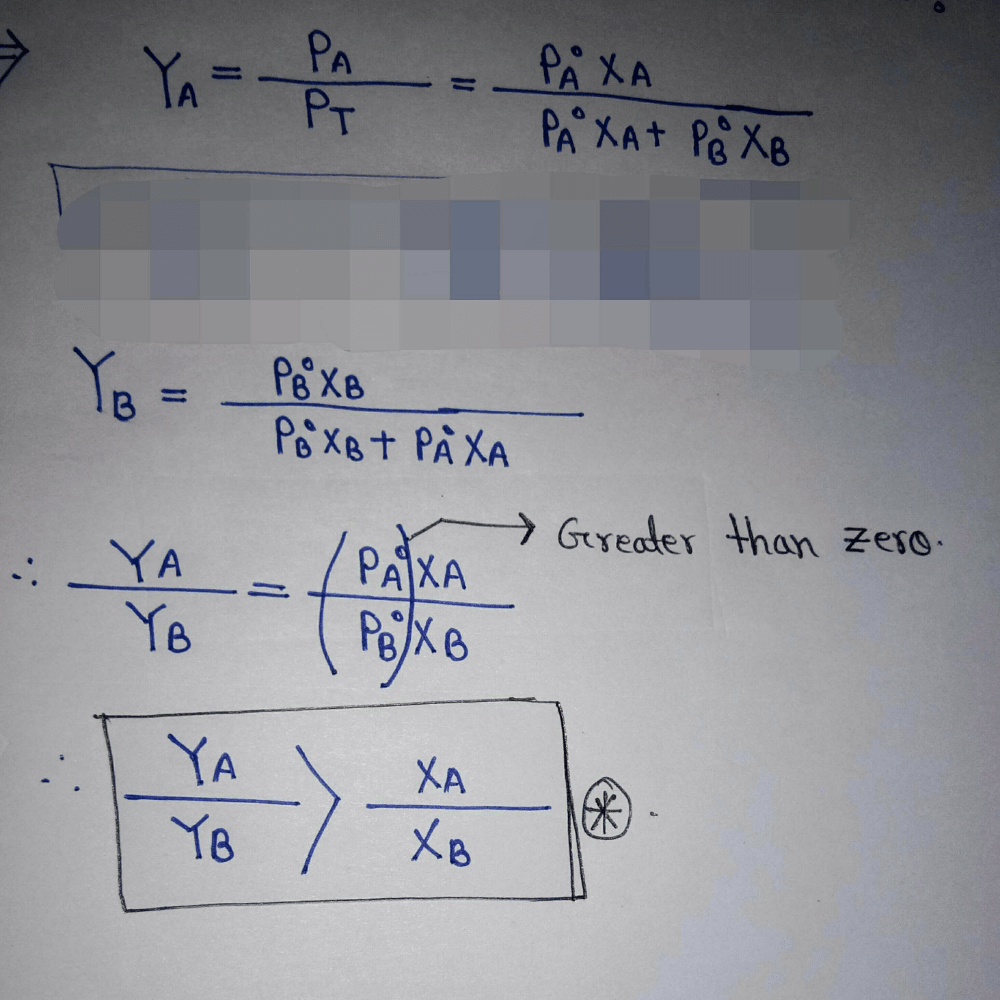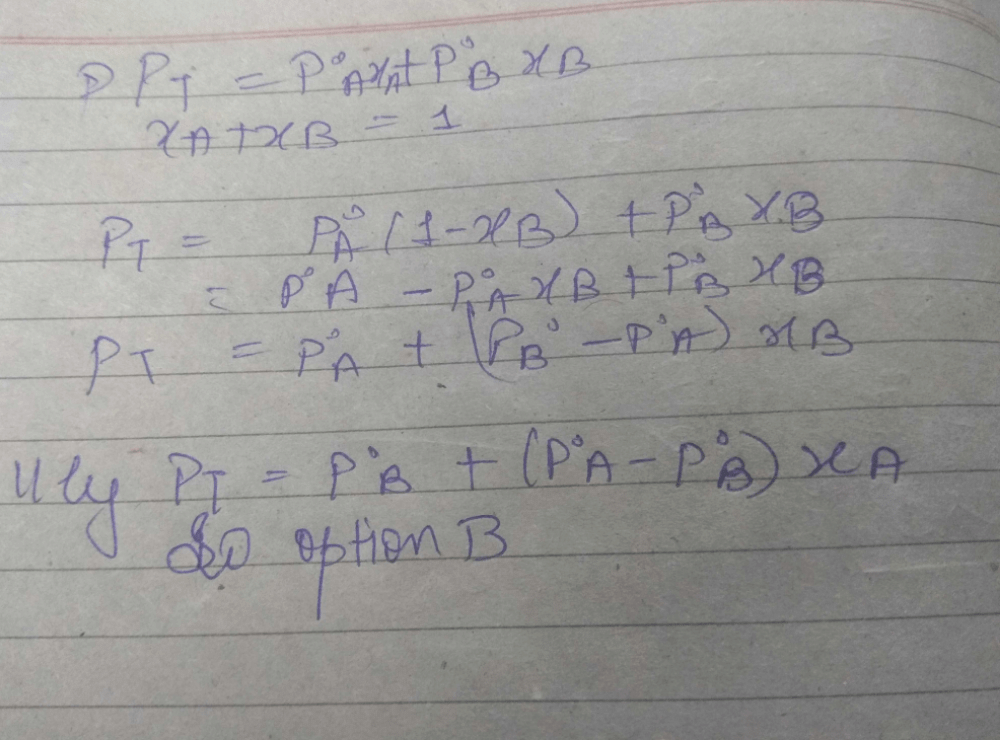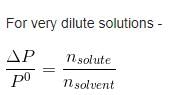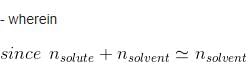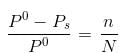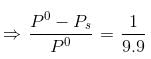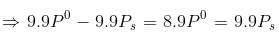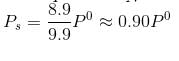All Exams >
Chemistry >
Physical Chemistry >
All Questions
All questions of Solutions & Colligative Properties for Chemistry Exam
An ideal solution was found to have a vapour pressure of 80 torr when the mole fraction of a non– volatile solute was 0.2. What would be the vapour pressure of the pure solvent at the same temperature?- a)64 torr
- b)80 torr
- c)100 torr
- d)400 torr
Correct answer is option 'C'. Can you explain this answer?
An ideal solution was found to have a vapour pressure of 80 torr when the mole fraction of a non– volatile solute was 0.2. What would be the vapour pressure of the pure solvent at the same temperature?
a)
64 torr
b)
80 torr
c)
100 torr
d)
400 torr

|
Baishali Bajaj answered |
Mole fraction of solute = 0.2
mole fraction of solvent = 1 - 0.2 = 0.8
Psolution = Xsolvent*Pdegsolvent
80 = 0.8*Pdeg solvent
Pdeg solvent = 100torr
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
- a)8 g
- b)5 g
- c)106.2 g
- d)None of these.
Correct answer is option 'A'. Can you explain this answer?
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
a)
8 g
b)
5 g
c)
106.2 g
d)
None of these.

|
Harshitha Sharma answered |
Let the vapour pressure of pure octane be p10.
Then, the vapour pressure of the octane after dissolving the non-volatile solute is 80/100 p10 = 0.8 p10.
Molar mass of solute, M2 = 40 g mol - 1
Mass of octane, w1 = 114 g
Molar mass of octane, (C8H18), M1 = 8 × 12 + 18 × 1 = 114 g mol - 1
Applying the relation,
(p10 - p1) / p10 = (w2 x M1 ) / (M2 x w1 )
⇒ (p10 - 0.8 p10) / p10 = (w2 x 114 ) / (40 x 114 )
⇒ 0.2 p10 / p10 = w2 / 40
⇒ 0.2 = w2 / 40
⇒ w2 = 8 g
Hence, the required mass of the solute is 8 g.
The vapour pressure of an aqueous solution of sucrose at 373 K is found to be 750 mm Hg. The molality of the solution at the same temperature will be:- a)0.26
- b)0.73
- c)0.74
- d)0.039
Correct answer is option 'C'. Can you explain this answer?
The vapour pressure of an aqueous solution of sucrose at 373 K is found to be 750 mm Hg. The molality of the solution at the same temperature will be:
a)
0.26
b)
0.73
c)
0.74
d)
0.039

|
Baishali Bajaj answered |
At 373K V.P of water is 750 mm Hg
We know that, (P0-P) / P0 = w x M / m x W
molality = (P0-P) / P0 X 1000/M
=> (760-750) / 760 X 1000/18
=> 0.73 m
Mole fraction = (P0-P) / P0 = 10/760 = 0.0131
A sample of liquid H2O of mass 18.0 g is injected into an evacuated 7.6 L flask maintained at 27.0°C. If vapour pressure of H2O at 27°C is 24.63 mm Hg. What weight percentage of the water will be vaporized when the system comes to equilibrium? Assume water vapours behaves as an ideal gas. The volume occupied by the liquid water is negligible compared to the volume of the container:- a)1%
- b)10%
- c)18%
- d)20%
Correct answer is option 'A'. Can you explain this answer?
A sample of liquid H2O of mass 18.0 g is injected into an evacuated 7.6 L flask maintained at 27.0°C. If vapour pressure of H2O at 27°C is 24.63 mm Hg. What weight percentage of the water will be vaporized when the system comes to equilibrium? Assume water vapours behaves as an ideal gas. The volume occupied by the liquid water is negligible compared to the volume of the container:
a)
1%
b)
10%
c)
18%
d)
20%

|
Swara Dasgupta answered |

The boiling point of C6H6, CH3OH, C6H5NH2 and C6H5NO2 are 80°C, 184°C and 212°C respectively. Which will show highest vapour pressure at room temperature:- a)C6H6
- b)CH3OH
- c)C6H5NH2
- d)C6H5NO2
Correct answer is option 'B'. Can you explain this answer?
The boiling point of C6H6, CH3OH, C6H5NH2 and C6H5NO2 are 80°C, 184°C and 212°C respectively. Which will show highest vapour pressure at room temperature:
a)
C6H6
b)
CH3OH
c)
C6H5NH2
d)
C6H5NO2

|
Kaavya Sengupta answered |
Boiling point is the temperature at which the vapour pressure of the liquid becomes equal to that of the atmospheric pressure. At boiling point, a substance is converted from the liquid phase to the vapoour phase.
Out of the given liquids, methanol has the lowest boiling point. Therefore at room temperature, methanol will show the highest vapour pressure.
The vapour pressure of a given liquid will decreases if:- a)Surface area of liquid is decreased.
- b)The volume of liquid in the container is decreased.
- c)The volume of the vapour phase is increased.
- d)The temperature is decreased.
Correct answer is option 'D'. Can you explain this answer?
The vapour pressure of a given liquid will decreases if:
a)
Surface area of liquid is decreased.
b)
The volume of liquid in the container is decreased.
c)
The volume of the vapour phase is increased.
d)
The temperature is decreased.
|
|
Rajeev Sharma answered |
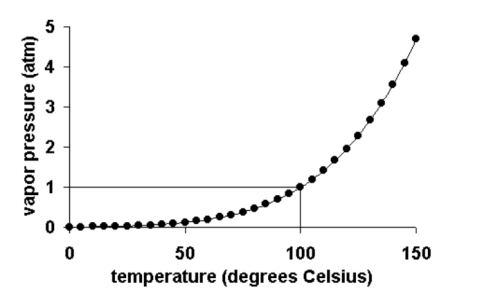
The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling temperature for water.
As the temperature of a liquid or solid increases its vapor pressure also increases. Conversely, vapor pressure decreases as the temperature decreases.
The values of observed and calculated molecular weights of silver nitrate are 92.64 and 170 respectively. The degree of dissociation of silver nitrate is:- a)60%
- b)83.5%
- c)46.7%
- d)60.23%
Correct answer is 'B'. Can you explain this answer?
The values of observed and calculated molecular weights of silver nitrate are 92.64 and 170 respectively. The degree of dissociation of silver nitrate is:
a)
60%
b)
83.5%
c)
46.7%
d)
60.23%
|
|
Mira Sharma answered |
I for AgNO3 = normal mol.wt/observed mol wt= 1+ α∴ α = i −1= (170/92.64 − 1)= 0.835 = 83.5%
For a dilute solut ion, Raoult’s law states that:- a)The lowering of vapour pressure is equal to the mole fraction of solute.
- b)The relative lowering of vapour pressure is equal to the mole fraction of solute.
- c)The relative lowering of vapour pressure is proportional to the amount of solute in solution.
- d)The vapour pressure of the solution is equal to the mole fraction of solvent.
Correct answer is option 'B'. Can you explain this answer?
For a dilute solut ion, Raoult’s law states that:
a)
The lowering of vapour pressure is equal to the mole fraction of solute.
b)
The relative lowering of vapour pressure is equal to the mole fraction of solute.
c)
The relative lowering of vapour pressure is proportional to the amount of solute in solution.
d)
The vapour pressure of the solution is equal to the mole fraction of solvent.
|
|
Aditya Deshmukh answered |
According to Raoult's law, for dilute solutions containing non volatile solute, the relative lowering of vapor pressure, Δp/po is equal to the mole fraction of solute, X2. * The lowering of vapor pressure is proportional to the mole fraction of solute or inversely related to mole fraction of solvent.
The solubility of a specific non–volatile salt is 4 g in 100 g of water at 25°C. If 2.0 g, 4.0 g and 6.0 g of the salt added of 100 g of water at 25°C, in system X, Y, and Z. The vapour pressure would be in the order:- a)X < Y < Z
- b)X > Y > Z
- c)Z > X = Y
- d)X > Y = Z
Correct answer is option 'D'. Can you explain this answer?
The solubility of a specific non–volatile salt is 4 g in 100 g of water at 25°C. If 2.0 g, 4.0 g and 6.0 g of the salt added of 100 g of water at 25°C, in system X, Y, and Z. The vapour pressure would be in the order:
a)
X < Y < Z
b)
X > Y > Z
c)
Z > X = Y
d)
X > Y = Z
|
|
Aditya Deshmukh answered |
Solution X is unsaturated so vapour pressure will be more, solution Y & Z are saturated so vapour pressure of Y = vapour pressure of Z and 2 gm of solute would be present in form of solid in system Z.
When a liquid that is immiscible with water was steam distilled at 95.2°C at a total pressure of 748 torr, the distillate contained 1.25 g of the liquid per gram of water. The vapour pressure of water is 648 torr at 95.2°C, what is the molar mass of liquid?- a)7.975 g/mol.
- b)166 g/mol.
- c)145.8 g/mol.
- d)None of these.
Correct answer is option 'C'. Can you explain this answer?
When a liquid that is immiscible with water was steam distilled at 95.2°C at a total pressure of 748 torr, the distillate contained 1.25 g of the liquid per gram of water. The vapour pressure of water is 648 torr at 95.2°C, what is the molar mass of liquid?
a)
7.975 g/mol.
b)
166 g/mol.
c)
145.8 g/mol.
d)
None of these.

|
Neha Basak answered |
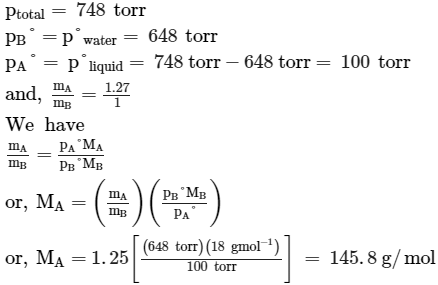
A very diluted saturated solution of a sparingly soluble salt X3Y4 has a vapour pressure of 20 mm Hg at temperature T, while pure water exerts a pressure of 20.0126 mm Hg at the same temperature. Calculate molality (m) at temperature T:- a)6.3 ×10-4
- b)3.5 ×10-2
- c)5 ×10-3
- d)None of these.
Correct answer is option 'C'. Can you explain this answer?
A very diluted saturated solution of a sparingly soluble salt X3Y4 has a vapour pressure of 20 mm Hg at temperature T, while pure water exerts a pressure of 20.0126 mm Hg at the same temperature. Calculate molality (m) at temperature T:
a)
6.3 ×10-4
b)
3.5 ×10-2
c)
5 ×10-3
d)
None of these.

|
Divyanshu Saini answered |
Correct Ans Is B not C
Chloro form, CHCl3, boils at 61.7°C. If the Kb for chloroform is 3.63°C/molal, what is the boiling point of a solution of 15.0 kg of CHCl3 and 0.616 kg of acenaphthalene, C12H10?- a)61.9
- b)62.0
- c)52.2
- d)62.67
Correct answer is option 'D'. Can you explain this answer?
Chloro form, CHCl3, boils at 61.7°C. If the Kb for chloroform is 3.63°C/molal, what is the boiling point of a solution of 15.0 kg of CHCl3 and 0.616 kg of acenaphthalene, C12H10?
a)
61.9
b)
62.0
c)
52.2
d)
62.67

|
Chirag Nambiar answered |
Given:
Boiling point of pure CHCl3 (Tb1) = 61.7°C
Kb for CHCl3 = 3.63°C/m
To find:
Boiling point of solution (Tb2) = ?
Solution:
1. Calculate the molality of the solution:
Molality (m) = moles of solute / kg of solvent
Molar mass of CHCl3 = 12 + 1 + 35.5x3 = 119.5 g/mol
Number of moles of CHCl3 = 15000 g / 119.5 g/mol = 125.52 mol
Molality of CHCl3 = 125.52 mol / 15 kg = 8.36 mol/kg
Molar mass of C12H10 = 156.2 g/mol
Number of moles of C12H10 = 616 g / 156.2 g/mol = 3.95 mol
Mass of solvent (CHCl3) = 15 kg
Molality of solution = 3.95 mol / (15 + 0.616) kg = 0.243 mol/kg
2. Calculate the change in boiling point:
ΔTb = Kb x m
ΔTb = 3.63°C/m x 0.243 mol/kg = 0.88149°C
3. Calculate the boiling point of solution:
Tb2 = Tb1 + ΔTb
Tb2 = 61.7°C + 0.88149°C = 62.58°C
Answer:
The boiling point of the solution is 62.58°C, option (D) is correct.
Boiling point of pure CHCl3 (Tb1) = 61.7°C
Kb for CHCl3 = 3.63°C/m
To find:
Boiling point of solution (Tb2) = ?
Solution:
1. Calculate the molality of the solution:
Molality (m) = moles of solute / kg of solvent
Molar mass of CHCl3 = 12 + 1 + 35.5x3 = 119.5 g/mol
Number of moles of CHCl3 = 15000 g / 119.5 g/mol = 125.52 mol
Molality of CHCl3 = 125.52 mol / 15 kg = 8.36 mol/kg
Molar mass of C12H10 = 156.2 g/mol
Number of moles of C12H10 = 616 g / 156.2 g/mol = 3.95 mol
Mass of solvent (CHCl3) = 15 kg
Molality of solution = 3.95 mol / (15 + 0.616) kg = 0.243 mol/kg
2. Calculate the change in boiling point:
ΔTb = Kb x m
ΔTb = 3.63°C/m x 0.243 mol/kg = 0.88149°C
3. Calculate the boiling point of solution:
Tb2 = Tb1 + ΔTb
Tb2 = 61.7°C + 0.88149°C = 62.58°C
Answer:
The boiling point of the solution is 62.58°C, option (D) is correct.
Estimate the lowering of vapour pressure due to the solute (glucose) in a 1.0 M aqueous solution at 100°C:- a)10 torr
- b)18 torr
- c)13.45 torr
- d)24 torr
Correct answer is option 'C'. Can you explain this answer?
Estimate the lowering of vapour pressure due to the solute (glucose) in a 1.0 M aqueous solution at 100°C:
a)
10 torr
b)
18 torr
c)
13.45 torr
d)
24 torr

|
Om Desai answered |
First of all find mole fraction of gas .
Let mole fraction of gas is x
we know, the relation between mole fraction and molality
molality = x x 1000/(1 - x)M
Here, M is Molecular weight of solvent .
for aqueous solution , solvent is water .
∴ Molecular weight of water , M = 18g/mol
Now, 1 = x x 1000/(1 - x) x 18
⇒18(1 - x) = 1000x
⇒18 = (1000 + 18)x
⇒x = 18/1018
Now, use formula ,
Relative lowering of vapor pressure = mole fraction of gas
∆P/P₀ = x
Here P₀ is initial pressure ,at STP , P₀ = 760 torr
so, ∆P = 760 � 18/1018 = 13.45 torr
Hence, lowering of vapor pressure = 13.45 torr
Equal weight of a solute are dissolved in equal weight of two solvents A and B formed very dilute solution. The relative lowering of vapour pressure for the solution B has twice the relative lowering of vapour pressure for the solut ion A. If MA and MB are the mo lecular weights of so lvents A and B respectively, then:- a)MA = MB
- b)MB = 2×MA
- c)MA = 4MB
- d)MA = 2MB
Correct answer is option 'B'. Can you explain this answer?
Equal weight of a solute are dissolved in equal weight of two solvents A and B formed very dilute solution. The relative lowering of vapour pressure for the solution B has twice the relative lowering of vapour pressure for the solut ion A. If MA and MB are the mo lecular weights of so lvents A and B respectively, then:
a)
MA = MB
b)
MB = 2×MA
c)
MA = 4MB
d)
MA = 2MB

|
Jaya Sen answered |
Explanation:
Relative lowering of vapour pressure is given by the formula:
(Relative lowering of vapour pressure) = (Mole fraction of solute) x (Molecular weight of solvent) / (1000 x Density of solvent)
Let the weight of solute be w and weight of solvent A and B be WA and WB respectively.
So, for solvent A:
Mole fraction of solute = w / (MA x WA)
Relative lowering of vapour pressure for A = (w / (MA x WA)) x MA / (1000 x Density of A)
For solvent B:
Mole fraction of solute = w / (MB x WB)
Relative lowering of vapour pressure for B = 2 x (w / (MB x WB)) x MB / (1000 x Density of B)
It is given that both the solvents have equal weight of solute and the relative lowering of vapour pressure for solution B is twice that of solution A.
Therefore,
(w / (MA x WA)) x MA / (1000 x Density of A) = 2 x (w / (MB x WB)) x MB / (1000 x Density of B)
Simplifying this expression, we get:
MA / WB = 2 x MB / WA
Dividing both sides by MA, we get:
1 / (MA x WB) = 2 / (WA x MB)
1 / MA = 2 / MB
Hence, MB = 2 x MA. Therefore, option B is the correct answer.
Relative lowering of vapour pressure is given by the formula:
(Relative lowering of vapour pressure) = (Mole fraction of solute) x (Molecular weight of solvent) / (1000 x Density of solvent)
Let the weight of solute be w and weight of solvent A and B be WA and WB respectively.
So, for solvent A:
Mole fraction of solute = w / (MA x WA)
Relative lowering of vapour pressure for A = (w / (MA x WA)) x MA / (1000 x Density of A)
For solvent B:
Mole fraction of solute = w / (MB x WB)
Relative lowering of vapour pressure for B = 2 x (w / (MB x WB)) x MB / (1000 x Density of B)
It is given that both the solvents have equal weight of solute and the relative lowering of vapour pressure for solution B is twice that of solution A.
Therefore,
(w / (MA x WA)) x MA / (1000 x Density of A) = 2 x (w / (MB x WB)) x MB / (1000 x Density of B)
Simplifying this expression, we get:
MA / WB = 2 x MB / WA
Dividing both sides by MA, we get:
1 / (MA x WB) = 2 / (WA x MB)
1 / MA = 2 / MB
Hence, MB = 2 x MA. Therefore, option B is the correct answer.
At 25°C, the vapour pressure of pure liquid A (mol. wt. = 40) is 100 torr, while that of pure liquid B is 40 torr, (mol. wt. = 80). The vapour pressure at 25°C of a solution containing 20 g of each A and B is:- a)80 torr
- b)59.8 torr
- c)68 torr
- d)48 torr
Correct answer is option 'A'. Can you explain this answer?
At 25°C, the vapour pressure of pure liquid A (mol. wt. = 40) is 100 torr, while that of pure liquid B is 40 torr, (mol. wt. = 80). The vapour pressure at 25°C of a solution containing 20 g of each A and B is:
a)
80 torr
b)
59.8 torr
c)
68 torr
d)
48 torr

|
Aditi Basak answered |
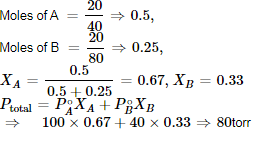
Raoult’s law is obeyed by each constituent of a binary liquid solution when:- a)The forces of attractions between like molecules are greater than those between unlike molecules.
- b)The forces of attractions between like molecules are smaller than those between unlike.
- c)The forces of attractions between like molecules are identical with those between unlike molecules.
- d)The volume occupied by unlike molecules are different.
Correct answer is option 'C'. Can you explain this answer?
Raoult’s law is obeyed by each constituent of a binary liquid solution when:
a)
The forces of attractions between like molecules are greater than those between unlike molecules.
b)
The forces of attractions between like molecules are smaller than those between unlike.
c)
The forces of attractions between like molecules are identical with those between unlike molecules.
d)
The volume occupied by unlike molecules are different.
|
|
Aditya Deshmukh answered |
Raoult's law is applicable when the forces of attractions between like molecules are more or less identical with those between unlike molecules.
Two liquids A and B from ideal solutions. At 300 K, the vapour pressure of solution containing 1 mole of A and 3 mole of B is 550 mm Hg. At the same temperature, if one more mole of B is added this solution, the vapour pressure of the solution increases by 10 mm Hg. Determine the vapour pressure of A and B in their pure states (in mm Hg):- a)400, 600
- b)500, 500
- c)600, 400
- d)None of these.
Correct answer is option 'A'. Can you explain this answer?
Two liquids A and B from ideal solutions. At 300 K, the vapour pressure of solution containing 1 mole of A and 3 mole of B is 550 mm Hg. At the same temperature, if one more mole of B is added this solution, the vapour pressure of the solution increases by 10 mm Hg. Determine the vapour pressure of A and B in their pure states (in mm Hg):
a)
400, 600
b)
500, 500
c)
600, 400
d)
None of these.
|
|
Aruna Singh answered |
Since P=xAP0A+xBP0B
⇒(11+3)P0A+(31+3)P∘B=550mm Hg
⇒(11+4)P0A+(41+4)P∘B=560mm Hg
That is,
0.25P0A+0.75P0B=550mm Hg
0.20P0A+0.8P0B=560mm Hg
Solving for P0A and P0B we get,
P0A=400 mm Hg
P0B=600 mm Hg
The boiling point elevation constant for toluene is 3.32 K kg mol–1. The normal boiling point of toluene is 110.7°C. The enthalpy of vaporization of toluene would be nearly:- a)17.0 kJ mol–1
- b)34.0 kJ mol–1
- c)51.0 kJ mol–1
- d)68.0 kJ mol–1
Correct answer is option 'B'. Can you explain this answer?
The boiling point elevation constant for toluene is 3.32 K kg mol–1. The normal boiling point of toluene is 110.7°C. The enthalpy of vaporization of toluene would be nearly:
a)
17.0 kJ mol–1
b)
34.0 kJ mol–1
c)
51.0 kJ mol–1
d)
68.0 kJ mol–1
|
|
Aditya Deshmukh answered |
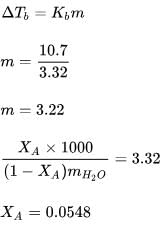
The relative lowering of vapour pressure of an aqueous solution of a non–volatile solute of molecular weight 60 (which neither dissociates nor associates in the solution) is 0.018. If Kf of water is 1.86º cm–1, the depression in freezing point will be ______ºC.
Correct answer is '1.89'. Can you explain this answer?
The relative lowering of vapour pressure of an aqueous solution of a non–volatile solute of molecular weight 60 (which neither dissociates nor associates in the solution) is 0.018. If Kf of water is 1.86º cm–1, the depression in freezing point will be ______ºC.

|
Abhijeet Majumdar answered |
Ans.
Method to Solve :
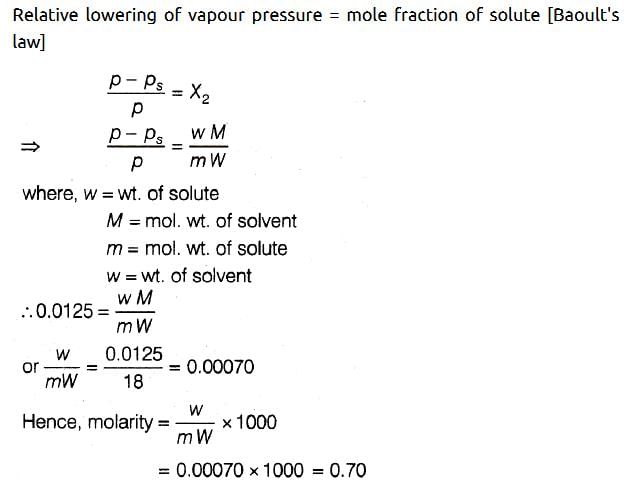
Water and Chlorobenzene are immiscible liquids. Their mixture boils at 89°C under a reduced pressure of 7.7×104 Pa. The vapour pressure of pure water at 89°C is 7×104 Pa. Weight per cent of Chlorobenzene in the distillate is:- a)50
- b)60
- c)78.3
- d)38.46
Correct answer is option 'D'. Can you explain this answer?
Water and Chlorobenzene are immiscible liquids. Their mixture boils at 89°C under a reduced pressure of 7.7×104 Pa. The vapour pressure of pure water at 89°C is 7×104 Pa. Weight per cent of Chlorobenzene in the distillate is:
a)
50
b)
60
c)
78.3
d)
38.46

|
Ipsita Chopra answered |
Given information:
- Water and Chlorobenzene are immiscible liquids.
- Their mixture boils at 89C under a reduced pressure of 7.7104 Pa.
- The vapour pressure of pure water at 89C is 7104 Pa.
To find: Weight per cent of Chlorobenzene in the distillate.
Solution:
1. Calculation of the total pressure in the system:
The reduced pressure given is the pressure at which the mixture boils. To find the total pressure in the system, we need to add the vapour pressure of pure water at 89C to the reduced pressure.
Total pressure = Vapour pressure of pure water + Reduced pressure
Total pressure = 7104 Pa + 7.7104 Pa
Total pressure = 7111.7104 Pa
2. Calculation of the composition of the vapour:
We assume that the vapour in equilibrium with the liquid mixture is composed of water and chlorobenzene in the same proportion as they exist in the liquid mixture. Let x be the weight per cent of chlorobenzene in the liquid mixture, then (100-x) is the weight per cent of water in the liquid mixture.
The vapour above the liquid mixture will also have the same composition as the liquid mixture.
Now, using Dalton's law of partial pressures, we can write:
Partial pressure of water in the vapour = mole fraction of water in the vapour x Total pressure
Partial pressure of chlorobenzene in the vapour = mole fraction of chlorobenzene in the vapour x Total pressure
We assume that the mole fraction of water in the vapour is equal to the mole fraction of water in the liquid mixture, and similarly for chlorobenzene.
Let's denote the mole fraction of water in the liquid mixture as y. Then, the mole fraction of chlorobenzene in the liquid mixture is (1-y).
Using the above assumptions and Dalton's law, we get:
Partial pressure of water in the vapour = y x 7111.7104 Pa
Partial pressure of chlorobenzene in the vapour = (1-y) x 7111.7104 Pa
3. Calculation of the weight per cent of chlorobenzene in the distillate:
We know that the mixture boils at 89C under the given reduced pressure. This means that the vapour pressure of the liquid mixture at 89C is equal to the reduced pressure.
Using Raoult's law, we can write:
Vapour pressure of the liquid mixture = mole fraction of water in the liquid mixture x Vapour pressure of pure water at 89C
+ mole fraction of chlorobenzene in the liquid mixture x Vapour pressure of chlorobenzene at 89C
Since water and chlorobenzene are immiscible, the mole fraction of each component in the liquid mixture is equal to its weight per cent in the liquid mixture.
At the boiling point, the vapour pressure of the liquid mixture is equal to the total pressure in the system. Therefore, we can write:
Total pressure = y x 7104 Pa + (1-y) x Vapour pressure of chlorobenzene at 89C
Solving for y, we get:
y = (7104 Pa - 7.7104 Pa)/(7104 Pa - Vapour pressure of chlor
- Water and Chlorobenzene are immiscible liquids.
- Their mixture boils at 89C under a reduced pressure of 7.7104 Pa.
- The vapour pressure of pure water at 89C is 7104 Pa.
To find: Weight per cent of Chlorobenzene in the distillate.
Solution:
1. Calculation of the total pressure in the system:
The reduced pressure given is the pressure at which the mixture boils. To find the total pressure in the system, we need to add the vapour pressure of pure water at 89C to the reduced pressure.
Total pressure = Vapour pressure of pure water + Reduced pressure
Total pressure = 7104 Pa + 7.7104 Pa
Total pressure = 7111.7104 Pa
2. Calculation of the composition of the vapour:
We assume that the vapour in equilibrium with the liquid mixture is composed of water and chlorobenzene in the same proportion as they exist in the liquid mixture. Let x be the weight per cent of chlorobenzene in the liquid mixture, then (100-x) is the weight per cent of water in the liquid mixture.
The vapour above the liquid mixture will also have the same composition as the liquid mixture.
Now, using Dalton's law of partial pressures, we can write:
Partial pressure of water in the vapour = mole fraction of water in the vapour x Total pressure
Partial pressure of chlorobenzene in the vapour = mole fraction of chlorobenzene in the vapour x Total pressure
We assume that the mole fraction of water in the vapour is equal to the mole fraction of water in the liquid mixture, and similarly for chlorobenzene.
Let's denote the mole fraction of water in the liquid mixture as y. Then, the mole fraction of chlorobenzene in the liquid mixture is (1-y).
Using the above assumptions and Dalton's law, we get:
Partial pressure of water in the vapour = y x 7111.7104 Pa
Partial pressure of chlorobenzene in the vapour = (1-y) x 7111.7104 Pa
3. Calculation of the weight per cent of chlorobenzene in the distillate:
We know that the mixture boils at 89C under the given reduced pressure. This means that the vapour pressure of the liquid mixture at 89C is equal to the reduced pressure.
Using Raoult's law, we can write:
Vapour pressure of the liquid mixture = mole fraction of water in the liquid mixture x Vapour pressure of pure water at 89C
+ mole fraction of chlorobenzene in the liquid mixture x Vapour pressure of chlorobenzene at 89C
Since water and chlorobenzene are immiscible, the mole fraction of each component in the liquid mixture is equal to its weight per cent in the liquid mixture.
At the boiling point, the vapour pressure of the liquid mixture is equal to the total pressure in the system. Therefore, we can write:
Total pressure = y x 7104 Pa + (1-y) x Vapour pressure of chlorobenzene at 89C
Solving for y, we get:
y = (7104 Pa - 7.7104 Pa)/(7104 Pa - Vapour pressure of chlor
One molal solution of a carboxylic acid in benzene shows the elevation of boiling point of 1.518 K. The degree of association for dimerization of the acid in benzene is (Kb for benzene = 2.53 K kg mol–1):- a)60%
- b)70%
- c)75%
- d)80%
Correct answer is option 'D'. Can you explain this answer?
One molal solution of a carboxylic acid in benzene shows the elevation of boiling point of 1.518 K. The degree of association for dimerization of the acid in benzene is (Kb for benzene = 2.53 K kg mol–1):
a)
60%
b)
70%
c)
75%
d)
80%

|
Shubham Rane answered |
Given information:
- One molal solution of a carboxylic acid in benzene
- Boiling point elevation = 1.518 K
- Kb for benzene = 2.53 K kg mol-1
To determine: Degree of association for dimerization of the acid in benzene
Formula:
ΔTb = Kb ∙ molality ∙ degree of association
where,
ΔTb = elevation of boiling point
Kb = ebullioscopic constant for solvent
molality = moles of solute per kg of solvent
degree of association = extent to which the solute associates or dissociates in solution
Solution:
Let's first calculate the molality of the solution.
Molality (m) = 1 mol of solute / 1000 g of solvent
We need to convert the mass of benzene (solvent) to kg.
Mass of benzene = 1000 g
Mass of solute = 1 mol
Molar mass of solute = unknown
Let's assume the molar mass of the solute to be M.
1 mol of solute = M g of solute
Total mass of solution = 1000 g (benzene) + M g (solute)
Molality (m) = 1 mol / (1000 g + M g)
Now, let's use the formula to calculate the degree of association.
ΔTb = Kb ∙ molality ∙ degree of association
1.518 = 2.53 ∙ (1 mol / (1000 g + M g)) ∙ degree of association
Simplifying the equation,
Degree of association = 1.518 ∙ (1000 g + M g) / (2.53 ∙ M)
We can further simplify the equation by assuming the molar mass of the solute to be twice its empirical formula weight (dimerization assumption).
Molar mass of solute = 2 ∙ Empirical formula weight
Degree of association = 1.518 ∙ (1000 g + 2 ∙ EFw g) / (2.53 ∙ 2 ∙ EFw)
Degree of association = (1.518 ∙ 1000) / (5.06 ∙ EFw + 1000)
Let's assume the empirical formula weight of the acid to be x.
Empirical formula weight (EFw) = x
Molar mass of solute = 2 ∙ x
Substituting the values,
Degree of association = (1.518 ∙ 1000) / (5.06 ∙ x + 1000)
We need to find the value of x that gives a degree of association of 80%.
Degree of association = 0.8 = (1.518 ∙ 1000) / (5.06 ∙ x + 1000)
Solving for x,
x = 90 g/mol
Therefore, the empirical formula of the acid is CH2O2 and the degree of association for dimerization of the acid in benzene is 80%.
- One molal solution of a carboxylic acid in benzene
- Boiling point elevation = 1.518 K
- Kb for benzene = 2.53 K kg mol-1
To determine: Degree of association for dimerization of the acid in benzene
Formula:
ΔTb = Kb ∙ molality ∙ degree of association
where,
ΔTb = elevation of boiling point
Kb = ebullioscopic constant for solvent
molality = moles of solute per kg of solvent
degree of association = extent to which the solute associates or dissociates in solution
Solution:
Let's first calculate the molality of the solution.
Molality (m) = 1 mol of solute / 1000 g of solvent
We need to convert the mass of benzene (solvent) to kg.
Mass of benzene = 1000 g
Mass of solute = 1 mol
Molar mass of solute = unknown
Let's assume the molar mass of the solute to be M.
1 mol of solute = M g of solute
Total mass of solution = 1000 g (benzene) + M g (solute)
Molality (m) = 1 mol / (1000 g + M g)
Now, let's use the formula to calculate the degree of association.
ΔTb = Kb ∙ molality ∙ degree of association
1.518 = 2.53 ∙ (1 mol / (1000 g + M g)) ∙ degree of association
Simplifying the equation,
Degree of association = 1.518 ∙ (1000 g + M g) / (2.53 ∙ M)
We can further simplify the equation by assuming the molar mass of the solute to be twice its empirical formula weight (dimerization assumption).
Molar mass of solute = 2 ∙ Empirical formula weight
Degree of association = 1.518 ∙ (1000 g + 2 ∙ EFw g) / (2.53 ∙ 2 ∙ EFw)
Degree of association = (1.518 ∙ 1000) / (5.06 ∙ EFw + 1000)
Let's assume the empirical formula weight of the acid to be x.
Empirical formula weight (EFw) = x
Molar mass of solute = 2 ∙ x
Substituting the values,
Degree of association = (1.518 ∙ 1000) / (5.06 ∙ x + 1000)
We need to find the value of x that gives a degree of association of 80%.
Degree of association = 0.8 = (1.518 ∙ 1000) / (5.06 ∙ x + 1000)
Solving for x,
x = 90 g/mol
Therefore, the empirical formula of the acid is CH2O2 and the degree of association for dimerization of the acid in benzene is 80%.
Osmotic pressure of 4% (w/v) urea solution is 1.64 atm and that of 3.42% (w/v) cane sugar is 2.46 atm. When equal volume of the above two solutions are mixed, the osmotic pressure of the resulting solution is:- a)1.64 atm
- b)2.46 atm
- c)4.1 atm
- d)2.05 atm
Correct answer is option 'D'. Can you explain this answer?
Osmotic pressure of 4% (w/v) urea solution is 1.64 atm and that of 3.42% (w/v) cane sugar is 2.46 atm. When equal volume of the above two solutions are mixed, the osmotic pressure of the resulting solution is:
a)
1.64 atm
b)
2.46 atm
c)
4.1 atm
d)
2.05 atm

|
Yashvi Roy answered |
Ans.
Method to Solve :
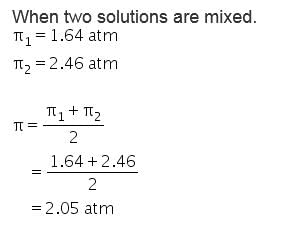
The van’t Hoff factor I for an electrolyte which undergoes dissociation and association in solvent are respectively:- a)Greater than one and less than one.
- b)Less than one and greater than one.
- c)Less than one and less than one.
- d)Greater than one and greater than one.
Correct answer is option 'A'. Can you explain this answer?
The van’t Hoff factor I for an electrolyte which undergoes dissociation and association in solvent are respectively:
a)
Greater than one and less than one.
b)
Less than one and greater than one.
c)
Less than one and less than one.
d)
Greater than one and greater than one.

|
Shruti Datta answered |
Explanation:
The vant Hoff factor, denoted by the symbol 'i', is a measure of the extent of dissociation or association of an electrolyte in a solvent. It is defined as the ratio of the moles of particles formed after dissociation or association to the moles of the electrolyte initially present.
When an electrolyte dissociates into ions in a solvent, the vant Hoff factor is greater than one. This is because each formula unit of the electrolyte breaks down into multiple ions.
On the other hand, when an electrolyte associates or combines with solvent molecules, the vant Hoff factor is less than one. This is because the number of particles formed after association is less than the number of formula units initially present.
Example:
Let's consider the example of table salt (sodium chloride, NaCl) in water. When NaCl dissolves in water, it dissociates into Na+ and Cl- ions. Each formula unit of NaCl dissociates into two ions. Therefore, the vant Hoff factor for NaCl in water is 2.
On the other hand, let's consider the example of acetic acid (CH3COOH) in water. Acetic acid is a weak electrolyte and does not completely dissociate into ions in water. Instead, it associates with water molecules to form hydronium ions (H3O+) and acetate ions (CH3COO-). The number of particles formed after association is greater than the number of formula units of acetic acid initially present. Therefore, the vant Hoff factor for acetic acid in water is less than one.
Conclusion:
In summary, the vant Hoff factor for an electrolyte undergoing dissociation is greater than one, while for an electrolyte undergoing association, it is less than one. Therefore, the correct answer is option 'A' - greater than one and less than one.
The vant Hoff factor, denoted by the symbol 'i', is a measure of the extent of dissociation or association of an electrolyte in a solvent. It is defined as the ratio of the moles of particles formed after dissociation or association to the moles of the electrolyte initially present.
When an electrolyte dissociates into ions in a solvent, the vant Hoff factor is greater than one. This is because each formula unit of the electrolyte breaks down into multiple ions.
On the other hand, when an electrolyte associates or combines with solvent molecules, the vant Hoff factor is less than one. This is because the number of particles formed after association is less than the number of formula units initially present.
Example:
Let's consider the example of table salt (sodium chloride, NaCl) in water. When NaCl dissolves in water, it dissociates into Na+ and Cl- ions. Each formula unit of NaCl dissociates into two ions. Therefore, the vant Hoff factor for NaCl in water is 2.
On the other hand, let's consider the example of acetic acid (CH3COOH) in water. Acetic acid is a weak electrolyte and does not completely dissociate into ions in water. Instead, it associates with water molecules to form hydronium ions (H3O+) and acetate ions (CH3COO-). The number of particles formed after association is greater than the number of formula units of acetic acid initially present. Therefore, the vant Hoff factor for acetic acid in water is less than one.
Conclusion:
In summary, the vant Hoff factor for an electrolyte undergoing dissociation is greater than one, while for an electrolyte undergoing association, it is less than one. Therefore, the correct answer is option 'A' - greater than one and less than one.
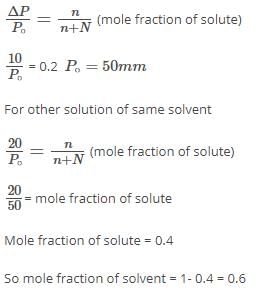
The vapour pressure of a solvent decreased by 10 mm Hg when a non-volatile solute was added to the solvent. The mole fraction of solute in solution is 0.2, what would be mole fraction of the solvent in decrease in vapour pressure is 20 mm of Hg:- a)0.8
- b)0.6
- c)0.4
- d)0.2
Correct answer is option 'B'. Can you explain this answer?
The vapour pressure of a solvent decreased by 10 mm Hg when a non-volatile solute was added to the solvent. The mole fraction of solute in solution is 0.2, what would be mole fraction of the solvent in decrease in vapour pressure is 20 mm of Hg:
a)
0.8
b)
0.6
c)
0.4
d)
0.2

|
Bijoy Kapoor answered |
The freezing point of a solution of acetic acid (mole fraction is 0.02) in benzene is 277.4 K. Acetic acid exists partly as a dimer. Freezing point of benzene is 278.4 K and Kf for benzene is 5 K kg mol–1. The equilibrium constant for dimerisation is ______ kg mol–1:
Correct answer is '3.19'. Can you explain this answer?
The freezing point of a solution of acetic acid (mole fraction is 0.02) in benzene is 277.4 K. Acetic acid exists partly as a dimer. Freezing point of benzene is 278.4 K and Kf for benzene is 5 K kg mol–1. The equilibrium constant for dimerisation is ______ kg mol–1:

|
Sarthak Chavan answered |
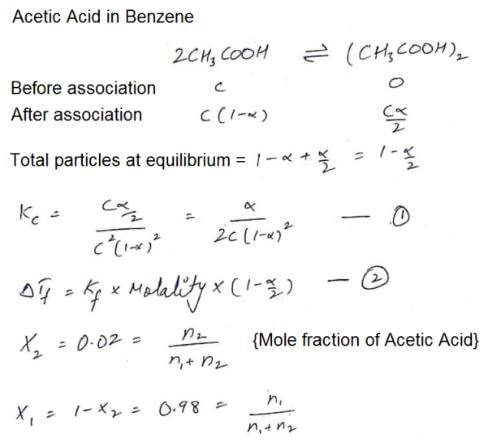
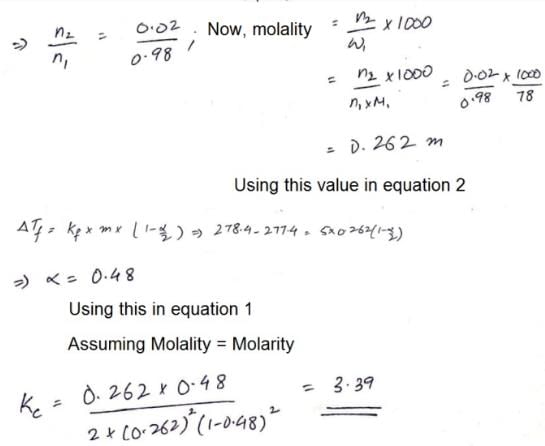
Hence 3.39 is correct
Which of the following aqueous solutions has the highest boiling point & minimum freezing point?- a)0.1 M KNO3
- b)0.1 M Na3PO4
- c)0.1 M BaCl2
- d)0.1 M K2SO4
Correct answer is option 'B'. Can you explain this answer?
Which of the following aqueous solutions has the highest boiling point & minimum freezing point?
a)
0.1 M KNO3
b)
0.1 M Na3PO4
c)
0.1 M BaCl2
d)
0.1 M K2SO4

|
Aashish Shrivastava answered |
Colligative property like elevation in boiling point and depression in freezing point is related to the no of moles or particles of substance, if the moles are larger the colligative properties are large
in this case Na3PO4 dissociate to give 4 ions for the same concentration 1M as:-
Na3PO4 -----> 3 Na+ + PO43-
total 4 ions more than that of other options
in this case Na3PO4 dissociate to give 4 ions for the same concentration 1M as:-
Na3PO4 -----> 3 Na+ + PO43-
total 4 ions more than that of other options
The ratio of the value of any colligative property for KCl solution to that for sugar is nearly ____ time:- a)0.1
- b)1
- c)2
- d)2.5
Correct answer is option 'C'. Can you explain this answer?
The ratio of the value of any colligative property for KCl solution to that for sugar is nearly ____ time:
a)
0.1
b)
1
c)
2
d)
2.5

|
Bijoy Kapoor answered |
Ionic compound will dissociate nd covlent will not
KCl======> K+ + Cl-
colligative property depends on no of particles
kcl have 2 particles bt sugar has only one therefore KCl/sugar = 2/1
When a non-volatile solute is added to a pure solvent, which statement(s) hold true:- a)V.P. of the solution becomes lower than V.P. of pure solvent.
- b)Rate of evaporation of pure solvent is reduced.
- c)Solute does not affect the rate of condensation.
- d)None of these.
Correct answer is option 'A,B,C'. Can you explain this answer?
When a non-volatile solute is added to a pure solvent, which statement(s) hold true:
a)
V.P. of the solution becomes lower than V.P. of pure solvent.
b)
Rate of evaporation of pure solvent is reduced.
c)
Solute does not affect the rate of condensation.
d)
None of these.
|
|
Pooja Choudhury answered |
-A non-volatile substance refers to a substance that does not readily evaporate into a gas under existing conditions. Non-volatile substances exhibit a low vapor pressure and a high boiling point. Sugar and salt are examples of non-volatile solutes.
-Pure solvent means absence of other substances or impurities. For example From Water and salt solution , Water is pure solvent while because of presence of salt it becomes impure.
-
When a non-volatile solute is added to a pure solvent,
V.P. of the solution becomes lower than V.P. of pure solvent.
Rate of evaporation of pure solvent is reduced.
Solute does not affect the rate of condensation.
An ideal solution has two components A and B. A is more volatile than B, i. e., 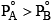 and also
and also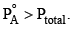 If XA and YA are mole fractions of components A in liquid and vapour phases, then
If XA and YA are mole fractions of components A in liquid and vapour phases, then- a)XA = YA
- b)XA > YA
- c)XA < YA
- d)Data insufficient.
Correct answer is option 'C'. Can you explain this answer?
An ideal solution has two components A and B. A is more volatile than B, i. e., 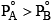 and also
and also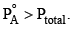 If XA and YA are mole fractions of components A in liquid and vapour phases, then
If XA and YA are mole fractions of components A in liquid and vapour phases, then
a)
XA = YA
b)
XA > YA
c)
XA < YA
d)
Data insufficient.

|
Dhanalakhsmi K N answered |
More volatile component have more its molecules at vapour Phase than in liquid phase..
So mole fraction of A will be higher in vapor phase than in liquid phase.
So mole fraction of A will be higher in vapor phase than in liquid phase.
An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase:- a)Addition of water.
- b)Addition of NaCl
- c)Addition of Na2SO4
- d)Addition of 1.0 molal KI
Correct answer is option 'A'. Can you explain this answer?
An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase:
a)
Addition of water.
b)
Addition of NaCl
c)
Addition of Na2SO4
d)
Addition of 1.0 molal KI

|
Sagarika Patel answered |
Addition of water
Vapour pressure depends upon the surface area of the solution. Larger the surface area, higher is the vapour pressure.
The addition of solute decreases the vapour pressure as some sites of the surface are occupied by solute particles, resulting in decreased surface area. However, the addition of solvent, ie, dilution increases the surface area of the liquid surface, thus results in increased vapour pressure.
Hence, the addition of water to the aqueous solution of (1 molal) KI, result in increased vapour pressure.
The Vant Hoff factor is:- a)Less than one in case of dissociation.
- b)Greater than one in case of dissociation
- c)Less than one in case of association.
- d)Greater than one in case of association
Correct answer is option 'B,C'. Can you explain this answer?
The Vant Hoff factor is:
a)
Less than one in case of dissociation.
b)
Greater than one in case of dissociation
c)
Less than one in case of association.
d)
Greater than one in case of association

|
Saranya Mehta answered |
In case of association of solute particles in solution, the observed molecular weight of solute being more than the normal, the value of factor 'i' is less than unity (i.e. i < 1), while for dissociation the value of i is greater than unity (i.e. i > 1), because the observed molecular weight has lesser value than normal molecular weight.
Moles of Na2SO4 to be dissolved in 12 mole water to lower its vapour pressure by 10 mm Hg at a temperature at which vapour pressure of pure water is 50 mm is:- a)1.5 mole.
- b)2 mole.
- c)3 mole.
- d)1 mole.
Correct answer is option 'C'. Can you explain this answer?
Moles of Na2SO4 to be dissolved in 12 mole water to lower its vapour pressure by 10 mm Hg at a temperature at which vapour pressure of pure water is 50 mm is:
a)
1.5 mole.
b)
2 mole.
c)
3 mole.
d)
1 mole.

|
Varun Yadav answered |
Given data:
Vapour pressure of pure water (P°) = 50 mm Hg
Change in vapour pressure (ΔP) = 10 mm Hg
Moles of water (n) = 12 mol
To calculate the moles of Na2SO4 required to lower the vapour pressure of water by 10 mm Hg, we can use the formula:
ΔP/P° = X2
where X2 is the mole fraction of solute (Na2SO4) in the solution.
Rearranging the formula, we get:
X2 = ΔP/P°
Substituting the given values, we get:
X2 = 10/50 = 0.2
Now, we know that the mole fraction of solute is given by:
X2 = moles of solute (Na2SO4)/(moles of solute + moles of solvent)
Since we are dissolving Na2SO4 in water, the solvent is water and the moles of solvent are given as 12 mol. Therefore, we can write:
0.2 = moles of Na2SO4/(moles of Na2SO4 + 12)
On solving, we get:
moles of Na2SO4 = 3
Hence, the correct option is (c) 3 mole.
Vapour pressure of pure water (P°) = 50 mm Hg
Change in vapour pressure (ΔP) = 10 mm Hg
Moles of water (n) = 12 mol
To calculate the moles of Na2SO4 required to lower the vapour pressure of water by 10 mm Hg, we can use the formula:
ΔP/P° = X2
where X2 is the mole fraction of solute (Na2SO4) in the solution.
Rearranging the formula, we get:
X2 = ΔP/P°
Substituting the given values, we get:
X2 = 10/50 = 0.2
Now, we know that the mole fraction of solute is given by:
X2 = moles of solute (Na2SO4)/(moles of solute + moles of solvent)
Since we are dissolving Na2SO4 in water, the solvent is water and the moles of solvent are given as 12 mol. Therefore, we can write:
0.2 = moles of Na2SO4/(moles of Na2SO4 + 12)
On solving, we get:
moles of Na2SO4 = 3
Hence, the correct option is (c) 3 mole.
Which solution has the highest vapour pressure?- a)0.02 M NaCl at 50°C
- b)0.03 M sucrose at 15°C
- c)0.005 M CaCl2 at 50°C
- d)0.005 M CaCl2 at 25°C
Correct answer is option 'C'. Can you explain this answer?
Which solution has the highest vapour pressure?
a)
0.02 M NaCl at 50°C
b)
0.03 M sucrose at 15°C
c)
0.005 M CaCl2 at 50°C
d)
0.005 M CaCl2 at 25°C

|
Athul Chaudhary answered |
Solution with highest vapour pressure
Vapour pressure is the pressure exerted by the vapours above the surface of the liquid when the liquid and its vapours are in dynamic equilibrium at a particular temperature. The higher the vapour pressure, the more easily the liquid will evaporate.
To determine which solution has the highest vapour pressure, we need to compare the mole fraction of the solute in each solution. The solution with the lowest mole fraction of solute will have the highest vapour pressure.
Mole fraction of solute
The mole fraction of solute in a solution is given by the following formula:
mole fraction of solute = moles of solute / total moles of solution
Let's calculate the mole fraction of solute for each solution:
a) 0.02 M NaCl at 50°C
NaCl is a strong electrolyte and dissociates completely in water. Therefore, 0.02 M NaCl solution contains 0.02 moles of Na+ ions and 0.02 moles of Cl- ions per litre of solution. The total moles of solution is 0.02 + 0.02 = 0.04 moles per litre.
mole fraction of NaCl = 0.02 / 0.04 = 0.5
b) 0.03 M sucrose at 15°C
Sucrose is a non-electrolyte and does not dissociate in water. Therefore, 0.03 M sucrose solution contains 0.03 moles of sucrose per litre of solution. The total moles of solution is 0.03 moles per litre.
mole fraction of sucrose = 0.03 / 0.03 = 1
c) 0.005 M CaCl2 at 50°C
CaCl2 is a strong electrolyte and dissociates completely in water. Therefore, 0.005 M CaCl2 solution contains 0.01 moles of Ca2+ ions and 0.01 moles of Cl- ions per litre of solution. The total moles of solution is 0.01 + 0.01 = 0.02 moles per litre.
mole fraction of CaCl2 = 0.005 / 0.02 = 0.25
d) 0.005 M CaCl2 at 25°C
The mole fraction of CaCl2 in this solution will be the same as in solution c) because the concentration of the solution is the same.
Conclusion
Comparing the mole fractions of solute in each solution, we can see that solution c) 0.005 M CaCl2 at 50°C has the lowest mole fraction of solute (0.25) and therefore the highest vapour pressure. This means that CaCl2 solution will evaporate more easily than the other solutions.
Vapour pressure is the pressure exerted by the vapours above the surface of the liquid when the liquid and its vapours are in dynamic equilibrium at a particular temperature. The higher the vapour pressure, the more easily the liquid will evaporate.
To determine which solution has the highest vapour pressure, we need to compare the mole fraction of the solute in each solution. The solution with the lowest mole fraction of solute will have the highest vapour pressure.
Mole fraction of solute
The mole fraction of solute in a solution is given by the following formula:
mole fraction of solute = moles of solute / total moles of solution
Let's calculate the mole fraction of solute for each solution:
a) 0.02 M NaCl at 50°C
NaCl is a strong electrolyte and dissociates completely in water. Therefore, 0.02 M NaCl solution contains 0.02 moles of Na+ ions and 0.02 moles of Cl- ions per litre of solution. The total moles of solution is 0.02 + 0.02 = 0.04 moles per litre.
mole fraction of NaCl = 0.02 / 0.04 = 0.5
b) 0.03 M sucrose at 15°C
Sucrose is a non-electrolyte and does not dissociate in water. Therefore, 0.03 M sucrose solution contains 0.03 moles of sucrose per litre of solution. The total moles of solution is 0.03 moles per litre.
mole fraction of sucrose = 0.03 / 0.03 = 1
c) 0.005 M CaCl2 at 50°C
CaCl2 is a strong electrolyte and dissociates completely in water. Therefore, 0.005 M CaCl2 solution contains 0.01 moles of Ca2+ ions and 0.01 moles of Cl- ions per litre of solution. The total moles of solution is 0.01 + 0.01 = 0.02 moles per litre.
mole fraction of CaCl2 = 0.005 / 0.02 = 0.25
d) 0.005 M CaCl2 at 25°C
The mole fraction of CaCl2 in this solution will be the same as in solution c) because the concentration of the solution is the same.
Conclusion
Comparing the mole fractions of solute in each solution, we can see that solution c) 0.005 M CaCl2 at 50°C has the lowest mole fraction of solute (0.25) and therefore the highest vapour pressure. This means that CaCl2 solution will evaporate more easily than the other solutions.
An azeotropic mixture of two liquids has a boiling point higher than either of them when it:- a)Shows positive deviation from Raoult’s law.
- b)Shows negative deviation from Raoult’s law.
- c)Shows ideal behaviour.
- d)Is saturated.
Correct answer is option 'B'. Can you explain this answer?
An azeotropic mixture of two liquids has a boiling point higher than either of them when it:
a)
Shows positive deviation from Raoult’s law.
b)
Shows negative deviation from Raoult’s law.
c)
Shows ideal behaviour.
d)
Is saturated.
|
|
Rajeev Sharma answered |
Azeotropic mixture of two liquids has lower B.P. if two liquids are dissolved and their boiling points will be different and they will increase either when there is one liquid the boiling point will be low.
So Option B is correct.
Among the following the solution which shows the highest osmotic pressure at 25ºC is:- a)0.05 M NaCl
- b)0.05 M FeCl3
- c)0.1 M BaCl2
- d)0.05 M Na2SO4
Correct answer is option 'C'. Can you explain this answer?
Among the following the solution which shows the highest osmotic pressure at 25ºC is:
a)
0.05 M NaCl
b)
0.05 M FeCl3
c)
0.1 M BaCl2
d)
0.05 M Na2SO4

|
Aryan Gupta answered |
Explanation:
Osmotic pressure is defined as the pressure required to stop the flow of solvent molecules across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration.
The osmotic pressure is directly proportional to the concentration of the solute in the solution. Therefore, the higher the concentration of the solute, the higher will be the osmotic pressure.
Among the given options, BaCl2 has the highest osmotic pressure at 25°C. This is because BaCl2 is a strong electrolyte, which dissociates completely into Ba2+ and 2Cl- ions in aqueous solution.
BaCl2 → Ba2+ + 2Cl-
Therefore, the concentration of solute particles in 0.1 M BaCl2 solution is three times higher than that in 0.05 M NaCl or Na2SO4 solutions, and twice as high as that in 0.05 M FeCl3 solution.
Hence, the correct answer is option C, 0.1 M BaCl2.
Osmotic pressure is defined as the pressure required to stop the flow of solvent molecules across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration.
The osmotic pressure is directly proportional to the concentration of the solute in the solution. Therefore, the higher the concentration of the solute, the higher will be the osmotic pressure.
Among the given options, BaCl2 has the highest osmotic pressure at 25°C. This is because BaCl2 is a strong electrolyte, which dissociates completely into Ba2+ and 2Cl- ions in aqueous solution.
BaCl2 → Ba2+ + 2Cl-
Therefore, the concentration of solute particles in 0.1 M BaCl2 solution is three times higher than that in 0.05 M NaCl or Na2SO4 solutions, and twice as high as that in 0.05 M FeCl3 solution.
Hence, the correct answer is option C, 0.1 M BaCl2.
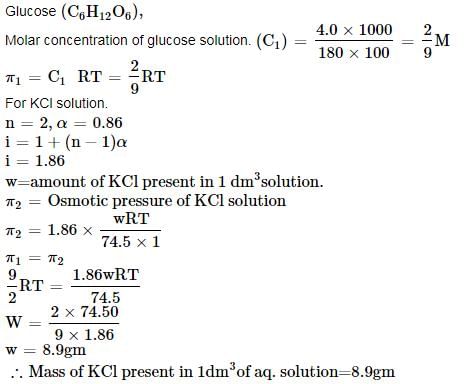
The apparent degree of ionization of KCl in water at 290 K is 0.86, the mass of KCl which must be made up to 1 dm3 of aqueous solution so as to produce the same osmotic pressure as the 4% solution of glucose at that temperature is ______g.
Correct answer is '8.9'. Can you explain this answer?
The apparent degree of ionization of KCl in water at 290 K is 0.86, the mass of KCl which must be made up to 1 dm3 of aqueous solution so as to produce the same osmotic pressure as the 4% solution of glucose at that temperature is ______g.

|
Soumya Sengupta answered |
Select correct statement:- a)Solution has more molecular randomness than a pure solvent has, the entropy change between solution and solid is larger than the entropy change between pure solvent and solid.
- b)Heat of fusion of solution and solvent are similar since similar forces of intermolecular forces are involved.
- c)Sugar containing solution freezes at a lower temperature than pure water.
- d)All are correct statements.
Correct answer is option 'D'. Can you explain this answer?
Select correct statement:
a)
Solution has more molecular randomness than a pure solvent has, the entropy change between solution and solid is larger than the entropy change between pure solvent and solid.
b)
Heat of fusion of solution and solvent are similar since similar forces of intermolecular forces are involved.
c)
Sugar containing solution freezes at a lower temperature than pure water.
d)
All are correct statements.
|
|
Pooja Choudhury answered |
A) Entropy of solution
>
Entropy of pure solvent
(
Δ
S
)
solution-solid
>
(
Δ
S
)
solvent-solid
In solution, number of particle is greater so solution have greater entropy.
(B) Only solvent involved in solid-liquid equilibrium.
D is the correct answer
The azeotropics mixture of water (B.P. = 100°C) and HCl (B.P. = 86°C) boils at about 120°C. During fractional distillation of this mixture it is possible to obtain:- a)Pure HCl
- b)Pure H2O
- c)Pure H2O as well as pure HCl
- d)Neither H2O nor HCl.
Correct answer is option 'D'. Can you explain this answer?
The azeotropics mixture of water (B.P. = 100°C) and HCl (B.P. = 86°C) boils at about 120°C. During fractional distillation of this mixture it is possible to obtain:
a)
Pure HCl
b)
Pure H2O
c)
Pure H2O as well as pure HCl
d)
Neither H2O nor HCl.

|
Amrendra Kumar answered |
It is a chemical rxn.When we mix water with acid product wiil be in ionic form. so we cannot separate .
A compound has the empirical formula C10H8Fe. A solution of 0.26 g of the compound in 11.2 g of benzene (C6H6) boils at 80.26°C. The boiling point of benzene is 80.10°C; the Kb is 2.53°C/molal. What is the molecular formula of the compound?- a)C30H24Fe3
- b)C10H8Fe
- c)C5H4Fe
- d)C20H16Fe2
Correct answer is option 'D'. Can you explain this answer?
A compound has the empirical formula C10H8Fe. A solution of 0.26 g of the compound in 11.2 g of benzene (C6H6) boils at 80.26°C. The boiling point of benzene is 80.10°C; the Kb is 2.53°C/molal. What is the molecular formula of the compound?
a)
C30H24Fe3
b)
C10H8Fe
c)
C5H4Fe
d)
C20H16Fe2

|
Stuti Patel answered |
Given data:
Empirical formula of the compound = C10H8Fe
Mass of the compound = 0.26 g
Mass of benzene = 11.2 g
Boiling point of benzene = 80.10C
Boiling point of solution = 80.26C
Kb of benzene = 2.53C/molal
To find: Molecular formula of the compound
Steps involved:
1. Calculate the molality of the solution
2. Calculate the change in boiling point of the solution
3. Calculate the number of moles of the compound in the solution
4. Calculate the molar mass of the compound
5. Find the molecular formula of the compound
1. Calculation of Molality:
Molality of the solution, m = (moles of solute) / (mass of solvent in kg)
Here, mass of solvent = 11.2 g = 0.0112 kg
Empirical formula weight of C10H8Fe = 160 g/mol
Moles of C10H8Fe in 0.26 g = 0.26 g / 160 g/mol = 0.001625 mol
Molality (m) = 0.001625 mol / 0.0112 kg = 0.145 mol/kg
2. Calculation of Change in Boiling Point (∆Tb):
∆Tb = Kb x m
Here, Kb = 2.53C/molal and m = 0.145 mol/kg
∆Tb = 2.53 C/molal x 0.145 mol/kg = 0.366 C
3. Calculation of Moles of the Compound:
∆Tb = Kb x m = (Kb x w2) / (Mf x Kb)
Here, w2 = mass of the compound = 0.26 g
Mf = molar mass of the compound
0.366 C = (2.53 C/molal x 0.26 g) / (Mf x 0.0112 kg)
Mf = 346.3 g/mol
Moles of the compound = 0.26 g / 346.3 g/mol = 0.000750 mol
4. Calculation of Molar Mass of the Compound:
Empirical formula weight of C10H8Fe = 160 g/mol
Molecular formula weight of the compound = 346.3 g/mol
Ratio of molecular formula weight to empirical formula weight = 346.3 g/mol / 160 g/mol = 2.165
The molecular formula of the compound should have a ratio of 2.165 times the empirical formula weight.
Therefore, the molecular formula of the compound = 2.165 x C10H8Fe = C20H16Fe2
Hence, the correct answer is option D, C20H16Fe2.
Empirical formula of the compound = C10H8Fe
Mass of the compound = 0.26 g
Mass of benzene = 11.2 g
Boiling point of benzene = 80.10C
Boiling point of solution = 80.26C
Kb of benzene = 2.53C/molal
To find: Molecular formula of the compound
Steps involved:
1. Calculate the molality of the solution
2. Calculate the change in boiling point of the solution
3. Calculate the number of moles of the compound in the solution
4. Calculate the molar mass of the compound
5. Find the molecular formula of the compound
1. Calculation of Molality:
Molality of the solution, m = (moles of solute) / (mass of solvent in kg)
Here, mass of solvent = 11.2 g = 0.0112 kg
Empirical formula weight of C10H8Fe = 160 g/mol
Moles of C10H8Fe in 0.26 g = 0.26 g / 160 g/mol = 0.001625 mol
Molality (m) = 0.001625 mol / 0.0112 kg = 0.145 mol/kg
2. Calculation of Change in Boiling Point (∆Tb):
∆Tb = Kb x m
Here, Kb = 2.53C/molal and m = 0.145 mol/kg
∆Tb = 2.53 C/molal x 0.145 mol/kg = 0.366 C
3. Calculation of Moles of the Compound:
∆Tb = Kb x m = (Kb x w2) / (Mf x Kb)
Here, w2 = mass of the compound = 0.26 g
Mf = molar mass of the compound
0.366 C = (2.53 C/molal x 0.26 g) / (Mf x 0.0112 kg)
Mf = 346.3 g/mol
Moles of the compound = 0.26 g / 346.3 g/mol = 0.000750 mol
4. Calculation of Molar Mass of the Compound:
Empirical formula weight of C10H8Fe = 160 g/mol
Molecular formula weight of the compound = 346.3 g/mol
Ratio of molecular formula weight to empirical formula weight = 346.3 g/mol / 160 g/mol = 2.165
The molecular formula of the compound should have a ratio of 2.165 times the empirical formula weight.
Therefore, the molecular formula of the compound = 2.165 x C10H8Fe = C20H16Fe2
Hence, the correct answer is option D, C20H16Fe2.
An ideal solution is formed by mixing two volatile liquids A and B, XA and XB are the mole fractions of A and B respectively in the solution and YA and YB are the mole fractions of A and B respectively in the vapour phase. A plot of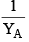 along y–axis against
along y–axis against 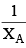 along x–axis gives a straight line. What is the slope of the straight line?
along x–axis gives a straight line. What is the slope of the straight line?- a)
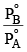
- b)

- c)
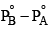
- d)
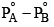
Correct answer is option 'A'. Can you explain this answer?
An ideal solution is formed by mixing two volatile liquids A and B, XA and XB are the mole fractions of A and B respectively in the solution and YA and YB are the mole fractions of A and B respectively in the vapour phase. A plot of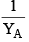 along y–axis against
along y–axis against 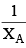 along x–axis gives a straight line. What is the slope of the straight line?
along x–axis gives a straight line. What is the slope of the straight line?
a)
b)
c)
d)
|
|
Kritika Dhingra answered |

An aqueous solution containing 288 g of a non–volatile compound having the stoichiometric composit ion CxH2xOx in 90 g water boils at 101.24ºC at 1 atmospheric pressure. What is the value of x? (Given that: Kb(H2O) = 0.512 K Kg mol–1):
Correct answer is '44'. Can you explain this answer?
An aqueous solution containing 288 g of a non–volatile compound having the stoichiometric composit ion CxH2xOx in 90 g water boils at 101.24ºC at 1 atmospheric pressure. What is the value of x? (Given that: Kb(H2O) = 0.512 K Kg mol–1):

|
Anagha Chauhan answered |
Ans.
Method to Solve :
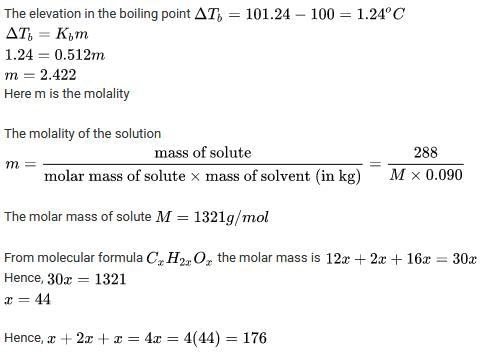
If a solute undergoes dimerisation and trimerisation, the minimum values of Vant Hoff factors respectively are:- a)0.5 and 1.5
- b)1.5 and 1.33
- c)0.25 and 0.67
- d)0.5 and 0.33
Correct answer is option 'D'. Can you explain this answer?
If a solute undergoes dimerisation and trimerisation, the minimum values of Vant Hoff factors respectively are:
a)
0.5 and 1.5
b)
1.5 and 1.33
c)
0.25 and 0.67
d)
0.5 and 0.33

|
Vandana Gupta answered |
Explanation:
The Vant Hoff factor is a measure of how many particles a solute dissociates into when it is dissolved in a solvent. For example, NaCl dissociates into two ions (Na+ and Cl-) when it dissolves in water, so its Vant Hoff factor is 2.
In the case of a solute that undergoes dimerisation and trimerisation, the Vant Hoff factor will be affected. Let's consider each case separately:
Dimerisation
When a solute undergoes dimerisation, it means that two molecules of the solute combine to form a dimer. This reduces the number of particles in solution, which in turn affects the Vant Hoff factor.
The minimum value of the Vant Hoff factor for dimerisation is 0.5. This is because the solute has combined into two particles, so the Vant Hoff factor is halved.
Trimerisation
When a solute undergoes trimerisation, it means that three molecules of the solute combine to form a trimer. This further reduces the number of particles in solution, which affects the Vant Hoff factor even more.
The minimum value of the Vant Hoff factor for trimerisation is 0.33. This is because the solute has combined into three particles, so the Vant Hoff factor is reduced to one third.
Overall, the minimum values of the Vant Hoff factors for dimerisation and trimerisation are 0.5 and 0.33, respectively. This means that when a solute undergoes both processes, the minimum value of the Vant Hoff factor will be 0.5 for dimerisation and 0.33 for trimerisation, giving a total minimum Vant Hoff factor of 0.5 + 0.33 = 0.83. However, option D suggests 0.5 and 0.33 as the minimum values for dimerisation and trimerisation, respectively, which is correct.
The Vant Hoff factor is a measure of how many particles a solute dissociates into when it is dissolved in a solvent. For example, NaCl dissociates into two ions (Na+ and Cl-) when it dissolves in water, so its Vant Hoff factor is 2.
In the case of a solute that undergoes dimerisation and trimerisation, the Vant Hoff factor will be affected. Let's consider each case separately:
Dimerisation
When a solute undergoes dimerisation, it means that two molecules of the solute combine to form a dimer. This reduces the number of particles in solution, which in turn affects the Vant Hoff factor.
The minimum value of the Vant Hoff factor for dimerisation is 0.5. This is because the solute has combined into two particles, so the Vant Hoff factor is halved.
Trimerisation
When a solute undergoes trimerisation, it means that three molecules of the solute combine to form a trimer. This further reduces the number of particles in solution, which affects the Vant Hoff factor even more.
The minimum value of the Vant Hoff factor for trimerisation is 0.33. This is because the solute has combined into three particles, so the Vant Hoff factor is reduced to one third.
Overall, the minimum values of the Vant Hoff factors for dimerisation and trimerisation are 0.5 and 0.33, respectively. This means that when a solute undergoes both processes, the minimum value of the Vant Hoff factor will be 0.5 for dimerisation and 0.33 for trimerisation, giving a total minimum Vant Hoff factor of 0.5 + 0.33 = 0.83. However, option D suggests 0.5 and 0.33 as the minimum values for dimerisation and trimerisation, respectively, which is correct.
Select correct statement?- a)Heats of vaporization for a pure solvent and for a solution are similar because similar intermolecular forces between solvent molecules must be overcome in both cases.
- b)Entropy change between solution and vapour is smaller than the entropy change between pure solvent and vapour.
- c)Boiling point of the solution is larger than that of the pure solvent.
- d)All are correct statements.
Correct answer is option 'D'. Can you explain this answer?
Select correct statement?
a)
Heats of vaporization for a pure solvent and for a solution are similar because similar intermolecular forces between solvent molecules must be overcome in both cases.
b)
Entropy change between solution and vapour is smaller than the entropy change between pure solvent and vapour.
c)
Boiling point of the solution is larger than that of the pure solvent.
d)
All are correct statements.
|
|
Pooja Choudhury answered |
Answer :
- d)All are correct statements.
option (a) is also a a correct statement because the heats of vaporization of pure solvent and solution are similar because similar forces of attraction must be overcome so that
the solvent molecules can break free from the surface and escape as vapours.
Entropy change between solution and vapour is smaller than the entropy change between pure solvent and vapour. Boiling point of the solution is larger than that of the pure solvent.
An aqueous solution of glucose boils at 100.01ºC. The mo lal elevat ion constant for water is 0.5 K Kg mol–1. The number of glucose molecules in the solution containing 100 g of water are x × 1021. The value of x is ______.
Correct answer is '1.2'. Can you explain this answer?
An aqueous solution of glucose boils at 100.01ºC. The mo lal elevat ion constant for water is 0.5 K Kg mol–1. The number of glucose molecules in the solution containing 100 g of water are x × 1021. The value of x is ______.

|
Sinjini Nair answered |
Solution:
Given, boiling point elevation = 0.01C
Molal elevation constant, Kf = 0.5 K Kg mol-1
Mass of water, w2 = 100 g
Number of glucose molecules in solution = x x 1021
We know that the boiling point elevation is given by the equation:
ΔTb = Kf × m
where ΔTb is the boiling point elevation, Kf is the molal elevation constant and m is the molality of the solution.
To find the molality of the solution, we need to know the mass of glucose in the solution and the mass of water in the solution.
Let the mass of glucose in the solution be w1.
Then, the total mass of the solution is given by:
w = w1 + w2
The molality of the solution is given by:
m = nglucose / mwater
where nglucose is the number of moles of glucose and mwater is the mass of water in kg.
The number of moles of glucose is given by:
nglucose = w1 / Mglucose
where Mglucose is the molar mass of glucose.
Substituting the values, we get:
ΔTb = Kf × nglucose / mwater
Solving for nglucose, we get:
nglucose = ΔTb × mwater / Kf
Substituting the given values, we get:
nglucose = 0.01 × 100 / 0.5
nglucose = 2
The number of moles of glucose in the solution is 2.
The number of glucose molecules in the solution is given by:
nglucose = N / Avogadro's number
where N is the number of glucose molecules and Avogadro's number is 6.022 x 1023 mol-1.
Substituting the values, we get:
N = nglucose × Avogadro's number
N = 2 × 6.022 x 1023
N = 1.2044 x 1024
The number of glucose molecules in the solution is 1.2044 x 1024.
Rounding off to one significant figure, we get:
x = 1.2
Therefore, the value of x is 1.2.
Given, boiling point elevation = 0.01C
Molal elevation constant, Kf = 0.5 K Kg mol-1
Mass of water, w2 = 100 g
Number of glucose molecules in solution = x x 1021
We know that the boiling point elevation is given by the equation:
ΔTb = Kf × m
where ΔTb is the boiling point elevation, Kf is the molal elevation constant and m is the molality of the solution.
To find the molality of the solution, we need to know the mass of glucose in the solution and the mass of water in the solution.
Let the mass of glucose in the solution be w1.
Then, the total mass of the solution is given by:
w = w1 + w2
The molality of the solution is given by:
m = nglucose / mwater
where nglucose is the number of moles of glucose and mwater is the mass of water in kg.
The number of moles of glucose is given by:
nglucose = w1 / Mglucose
where Mglucose is the molar mass of glucose.
Substituting the values, we get:
ΔTb = Kf × nglucose / mwater
Solving for nglucose, we get:
nglucose = ΔTb × mwater / Kf
Substituting the given values, we get:
nglucose = 0.01 × 100 / 0.5
nglucose = 2
The number of moles of glucose in the solution is 2.
The number of glucose molecules in the solution is given by:
nglucose = N / Avogadro's number
where N is the number of glucose molecules and Avogadro's number is 6.022 x 1023 mol-1.
Substituting the values, we get:
N = nglucose × Avogadro's number
N = 2 × 6.022 x 1023
N = 1.2044 x 1024
The number of glucose molecules in the solution is 1.2044 x 1024.
Rounding off to one significant figure, we get:
x = 1.2
Therefore, the value of x is 1.2.
Chapter doubts & questions for Solutions & Colligative Properties - Physical Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Solutions & Colligative Properties - Physical Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Physical Chemistry
83 videos|142 docs|67 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup