All Exams >
Mechanical Engineering >
GATE Mechanical (ME) Mock Test Series 2026 >
All Questions
All questions of Thermodynamics for Mechanical Engineering Exam
Assertion (a): If an alcohol and a mercury thermom... moreeter read exactly 0°C at the ice point and 100°C at the steam point and the distance between the two points is divided into 100 equal parts in both thermometers, the two thermometers will give exactly the same reading at 50°C.Reason (R): Temperature scales are arbitrary.a)Both A and R are individually true and R is the correct explanation of Ab)Both A and R are individually true but R is NOT the correct explanation of Ac)A is true but R is falsed)A is false but R is trueCorrect answer is option 'D'. Can you explain this answer?
a)Both A and R are individually true and R is the correct explanation of A
b)Both A and R are individually true but R is NOT the correct explanation of A
c)A is true but R is false
d)A is false but R is true
Correct answer is option 'D'. Can you explain this answer?
|
|
Nilanjan Goyal answered |
Because firstly they are of different material they have different thermal expansion etc and secondly no 2 things whether of same company can tel u the same.
Eg. if u purchase the weighing machine of 2 companies even they can' t tell the same weight .. i.e some amt of deflection will be there
Eg. if u purchase the weighing machine of 2 companies even they can' t tell the same weight .. i.e some amt of deflection will be there
Heat and work are - a)intensive properties
- b)extensive properties
- c)point functions
- d)path functions
Correct answer is option 'D'. Can you explain this answer?
Heat and work are
a)
intensive properties
b)
extensive properties
c)
point functions
d)
path functions
|
|
Neha Joshi answered |
Heat and work depend upon the path traversed to move from one thermodynamic state to another. This is why they are known as path functions.
Which of the following are intensive properties?1. Kinetic Energy2. Specific Enthalpy3. Pressure4. EntropySelect the correct answer using the code given below: - a)1 and 3
- b)2 and 3
- c)1, 3 and 4
- d)2 and 4
Correct answer is option 'B'. Can you explain this answer?
Which of the following are intensive properties?
1. Kinetic Energy
2. Specific Enthalpy
3. Pressure
4. Entropy
Select the correct answer using the code given below:
a)
1 and 3
b)
2 and 3
c)
1, 3 and 4
d)
2 and 4
|
|
Sanya Agarwal answered |
Option (b) 2 and 3 is correct.
Specific enthalpy and pressure
Are both intensive properties.
Explanation:-
{ Intensive properties are those that do not depend on the size of the system. Specific enthalpy is the enthalpy on a per gram basis. Since this value is normalized to the per gram basis it does not depend on the size of the system being measured. }
So, both are intensive properties.
So, both are intensive properties.
Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the- a)Zeroth law of thermodynamics
- b)First law of thermodynamics
- c)Second law of thermodynamics
- d)Third law of thermodynamics
Correct answer is option 'A'. Can you explain this answer?
Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the
a)
Zeroth law of thermodynamics
b)
First law of thermodynamics
c)
Second law of thermodynamics
d)
Third law of thermodynamics
|
|
Rhea Reddy answered |
Using conservation of law may find the final temperature.
Assertion (A): Thermodynamic work is path-dependent except for an adiabatic process.Reason(R): It is always possible to take a system from a given initial state to any final state by performing adiabatic work only.- a)Both A and R are individually true and R is the correct explanation of A
- b)Both A and R are individually true but R is NOT the correct explanation of A
- c)A is true but R is false
- d)A is false but R is true
Correct answer is option 'C'. Can you explain this answer?
Assertion (A): Thermodynamic work is path-dependent except for an adiabatic process.
Reason(R): It is always possible to take a system from a given initial state to any final state by performing adiabatic work only.
a)
Both A and R are individually true and R is the correct explanation of A
b)
Both A and R are individually true but R is NOT the correct explanation of A
c)
A is true but R is false
d)
A is false but R is true
|
|
Neha Joshi answered |
It is not possible to take a system from a given initial state to any final state by performing adiabatic work only.
The assertion is true since in an adiabatic process heat transfer is zero and work is equal to change in internal energy.
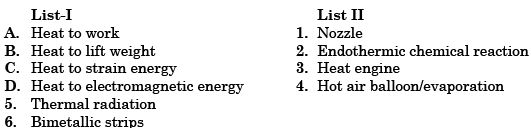
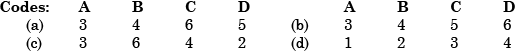
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'A'. Can you explain this answer?

a)
A
b)
B
c)
C
d)
D
|
|
Neha Joshi answered |
A heat engine is a system that converts heat into work by taking heat from the reservoir ( hot body) to carry out some work. There is a discharge of some heat to the sink (cold body).
Hot Air Balloon is used to lift the weight using heat.
A bimetallic strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated. ... This effect is used in a range of mechanical and electrical devices.
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:- a)– 8
- b)Zero
- c)10
- d)–10
Correct answer is option 'B'. Can you explain this answer?
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:
a)
– 8
b)
Zero
c)
10
d)
–10

|
Bhaskar Unni answered |
Internal energy is a property of a system so 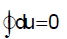
A system comprising a single phase is called a- a)closed system
- b)open system
- c)isolated system
- d)homogeneous system
Correct answer is option 'D'. Can you explain this answer?
A system comprising a single phase is called a
a)
closed system
b)
open system
c)
isolated system
d)
homogeneous system

|
Manasa Bose answered |
Definition of a Single Phase System
A single phase system is a thermodynamic system consisting of a single phase, which means that the system has a uniform physical state throughout. In other words, the system has a uniform composition, temperature, pressure, and density, and there are no phase changes occurring within the system.
Explanation of Each Option
a) Closed System: A closed system is a thermodynamic system that allows energy transfer but not mass transfer across its boundaries. This means that the system is isolated from its surroundings in terms of matter exchange, but energy can be exchanged between the system and its surroundings. A single phase system can be a closed system, but not all closed systems are single phase systems.
b) Open System: An open system is a thermodynamic system that allows both energy and matter transfer across its boundaries. This means that the system can exchange both energy and matter with its surroundings. A single phase system cannot be an open system because it has a uniform physical state throughout and there is no matter exchange.
c) Isolated System: An isolated system is a thermodynamic system that does not allow energy or matter transfer across its boundaries. This means that the system is completely isolated from its surroundings. A single phase system cannot be an isolated system because it has a uniform physical state throughout and there is no energy or matter exchange.
d) Homogeneous System: A homogeneous system is a thermodynamic system that has a uniform physical state throughout. This means that the system has a uniform composition, temperature, pressure, and density, and there are no phase changes occurring within the system. A single phase system is a homogeneous system because it has a uniform physical state throughout.
Conclusion
Therefore, the correct answer is option 'D' - homogeneous system. A single phase system is a thermodynamic system that has a uniform physical state throughout, and therefore, it is a homogeneous system.
A single phase system is a thermodynamic system consisting of a single phase, which means that the system has a uniform physical state throughout. In other words, the system has a uniform composition, temperature, pressure, and density, and there are no phase changes occurring within the system.
Explanation of Each Option
a) Closed System: A closed system is a thermodynamic system that allows energy transfer but not mass transfer across its boundaries. This means that the system is isolated from its surroundings in terms of matter exchange, but energy can be exchanged between the system and its surroundings. A single phase system can be a closed system, but not all closed systems are single phase systems.
b) Open System: An open system is a thermodynamic system that allows both energy and matter transfer across its boundaries. This means that the system can exchange both energy and matter with its surroundings. A single phase system cannot be an open system because it has a uniform physical state throughout and there is no matter exchange.
c) Isolated System: An isolated system is a thermodynamic system that does not allow energy or matter transfer across its boundaries. This means that the system is completely isolated from its surroundings. A single phase system cannot be an isolated system because it has a uniform physical state throughout and there is no energy or matter exchange.
d) Homogeneous System: A homogeneous system is a thermodynamic system that has a uniform physical state throughout. This means that the system has a uniform composition, temperature, pressure, and density, and there are no phase changes occurring within the system. A single phase system is a homogeneous system because it has a uniform physical state throughout.
Conclusion
Therefore, the correct answer is option 'D' - homogeneous system. A single phase system is a thermodynamic system that has a uniform physical state throughout, and therefore, it is a homogeneous system.
A frictionless piston-cylinder device contains a gas initially at 0.8 MPa and 0.015 m3. It expands quasi-statically at constant temperature to a final volume of 0.030 m3. The work output (in kJ/kg) during this process will be: - a)8.32
- b)12.00
- c)554.67
- d)8320.00
Correct answer is option 'A'. Can you explain this answer?
A frictionless piston-cylinder device contains a gas initially at 0.8 MPa and 0.015 m3. It expands quasi-statically at constant temperature to a final volume of 0.030 m3. The work output (in kJ/kg) during this process will be:
a)
8.32
b)
12.00
c)
554.67
d)
8320.00
|
|
Aditya Deshmukh answered |
In an isothermal process
W D=P1V1 ln (V1/ V1)=(0.810^6) (0.015) ln (0.030/0.015) = 8.32kJ
Consider the following properties:1. Entropy2. Viscosity3. Temperature4. Specific heat at constant volumeWhich of the above properties of a system is/are extensive? - a)1 only
- b)1 and 2 only
- c)2, 3 and 4
- d)1, 2 and 4
Correct answer is option 'A'. Can you explain this answer?
Consider the following properties:
1. Entropy
2. Viscosity
3. Temperature
4. Specific heat at constant volume
Which of the above properties of a system is/are extensive?
a)
1 only
b)
1 and 2 only
c)
2, 3 and 4
d)
1, 2 and 4
|
|
Garima Mehta answered |
Extensive property: Whose value depends on the size or extent i.e. mass of the system (upper case letters as the symbols) e.g., Volume, Mass (V, M). If mass is increased, the value of extensive property also increases.
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statements is TRUE at the end of above process? - a)The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
- b)The internal energy of the gas increases from its initial value, but the enthalpy remains constant
- c)Both internal energy and enthalpy of the gas remain constant
- d)Both internal energy and enthalpy of the gas increase
Correct answer is option 'C'. Can you explain this answer?
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statements is TRUE at the end of above process?
a)
The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
b)
The internal energy of the gas increases from its initial value, but the enthalpy remains constant
c)
Both internal energy and enthalpy of the gas remain constant
d)
Both internal energy and enthalpy of the gas increase
|
|
Aditya Deshmukh answered |
h = u + pV
The balloon containing the ideal gas is initially kept in an evacuated and insulated room. Since internal energy is during the process is constant, the flow work pV must also remain constant thus we may conclude that during free expansion process pV, ie. Product of specific volume and pressure change is such a way that their product remains constant. It may however called constant internal energy and constant enthalpy process.
If the clothes of a person working in the kitchen catch fire,the person is immediately covered with a blanket.When the burning clothes of a person are covered with a blanket,the supply of air to the burning clothes is cut off and hence the burning stops.
A control volume is - a)An isolated system
- b)A closed system but heat and work can cross the boundary
- c)A specific amount of mass in space
- d)A fixed region in space where mass, heat and work can cross the boundary of that region
Correct answer is option 'D'. Can you explain this answer?
A control volume is
a)
An isolated system
b)
A closed system but heat and work can cross the boundary
c)
A specific amount of mass in space
d)
A fixed region in space where mass, heat and work can cross the boundary of that region

|
Anirban Khanna answered |
A control volume is a mathematical abstraction employed in the process of creating mathematical models of physical processes. In an inertial frame of reference, it is a volume fixed in space or moving with constant flow velocity through which the continuum (gas, liquid or solid) flows. The surface enclosing the control volume is referred to as the control surface.
At steady state, a control volume can be thought of as an arbitrary volume in which the mass of the continuum remains constant. As a continuum moves through the control volume, the mass entering the control volume is equal to the mass leaving the control volume. At steady state, and in the absence of work and heat transfer, the energy within the control volume remains constant. It is analogous to the classical mechanics concept of the free body diagram.
The entropy of a mixture of ideal gases is the sum of the entropies of constituents evaluated at:- a)Temperature and pressure of the mixture
- b)Temperature of the mixture and the partial pressure of the constituents
- c)Temperature and volume of the mixture
- d)Pressure and volume of the mixture
Correct answer is option 'C'. Can you explain this answer?
The entropy of a mixture of ideal gases is the sum of the entropies of constituents evaluated at:
a)
Temperature and pressure of the mixture
b)
Temperature of the mixture and the partial pressure of the constituents
c)
Temperature and volume of the mixture
d)
Pressure and volume of the mixture

|
Anirban Khanna answered |
The entropy of a mixture of ideal gases is equal to the sum of the entropies of the component gases as they exist in the mixture. We employ the Gibbs-Dalton law that says each gas behaves as if it alone occupies the volume of the system at the mixture temperature. That is, the pressure of each component is the partial pressure.
A gas expands in a frictionless piston-cylinder arrangement. The expansion process is very slow, and is resisted by an ambient pressure of 100 kPa. During the expansion process, the pressure of the system (gas) remains constant at 300 kPa. The change in volume of the gas is 0.01 m3. The maximum amount of work that could be utilized from the above process is: - a)0 kJ
- b)1 kJ
- c)2 kJ
- d)3 kJ
Correct answer is option 'C'. Can you explain this answer?
A gas expands in a frictionless piston-cylinder arrangement. The expansion process is very slow, and is resisted by an ambient pressure of 100 kPa. During the expansion process, the pressure of the system (gas) remains constant at 300 kPa. The change in volume of the gas is 0.01 m3. The maximum amount of work that could be utilized from the above process is:
a)
0 kJ
b)
1 kJ
c)
2 kJ
d)
3 kJ
|
|
Rajeev Menon answered |
Net pressure action on the piston = 300 KPa - 100 KPa
= 200 KPa
(which is constant throughout the expansion process)
and change in volume = dV = 0.01 m^3
W.D in expansion process = pdv
={200*10^3(N/m^2}x(0.01m^3)
= 2 * 10^3 Nm
= 2 KJ
Consider the following: 1. Kinetic energy2. Entropy3. Thermal conductivity4. PressureWhich of these are intensive properties? - a)1, 2 and 3 only
- b)2 and 4 only
- c)3 and 4 only
- d)1, 2, 3 and 4
Correct answer is option 'C'. Can you explain this answer?
Consider the following:
1. Kinetic energy
2. Entropy
3. Thermal conductivity
4. Pressure
Which of these are intensive properties?
a)
1, 2 and 3 only
b)
2 and 4 only
c)
3 and 4 only
d)
1, 2, 3 and 4
|
|
Ravi Singh answered |
Kinetic energy 1/2mv2 depends on mass, Entropy kJ/k depends on mass so Entropy is extensive property but specific entropy kJ/kg K is an intensive property.
The door of a running refrigerator inside a room was left open. Which of the following statements is correct?- a)The room will be cooled to the temperature inside the refrigerator.
- b)The room will be cooled very slightly.
- c)The room will be gradually warmed up.
- d)The temperature of the air in room will remain unaffected.
Correct answer is option 'C'. Can you explain this answer?
The door of a running refrigerator inside a room was left open. Which of the following statements is correct?
a)
The room will be cooled to the temperature inside the refrigerator.
b)
The room will be cooled very slightly.
c)
The room will be gradually warmed up.
d)
The temperature of the air in room will remain unaffected.

|
Paranthaman Sagadevan answered |
We give some energy in form of electricity and it is coverted into heat energy ( amount of heat observed by evaporation) then this heat is dissipated to our environment (room) .
If a closed system is undergoing an irreversible process, the entropy of the system- a)Must increase
- b)Always remains constant
- c)Must decrease
- d)Can increase, decrease or remain constant
Correct answer is option 'D'. Can you explain this answer?
If a closed system is undergoing an irreversible process, the entropy of the system
a)
Must increase
b)
Always remains constant
c)
Must decrease
d)
Can increase, decrease or remain constant

|
Pk Academy answered |
If a closed system is undergoing an irreversible process, the change in entropy of the system is given by
dS > 0, or dS = 0, or dS < 0
In an irreversible process in which heat is removed from the system then its entropy can decrease.
The entropy of a closed system is given by
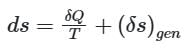
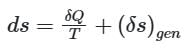
When the process is irreversible then entropy generation in the system (δs)gen is always positive, the heat transfer will decide whether the entropy will increase or decrease.
When heat is added to the system
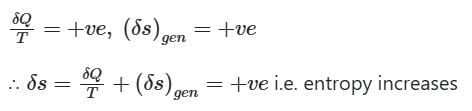
When Heat is removed from the system

When the process is adiabatic, dQ = 0,
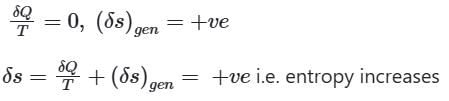
∴ when a closed system is undergoing an irreversible process the entropy may increase, decrease or remain constant.
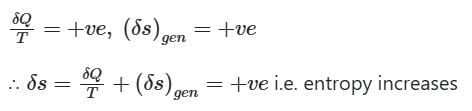
When Heat is removed from the system

When the process is adiabatic, dQ = 0,
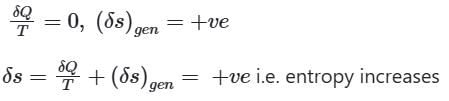
∴ when a closed system is undergoing an irreversible process the entropy may increase, decrease or remain constant.
Which of the following is/are reversible process(es)?1. Isentropic expansion2. Slow heating of water from a hot source3. Constant pressure heating of an ideal gas from a constant temperature source4. Evaporation of a liquid at constant temperatureSelect the correct answer using the code given below:- a)1 only
- b)1 and 2
- c)2 and 3
- d)1 and 4
Correct answer is option 'D'. Can you explain this answer?
Which of the following is/are reversible process(es)?
1. Isentropic expansion
2. Slow heating of water from a hot source
3. Constant pressure heating of an ideal gas from a constant temperature source
4. Evaporation of a liquid at constant temperature
Select the correct answer using the code given below:
a)
1 only
b)
1 and 2
c)
2 and 3
d)
1 and 4
|
|
Srestha Chavan answered |
Isentropic means reversible adiabatic. Heat transfer in any finite temp difference is irreversible.
Ice kept in a well insulated thermo flask is an example of which system? - a)Closed system
- b)Isolated systems
- c)Open system
- d)Non-flow adiabatic system
Correct answer is option 'B'. Can you explain this answer?
Ice kept in a well insulated thermo flask is an example of which system?
a)
Closed system
b)
Isolated systems
c)
Open system
d)
Non-flow adiabatic system
|
|
Sanskriti Chakraborty answered |
Isolated System - in which there is no interaction between system and the
surroundings. It is of fixed mass and energy, and hence there is no mass and energy transfer across the system boundary.
surroundings. It is of fixed mass and energy, and hence there is no mass and energy transfer across the system boundary.
The contents of a well-insulated tank are heated by a resistor of 2Ω in which 10 A current is flowing. Consider the tank along with its contents as a thermodynamic system. The work done by the system and the heat transfer to the system are positive. The rates of heat (Q), work (W) and change in internal energy (ΔU) during the process in kW are - a)
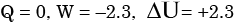
- b)
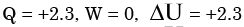
- c)
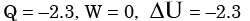
- d)
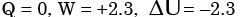
Correct answer is option 'A'. Can you explain this answer?
The contents of a well-insulated tank are heated by a resistor of 2Ω in which 10 A current is flowing. Consider the tank along with its contents as a thermodynamic system. The work done by the system and the heat transfer to the system are positive. The rates of heat (Q), work (W) and change in internal energy (ΔU) during the process in kW are
a)
b)
c)
d)

|
Anirban Khanna answered |
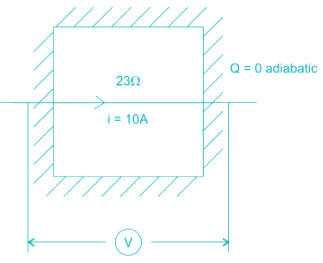
electric work = I2R = 2.3 kW
On the system ⇒ W = -2.3 kW
& ΔU = -W = 2.3 kW
A saturated vapour is compressed to half its volume without changing its temperature. The result is that:- a)All the vapour condenses to liquid
- b)Some of the liquid evaporates and the pressure does not change
- c)The pressure is double its initial value
- d)Some of the vapour condenses and the pressure does not change
Correct answer is option 'D'. Can you explain this answer?
A saturated vapour is compressed to half its volume without changing its temperature. The result is that:
a)
All the vapour condenses to liquid
b)
Some of the liquid evaporates and the pressure does not change
c)
The pressure is double its initial value
d)
Some of the vapour condenses and the pressure does not change
|
|
Anirudh Banerjee answered |
By compressing a saturated vapour, its vapours condense and pressure remains unchanged. Remember it is not gas.
A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is:- a)18000 kJ
- b)9000 kJ
- c)6000 kJ
- d)None of the above
Correct answer is option 'D'. Can you explain this answer?
A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is:
a)
18000 kJ
b)
9000 kJ
c)
6000 kJ
d)
None of the above
|
|
Sandeep Sengupta answered |
Loss in availability due to the process
The availability of a system refers to the maximum useful work that can be obtained from it. When heat is transferred between two reservoirs at different temperatures, there is a loss in availability due to the irreversibility of the process.
Calculation of loss in availability
The loss in availability can be calculated using the equation:
ΔA = Q/Th - Q/Tc
Where:
ΔA = loss in availability
Q = heat transferred
Th = temperature of the hot reservoir
Tc = temperature of the cold reservoir
In this case, the heat reservoir is at 900 K and the ambient (cold reservoir) is at 300 K. The heat transfer is given as 9000 kJ.
ΔA = 9000 kJ/900 K - 9000 kJ/300 K
ΔA = 10 kJ/K - 30 kJ/K
ΔA = -20 kJ/K
Interpretation of the result
The negative sign indicates that there is a loss in availability during the heat transfer process. Since the loss in availability is -20 kJ/K, the total loss in availability due to this process can be calculated by multiplying the loss per Kelvin by the temperature difference between the hot and cold reservoirs:
Total loss in availability = ΔA * (Th - Tc)
Total loss in availability = -20 kJ/K * (900 K - 300 K)
Total loss in availability = -20 kJ/K * 600 K
Total loss in availability = -12,000 kJ
The negative sign indicates that the loss in availability is a decrease in the system's ability to do useful work. Therefore, the correct answer is option D, "None of the above."
The availability of a system refers to the maximum useful work that can be obtained from it. When heat is transferred between two reservoirs at different temperatures, there is a loss in availability due to the irreversibility of the process.
Calculation of loss in availability
The loss in availability can be calculated using the equation:
ΔA = Q/Th - Q/Tc
Where:
ΔA = loss in availability
Q = heat transferred
Th = temperature of the hot reservoir
Tc = temperature of the cold reservoir
In this case, the heat reservoir is at 900 K and the ambient (cold reservoir) is at 300 K. The heat transfer is given as 9000 kJ.
ΔA = 9000 kJ/900 K - 9000 kJ/300 K
ΔA = 10 kJ/K - 30 kJ/K
ΔA = -20 kJ/K
Interpretation of the result
The negative sign indicates that there is a loss in availability during the heat transfer process. Since the loss in availability is -20 kJ/K, the total loss in availability due to this process can be calculated by multiplying the loss per Kelvin by the temperature difference between the hot and cold reservoirs:
Total loss in availability = ΔA * (Th - Tc)
Total loss in availability = -20 kJ/K * (900 K - 300 K)
Total loss in availability = -20 kJ/K * 600 K
Total loss in availability = -12,000 kJ
The negative sign indicates that the loss in availability is a decrease in the system's ability to do useful work. Therefore, the correct answer is option D, "None of the above."
Measurement of temperature is based on which law of thermodynamics? - a)Zeroth law of thermodynamics
- b)First law of thermodynamics
- c)Second law of thermodynamics
- d)Third law of thermodynamics
Correct answer is option 'A'. Can you explain this answer?
Measurement of temperature is based on which law of thermodynamics?
a)
Zeroth law of thermodynamics
b)
First law of thermodynamics
c)
Second law of thermodynamics
d)
Third law of thermodynamics

|
Bijoy Kapoor answered |
The Zeroth law of thermodynamics is the basis for measurement of temperature and setting its scale. In simple word, Zeroth law of thermodynamics says that “When two bodies are separately in thermal equilibrium with the third body, then the two are also in thermal equilibrium with each other."
Assertion (A): A thermodynamic system may be considered as a quantity of working substance with which interactions of heat and work are studied.
Reason (R): Energy in the form of work and heat are mutually convertible.- a)Both A and R are individually true and R is the correct explanation of A
- b)Both A and R are individually true but R is NOT the correct explanation of A
- c)A is true but R is false
- d)A is false but R is true
Correct answer is option 'D'. Can you explain this answer?
Assertion (A): A thermodynamic system may be considered as a quantity of working substance with which interactions of heat and work are studied.
Reason (R): Energy in the form of work and heat are mutually convertible.
Reason (R): Energy in the form of work and heat are mutually convertible.
a)
Both A and R are individually true and R is the correct explanation of A
b)
Both A and R are individually true but R is NOT the correct explanation of A
c)
A is true but R is false
d)
A is false but R is true
|
|
Shivani Reddy answered |
- But remember 100% heat can‟t be convertible to work but 100% work can be converted to heat. It depends on second law of thermodynamics.
- A thermodynamic system is defined as a definite quantity of matter or a region in space upon which attention is focused in the analysis of a problem.
- The system is a macroscopically identifiable collection of matter on which we focus our attention
Consider the following statements:1. The first law of thermodynamics is a law of conservation of energy.2. Perpetual motion machine of the first kind converts energy into equivalent work.3. A closed system does not exchange work or energy with its surroundings.4. The second law of thermodynamics stipulates the law of conservation of energy and entropy.Which of the statements are correct?- a)1 and 2
- b)2 and 4
- c)2, 3 and 4
- d)1, 2 and 3
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements:
1. The first law of thermodynamics is a law of conservation of energy.
2. Perpetual motion machine of the first kind converts energy into equivalent work.
3. A closed system does not exchange work or energy with its surroundings.
4. The second law of thermodynamics stipulates the law of conservation of energy and entropy.
Which of the statements are correct?
a)
1 and 2
b)
2 and 4
c)
2, 3 and 4
d)
1, 2 and 3
|
|
Arshiya Dey answered |
Statement Analysis:
Statement 1: The first law of thermodynamics is a law of conservation of energy.
Statement 2: Perpetual motion machine of the first kind converts energy into equivalent work.
Statement 3: A closed system does not exchange work or energy with its surroundings.
Statement 4: The second law of thermodynamics stipulates the law of conservation of energy and entropy.
Correct Answer: Option A (1 and 2)
Explanation:
First Law of Thermodynamics:
- The first law of thermodynamics is also known as the law of conservation of energy.
- It states that energy cannot be created nor destroyed, but it can be converted from one form to another.
- This law is applied to all systems, including closed, open, and isolated systems.
Perpetual Motion Machine:
- A perpetual motion machine is a hypothetical device that can operate indefinitely without any external energy source.
- According to the second law of thermodynamics, such a machine is impossible because it violates the law of conservation of energy.
- The statement 2 is incorrect because a perpetual motion machine of the first kind does not exist.
Closed System:
- A closed system is a system that does not exchange matter with its surroundings but can exchange energy.
- The statement 3 is incorrect because a closed system can exchange energy with its surroundings, but it cannot exchange matter.
Second Law of Thermodynamics:
- The second law of thermodynamics states that in any energy transfer, the total entropy of a closed system will always increase over time.
- This law is applied to all systems, including closed, open, and isolated systems.
- The statement 4 is incorrect because the second law of thermodynamics stipulates the law of conservation of energy and entropy, not just the law of conservation of energy.
Conclusion:
- The only correct statements are 1 and 2.
- The first law of thermodynamics is a law of conservation of energy, and perpetual motion machine of the first kind is impossible.
Statement 1: The first law of thermodynamics is a law of conservation of energy.
Statement 2: Perpetual motion machine of the first kind converts energy into equivalent work.
Statement 3: A closed system does not exchange work or energy with its surroundings.
Statement 4: The second law of thermodynamics stipulates the law of conservation of energy and entropy.
Correct Answer: Option A (1 and 2)
Explanation:
First Law of Thermodynamics:
- The first law of thermodynamics is also known as the law of conservation of energy.
- It states that energy cannot be created nor destroyed, but it can be converted from one form to another.
- This law is applied to all systems, including closed, open, and isolated systems.
Perpetual Motion Machine:
- A perpetual motion machine is a hypothetical device that can operate indefinitely without any external energy source.
- According to the second law of thermodynamics, such a machine is impossible because it violates the law of conservation of energy.
- The statement 2 is incorrect because a perpetual motion machine of the first kind does not exist.
Closed System:
- A closed system is a system that does not exchange matter with its surroundings but can exchange energy.
- The statement 3 is incorrect because a closed system can exchange energy with its surroundings, but it cannot exchange matter.
Second Law of Thermodynamics:
- The second law of thermodynamics states that in any energy transfer, the total entropy of a closed system will always increase over time.
- This law is applied to all systems, including closed, open, and isolated systems.
- The statement 4 is incorrect because the second law of thermodynamics stipulates the law of conservation of energy and entropy, not just the law of conservation of energy.
Conclusion:
- The only correct statements are 1 and 2.
- The first law of thermodynamics is a law of conservation of energy, and perpetual motion machine of the first kind is impossible.
Isothermal compression of air in a Stirling engine is an example of - a)Open system
- b)Steady flow diabatic system
- c)Closed system with a movable boundary
- d)Closed system with fixed boundary
Correct answer is option 'C'. Can you explain this answer?
Isothermal compression of air in a Stirling engine is an example of
a)
Open system
b)
Steady flow diabatic system
c)
Closed system with a movable boundary
d)
Closed system with fixed boundary
|
|
Rajat Basu answered |
Isothermal Compression of Air in a Stirling Engine - Closed System with a Movable Boundary
Definition of Isothermal Compression
Isothermal compression refers to a thermodynamic process where a gas is compressed at a constant temperature. The term "isothermal" means that the temperature of the gas remains the same during the compression process.
Stirling Engine
A Stirling engine is a heat engine that operates by cyclic compression and expansion of air or other gas at different temperatures. The working fluid is contained within the engine and is alternately heated and cooled, causing it to expand and contract. This motion drives a piston or other mechanical device, producing useful work.
Closed System with a Movable Boundary
In a closed system, the working fluid is contained within the system and does not exchange matter with the surroundings. The boundary of the system can be fixed or movable. In the case of a Stirling engine, the working fluid (air) is contained within the engine and the boundary of the system is movable, as the piston moves back and forth during the cyclic compression and expansion process.
In an isothermal compression process, the working fluid (air) is compressed at a constant temperature, which means that the heat energy added to the system during the compression process is removed by cooling the system. This heat transfer occurs through the engine's heat exchanger, which acts as a thermal bridge between the hot and cold zones of the engine.
Conclusion
Therefore, the isothermal compression of air in a Stirling engine is an example of a closed system with a movable boundary, as the working fluid is contained within the engine and the boundary of the system is movable. The compression process is isothermal, meaning that the temperature of the working fluid remains constant, and the heat transfer occurs through the engine's heat exchanger, which acts as a thermal bridge between the hot and cold zones of the engine.
Definition of Isothermal Compression
Isothermal compression refers to a thermodynamic process where a gas is compressed at a constant temperature. The term "isothermal" means that the temperature of the gas remains the same during the compression process.
Stirling Engine
A Stirling engine is a heat engine that operates by cyclic compression and expansion of air or other gas at different temperatures. The working fluid is contained within the engine and is alternately heated and cooled, causing it to expand and contract. This motion drives a piston or other mechanical device, producing useful work.
Closed System with a Movable Boundary
In a closed system, the working fluid is contained within the system and does not exchange matter with the surroundings. The boundary of the system can be fixed or movable. In the case of a Stirling engine, the working fluid (air) is contained within the engine and the boundary of the system is movable, as the piston moves back and forth during the cyclic compression and expansion process.
In an isothermal compression process, the working fluid (air) is compressed at a constant temperature, which means that the heat energy added to the system during the compression process is removed by cooling the system. This heat transfer occurs through the engine's heat exchanger, which acts as a thermal bridge between the hot and cold zones of the engine.
Conclusion
Therefore, the isothermal compression of air in a Stirling engine is an example of a closed system with a movable boundary, as the working fluid is contained within the engine and the boundary of the system is movable. The compression process is isothermal, meaning that the temperature of the working fluid remains constant, and the heat transfer occurs through the engine's heat exchanger, which acts as a thermal bridge between the hot and cold zones of the engine.
Which one of the following expresses the reversible work done by the system (steady flow) between states 1 and 2? - a)
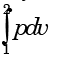
- b)
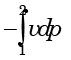
- c)
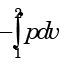
- d)
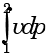
Correct answer is option 'B'. Can you explain this answer?
Which one of the following expresses the reversible work done by the system (steady flow) between states 1 and 2?
a)
b)
c)
d)
|
|
Dipika Bose answered |
For steady flow process, reversible work given by 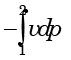
Which one of the following sets of thermodynamic laws/relations is directly involved in determining the final properties during an adiabatic mixing process?- a)The first and second laws of thermodynamics
- b)The second law of thermodynamics and steady flow relations
- c)Perfect gas relationship and steady flow relations
- d)The first law of thermodynamics and perfect gas relationship
Correct answer is option 'A'. Can you explain this answer?
Which one of the following sets of thermodynamic laws/relations is directly involved in determining the final properties during an adiabatic mixing process?
a)
The first and second laws of thermodynamics
b)
The second law of thermodynamics and steady flow relations
c)
Perfect gas relationship and steady flow relations
d)
The first law of thermodynamics and perfect gas relationship
|
|
Shail Mukherjee answered |
If we adiabatically mix two liquid then perfect gas law is not necessary. But entropy change in the universe must be calculated by Second law of thermodynamics. Final entropy of then system is also a property. That so why we need second law.
The time constant of a thermocouple is the time taken to attain:- a)The final value to he measured
- b)50% of the value of the initial temperature difference
- c)63.2% of the value of the initial temperature difference
- d)98.8% of the value of the initial temperature difference
Correct answer is option 'C'. Can you explain this answer?
The time constant of a thermocouple is the time taken to attain:
a)
The final value to he measured
b)
50% of the value of the initial temperature difference
c)
63.2% of the value of the initial temperature difference
d)
98.8% of the value of the initial temperature difference

|
Shivam Sharma answered |
(q/q0 = e^-t/t when t = t
(q/q0) = e^-1 = 0.368
So after time period equal to t (time constant) the initial temperature difference q0 is reduced to 36.8% so it can also be defined time constant as the equivalent time required to reduce the initial temperature difference q0 by 63.2%
With increase of pressure, the latent heat of steam - a)Remains same
- b)Increases
- c)Decreases
- d)Behaves unpredictably
Correct answer is option 'C'. Can you explain this answer?
With increase of pressure, the latent heat of steam
a)
Remains same
b)
Increases
c)
Decreases
d)
Behaves unpredictably

|
Anirban Khanna answered |
Prresure increases at 100 degree latent heat of vapourisation also increases while as pressure increases latent heat of condensation decreases. When temperature of the water in the beaker increases, the bond b/w molecules releases and water starts vaporizing.
Thermodynamic work is the product of - a)Two intensive properties
- b)Two extensive properties
- c)An intensive property and change in an extensive property
- d)An extensive property and change in an intensive property
Correct answer is option 'C'. Can you explain this answer?
Thermodynamic work is the product of
a)
Two intensive properties
b)
Two extensive properties
c)
An intensive property and change in an extensive property
d)
An extensive property and change in an intensive property
|
|
Ipsita Sengupta answered |
Which one of the following is correct on basis of the second law of thermodynamics?- a)For any spontaneous process, the entropy of the universe increases
- b)∆S =qrev/T at constant temperature
- c)Efficiency of the Stirling cycle is more than that of a Carnot cycle
- d)∆E=q+w (The symbols have their usual meaning)
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is correct on basis of the second law of thermodynamics?
a)
For any spontaneous process, the entropy of the universe increases
b)
∆S =qrev/T at constant temperature
c)
Efficiency of the Stirling cycle is more than that of a Carnot cycle
d)
∆E=q+w (The symbols have their usual meaning)

|
Siddharth Bajaj answered |
Explanation:
The second law of thermodynamics states that the entropy of the universe always increases for any spontaneous process. This can be explained in terms of the following:
Spontaneous Processes and Entropy:
A spontaneous process is a process that occurs naturally without any external intervention. For example, when a hot cup of coffee is left on a table, it cools down naturally without any external intervention. The second law of thermodynamics states that for any spontaneous process, the entropy of the universe always increases. Entropy is the measure of disorder or randomness in a system. Thus, the second law of thermodynamics implies that the natural tendency of any system is to move towards a state of maximum disorder or randomness.
S = qrev/T:
The equation S = qrev/T is the definition of entropy. It states that the entropy change in a system is equal to the heat transferred in a reversible process divided by the absolute temperature of the system. This equation is a consequence of the second law of thermodynamics and is valid only for reversible processes.
Efficiency of Stirling and Carnot Cycles:
The efficiency of a Stirling cycle is less than that of a Carnot cycle. This is because a Carnot cycle is the most efficient cycle possible and is based on the Carnot theorem, which states that no engine can be more efficient than a reversible engine operating between the same two temperatures.
E=q+w:
The equation E = q + w is the first law of thermodynamics, which states that the internal energy of a system is equal to the heat transferred into the system plus the work done on the system. This equation does not directly relate to the second law of thermodynamics.
Therefore, option 'A' is correct as it is in accordance with the second law of thermodynamics.
The second law of thermodynamics states that the entropy of the universe always increases for any spontaneous process. This can be explained in terms of the following:
Spontaneous Processes and Entropy:
A spontaneous process is a process that occurs naturally without any external intervention. For example, when a hot cup of coffee is left on a table, it cools down naturally without any external intervention. The second law of thermodynamics states that for any spontaneous process, the entropy of the universe always increases. Entropy is the measure of disorder or randomness in a system. Thus, the second law of thermodynamics implies that the natural tendency of any system is to move towards a state of maximum disorder or randomness.
S = qrev/T:
The equation S = qrev/T is the definition of entropy. It states that the entropy change in a system is equal to the heat transferred in a reversible process divided by the absolute temperature of the system. This equation is a consequence of the second law of thermodynamics and is valid only for reversible processes.
Efficiency of Stirling and Carnot Cycles:
The efficiency of a Stirling cycle is less than that of a Carnot cycle. This is because a Carnot cycle is the most efficient cycle possible and is based on the Carnot theorem, which states that no engine can be more efficient than a reversible engine operating between the same two temperatures.
E=q+w:
The equation E = q + w is the first law of thermodynamics, which states that the internal energy of a system is equal to the heat transferred into the system plus the work done on the system. This equation does not directly relate to the second law of thermodynamics.
Therefore, option 'A' is correct as it is in accordance with the second law of thermodynamics.
Which of the following statement is correct?- a)The increase in entropy is obtained from a given quantity of heat transfer at a low temperature.
- b)The change in entropy may be regarded as a measure of the rate of the availability of heat for transformation into work.
- c)The entropy represents the maximum amount of work obtainable per degree drop in temperature
- d)All of the above
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement is correct?
a)
The increase in entropy is obtained from a given quantity of heat transfer at a low temperature.
b)
The change in entropy may be regarded as a measure of the rate of the availability of heat for transformation into work.
c)
The entropy represents the maximum amount of work obtainable per degree drop in temperature
d)
All of the above
|
|
Lekshmi Khanna answered |
The change of entropy may be regarded as a measure of the rate of availability of heat for transformation into work.
Which one of the following statements is correct when saturation pressure of a vapour increases?- a)Saturation temperature decreases
- b)Enthalpy of evaporation decreases
- c)Enthalpy of evaporation increases
- d)Specific volume change of phase increases
Correct answer is option 'B'. Can you explain this answer?
Which one of the following statements is correct when saturation pressure of a vapour increases?
a)
Saturation temperature decreases
b)
Enthalpy of evaporation decreases
c)
Enthalpy of evaporation increases
d)
Specific volume change of phase increases
|
|
Hrishikesh Chakraborty answered |
Explanation:
When the saturation pressure of a vapor increases, there are changes in various thermodynamic properties. The correct statement among the given options is:
b) Enthalpy of evaporation decreases
Enthalpy of evaporation:
Enthalpy of evaporation, also known as latent heat of vaporization, is the amount of energy required to change a given amount of liquid into vapor at a constant temperature. It is a thermodynamic property that depends on the pressure and temperature of the system.
Effect of saturation pressure on enthalpy of evaporation:
When the saturation pressure of a vapor increases, the enthalpy of evaporation decreases. This is because the energy required to change the liquid into vapor decreases as the pressure increases.
The following are the effects of saturation pressure on various thermodynamic properties:
Saturation temperature:
Saturation temperature is the temperature at which a given substance changes from a liquid state to a vapor state at a constant pressure. When the saturation pressure of a vapor increases, the saturation temperature also increases. This is because the pressure and temperature of a vapor are directly proportional.
Enthalpy of evaporation:
As explained earlier, the enthalpy of evaporation decreases when the saturation pressure of a vapor increases.
Specific volume change of phase:
The specific volume change of phase is the change in volume of a substance during a phase change. When the saturation pressure of a vapor increases, the specific volume change of phase also increases. This is because the volume occupied by a vapor increases as the pressure increases.
Conclusion:
When the saturation pressure of a vapor increases, the enthalpy of evaporation decreases. This is because the energy required to change the liquid into vapor decreases as the pressure increases.
When the saturation pressure of a vapor increases, there are changes in various thermodynamic properties. The correct statement among the given options is:
b) Enthalpy of evaporation decreases
Enthalpy of evaporation:
Enthalpy of evaporation, also known as latent heat of vaporization, is the amount of energy required to change a given amount of liquid into vapor at a constant temperature. It is a thermodynamic property that depends on the pressure and temperature of the system.
Effect of saturation pressure on enthalpy of evaporation:
When the saturation pressure of a vapor increases, the enthalpy of evaporation decreases. This is because the energy required to change the liquid into vapor decreases as the pressure increases.
The following are the effects of saturation pressure on various thermodynamic properties:
Saturation temperature:
Saturation temperature is the temperature at which a given substance changes from a liquid state to a vapor state at a constant pressure. When the saturation pressure of a vapor increases, the saturation temperature also increases. This is because the pressure and temperature of a vapor are directly proportional.
Enthalpy of evaporation:
As explained earlier, the enthalpy of evaporation decreases when the saturation pressure of a vapor increases.
Specific volume change of phase:
The specific volume change of phase is the change in volume of a substance during a phase change. When the saturation pressure of a vapor increases, the specific volume change of phase also increases. This is because the volume occupied by a vapor increases as the pressure increases.
Conclusion:
When the saturation pressure of a vapor increases, the enthalpy of evaporation decreases. This is because the energy required to change the liquid into vapor decreases as the pressure increases.
Which one of the following is the correct sequence of the three processes A, B and C in the increasing order of the amount of work done by a gas following idealgas expansions by these processes?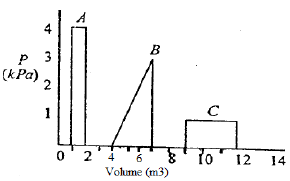
- a)A – B – C
- b)B – A – C
- c)A – C – B
- d)C – A – B
Correct answer is option 'D'. Can you explain this answer?
Which one of the following is the correct sequence of the three processes A, B and C in the increasing order of the amount of work done by a gas following idealgas expansions by these processes?
a)
A – B – C
b)
B – A – C
c)
A – C – B
d)
C – A – B

|
Varun Banerjee answered |
Which of the following is NOT a path function?- a) Internal energy
- b) Heat energy
- c) Work energy
- d) none of the above
Correct answer is option 'A'. Can you explain this answer?
Which of the following is NOT a path function?
a)
Internal energy
b)
Heat energy
c)
Work energy
d)
none of the above
|
|
Nisha Singh answered |
In thermodynamics, energy can be in two forms, energy in transit and energy in storage. Energy in transit is a path function as the transfer of this energy through the boundaries of the system depends on the path which is followed by the system in the process. But energy in storage does not cross the boundaries of the system; hence it is a point function. Heat transfer as well as work transfer between the system and surrounding depends upon the path by which the process is occurred. Therefore heat energy and work energy are the path functions. Energy in storage is the internal energy. The change in internal energy (ΔE) remains constant, no matter which path is followed by a system to undergo a change of a certain state. Thus internal energy is a point function or state function.
Assigning the basic dimensions to mass, length, time and temperature respectively as M, L, T and θ (Temperature), what are the dimensions of entropy?- a) M LT-2 θ
- b)M L2 T-1 θ-1
- c)M L2 T-2θ-1
- d)M L3T-2 θ -1
Correct answer is option 'C'. Can you explain this answer?
Assigning the basic dimensions to mass, length, time and temperature respectively as M, L, T and θ (Temperature), what are the dimensions of entropy?
a)
M LT-2 θ
b)
M L2 T-1 θ-1
c)
M L2 T-2θ-1
d)
M L3T-2 θ -1

|
Bhaskar Unni answered |
dS=dQ/T
where,
Q= ML2T-2
T=θ
Assertion (A): No machine would continuously supply work without expenditure of some other form of energy.Reason (R): Energy can be neither created nor destroyed, but it can only be transformed from one form into another.- a)Both A and R are individually true and R is the correct explanation of A
- b)Both A and R are individually true but R is NOT the correct explanation of A
- c)A is true but R is false
- d)A is false but R is true
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): No machine would continuously supply work without expenditure of some other form of energy.
Reason (R): Energy can be neither created nor destroyed, but it can only be transformed from one form into another.
a)
Both A and R are individually true and R is the correct explanation of A
b)
Both A and R are individually true but R is NOT the correct explanation of A
c)
A is true but R is false
d)
A is false but R is true

|
Trilochan Thakur answered |
1st statement is PMM1 and it is correct and 2nd statement is 1st law..both are true.
The constant volume gas thermometer works on the principle that - a)at low pressure, the temperature of the gas is independent of its pressure at constant volume
- b)at high pressure, the temperature of the gas is independent of its pressure at constant volume
- c)at low pressure, the temperature of the gas is proportional to its pressure at constant volume
- d)at high pressure, the temperature of the gas is proportional to its pressure at constant volume
Correct answer is option 'C'. Can you explain this answer?
The constant volume gas thermometer works on the principle that
a)
at low pressure, the temperature of the gas is independent of its pressure at constant volume
b)
at high pressure, the temperature of the gas is independent of its pressure at constant volume
c)
at low pressure, the temperature of the gas is proportional to its pressure at constant volume
d)
at high pressure, the temperature of the gas is proportional to its pressure at constant volume
|
|
Ashutosh Sharma answered |
The Constant Volume Gas Thermometer
The constant volume gas thermometer is a device used to measure the temperature of a gas at a constant volume. It works on the principle that at low pressure, the temperature of the gas is proportional to its pressure at constant volume.
Pressure-Temperature Relationship
According to Gay-Lussac's law, the pressure of a gas is directly proportional to its temperature when the volume is kept constant. This means that if the temperature of a gas is increased, its pressure will also increase. Similarly, if the temperature of a gas is decreased, its pressure will also decrease.
The Relationship in the Constant Volume Gas Thermometer
In the constant volume gas thermometer, the volume of the gas is kept constant. Therefore, according to Gay-Lussac's law, the pressure of the gas will be directly proportional to its temperature. This allows the thermometer to measure the temperature of the gas by measuring its pressure.
Advantages of the Constant Volume Gas Thermometer
The constant volume gas thermometer has several advantages over other types of thermometers. These include:
- High accuracy: The constant volume gas thermometer can measure temperatures with a very high degree of accuracy.
- Wide range: The constant volume gas thermometer can measure temperatures over a wide range, from very low temperatures to very high temperatures.
- No calibration needed: The constant volume gas thermometer does not need to be calibrated, as the pressure-temperature relationship is well known and does not change over time.
Conclusion
In conclusion, the constant volume gas thermometer works on the principle that at low pressure, the temperature of the gas is proportional to its pressure at constant volume. This allows the thermometer to measure the temperature of a gas with a high degree of accuracy over a wide range of temperatures.
The constant volume gas thermometer is a device used to measure the temperature of a gas at a constant volume. It works on the principle that at low pressure, the temperature of the gas is proportional to its pressure at constant volume.
Pressure-Temperature Relationship
According to Gay-Lussac's law, the pressure of a gas is directly proportional to its temperature when the volume is kept constant. This means that if the temperature of a gas is increased, its pressure will also increase. Similarly, if the temperature of a gas is decreased, its pressure will also decrease.
The Relationship in the Constant Volume Gas Thermometer
In the constant volume gas thermometer, the volume of the gas is kept constant. Therefore, according to Gay-Lussac's law, the pressure of the gas will be directly proportional to its temperature. This allows the thermometer to measure the temperature of the gas by measuring its pressure.
Advantages of the Constant Volume Gas Thermometer
The constant volume gas thermometer has several advantages over other types of thermometers. These include:
- High accuracy: The constant volume gas thermometer can measure temperatures with a very high degree of accuracy.
- Wide range: The constant volume gas thermometer can measure temperatures over a wide range, from very low temperatures to very high temperatures.
- No calibration needed: The constant volume gas thermometer does not need to be calibrated, as the pressure-temperature relationship is well known and does not change over time.
Conclusion
In conclusion, the constant volume gas thermometer works on the principle that at low pressure, the temperature of the gas is proportional to its pressure at constant volume. This allows the thermometer to measure the temperature of a gas with a high degree of accuracy over a wide range of temperatures.
Pressure reaches a value of absolute zero- a)At a temperature of – 273 K
- b)Under vacuum condition
- c)At the earth's centre
- d)When molecular momentum of system becomes zero
Correct answer is option 'D'. Can you explain this answer?
Pressure reaches a value of absolute zero
a)
At a temperature of – 273 K
b)
Under vacuum condition
c)
At the earth's centre
d)
When molecular momentum of system becomes zero

|
Bijoy Kapoor answered |
Absolute zero, temperature at which a thermodynamic system has the lowest energy. ... It appeared that an “ideal gas” at constant pressure would reach zero volume at what is now called the absolute zero of temperature.
In a test of a water-jacketed compressor, the shaft work required is 90 kN-m/kg of air compressed. During compression, increase in enthalpy of air is 30 kJ/kg of air and increase in enthalpy of circulating cooling water is 40 kJ/ kg of air. The change is velocity is negligible. The amount of heat lost to the atmosphere from the compressor per kg of air is:- a)20kJ
- b)60kJ
- c)80 kJ
- d)120kJ
Correct answer is option 'A'. Can you explain this answer?
In a test of a water-jacketed compressor, the shaft work required is 90 kN-m/kg of air compressed. During compression, increase in enthalpy of air is 30 kJ/kg of air and increase in enthalpy of circulating cooling water is 40 kJ/ kg of air. The change is velocity is negligible. The amount of heat lost to the atmosphere from the compressor per kg of air is:
a)
20kJ
b)
60kJ
c)
80 kJ
d)
120kJ

|
Dibyendu Singha answered |
I think 80
A tank containing air is stirred by a paddle wheel. The work input to the paddle wheel is 9000 kJ and the heat transferred to the surroundings from the tank is 3000 kJ. The external work done by the system is:- a)Zero
- b)3000 kJ
- c)6000 kJ
- d)9000 kJ
Correct answer is option 'A'. Can you explain this answer?
A tank containing air is stirred by a paddle wheel. The work input to the paddle wheel is 9000 kJ and the heat transferred to the surroundings from the tank is 3000 kJ. The external work done by the system is:
a)
Zero
b)
3000 kJ
c)
6000 kJ
d)
9000 kJ

|
Dhirajkumar Bhondekar answered |
Mechanical or flow work is done on the system..from 1st law its internal energy increases by6000kj,,raises its temp. but there is no work by system to surrounding as no change is boundry of system or volume..so no pdv work...
Assertion (A): In thermodynamic analysis, the concept of reversibility is that, a reversible process is the most efficient process.Reason (R): The energy transfer as heat and work during the forward process as always identically equal to the energy transfer is heat and work during the reversal or the process. - a)Both A and R are individually true and R is the correct explanation of A
- b)Both A and R are individually true but R is NOT the correct explanation of A
- c)A is true but R is false
- d)A is false but R is true
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): In thermodynamic analysis, the concept of reversibility is that, a reversible process is the most efficient process.
Reason (R): The energy transfer as heat and work during the forward process as always identically equal to the energy transfer is heat and work during the reversal or the process.
a)
Both A and R are individually true and R is the correct explanation of A
b)
Both A and R are individually true but R is NOT the correct explanation of A
c)
A is true but R is false
d)
A is false but R is true
|
|
Pragati Sengupta answered |
The energy transfer as heat and work during the forward process as always
identically equal to the energy transfer is heat and work during the reversal or the process is the correct reason for maximum efficiency because it is conservative system.
identically equal to the energy transfer is heat and work during the reversal or the process is the correct reason for maximum efficiency because it is conservative system.
The inlet and the outlet conditions of steam for an adiabatic steam turbine are as indicated in the figure. The notations are as usually followed. 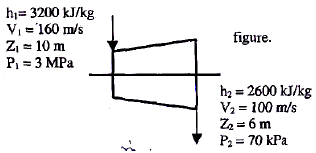 If mass flow rate of steam through the turbine is 20 kg/s the power output of the turbine (in MW) is:
If mass flow rate of steam through the turbine is 20 kg/s the power output of the turbine (in MW) is:- a)12.157
- b)12.941
- c)168.001
- d)168.785
Correct answer is option 'A'. Can you explain this answer?
The inlet and the outlet conditions of steam for an adiabatic steam turbine are as indicated in the figure. The notations are as usually followed.
If mass flow rate of steam through the turbine is 20 kg/s the power output of the turbine (in MW) is:
a)
12.157
b)
12.941
c)
168.001
d)
168.785

|
Vertex Academy answered |
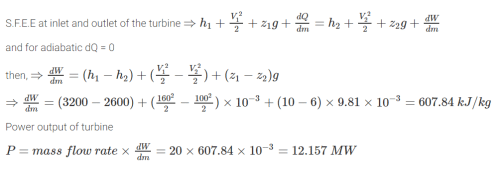
Which of the following is the extensive property of a thermodynamic system?- a) Pressure
- b)Volume
- c)Temperature
- d)Density
Correct answer is option 'B'. Can you explain this answer?
Which of the following is the extensive property of a thermodynamic system?
a)
Pressure
b)
Volume
c)
Temperature
d)
Density
|
|
Anirudh Banerjee answered |
Extensive Property of a Thermodynamic System
A thermodynamic system is a region in space containing matter and energy, which can be studied using thermodynamics. Thermodynamics is a branch of physics that deals with energy exchange and its transformations.
An extensive property is a physical quantity that depends on the size or amount of a system. It is proportional to the size or amount of the system. For example, the mass of a system is an extensive property. The larger the system, the greater its mass.
Among the given options, the extensive property of a thermodynamic system is volume.
Explanation
Volume is an extensive property because its value depends on the size of the system. The larger the system, the greater its volume. For example, a 1-liter container will have a smaller volume than a 10-liter container.
On the other hand, pressure, temperature, and density are intensive properties. Their values do not depend on the size or amount of the system. For example, the pressure inside a 1-liter container is the same as the pressure inside a 10-liter container, provided the temperature and the amount of gas are the same.
Conclusion
In conclusion, volume is the extensive property of a thermodynamic system. It is proportional to the size or amount of the system. The larger the system, the greater its volume. On the other hand, pressure, temperature, and density are intensive properties. Their values do not depend on the size or amount of the system.
A thermodynamic system is a region in space containing matter and energy, which can be studied using thermodynamics. Thermodynamics is a branch of physics that deals with energy exchange and its transformations.
An extensive property is a physical quantity that depends on the size or amount of a system. It is proportional to the size or amount of the system. For example, the mass of a system is an extensive property. The larger the system, the greater its mass.
Among the given options, the extensive property of a thermodynamic system is volume.
Explanation
Volume is an extensive property because its value depends on the size of the system. The larger the system, the greater its volume. For example, a 1-liter container will have a smaller volume than a 10-liter container.
On the other hand, pressure, temperature, and density are intensive properties. Their values do not depend on the size or amount of the system. For example, the pressure inside a 1-liter container is the same as the pressure inside a 10-liter container, provided the temperature and the amount of gas are the same.
Conclusion
In conclusion, volume is the extensive property of a thermodynamic system. It is proportional to the size or amount of the system. The larger the system, the greater its volume. On the other hand, pressure, temperature, and density are intensive properties. Their values do not depend on the size or amount of the system.
A gas contained in a cylinder is compressed, the work required for compression being 5000 kJ. During the process, heat interaction of 2000 kJ causes the surroundings to the heated. The change in internal energy of the gas during the process is:- a)– 7000 kJ
- b)– 3000 kJ
- c)+ 3000 kJ
- d)+ 7000 kJ
Correct answer is option 'C'. Can you explain this answer?
A gas contained in a cylinder is compressed, the work required for compression being 5000 kJ. During the process, heat interaction of 2000 kJ causes the surroundings to the heated. The change in internal energy of the gas during the process is:
a)
– 7000 kJ
b)
– 3000 kJ
c)
+ 3000 kJ
d)
+ 7000 kJ
|
|
Pritam Chavan answered |
An insulated tank initially contains 0.25 kg of a gas with an internal energy of 200 kJ/kg .Additional gas with an internal energy of 300 kJ/kg and an enthalpy of 400 kJ/kg enters the tank until the total mass of gas contained is 1 kg. What is the final internal energy(in kJ/kg) of the gas in the tank?- a)250
- b)275
- c) 350
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
An insulated tank initially contains 0.25 kg of a gas with an internal energy of 200 kJ/kg .Additional gas with an internal energy of 300 kJ/kg and an enthalpy of 400 kJ/kg enters the tank until the total mass of gas contained is 1 kg. What is the final internal energy(in kJ/kg) of the gas in the tank?
a)
250
b)
275
c)
350
d)
None of the above
|
|
Neha Joshi answered |
Given: Quantity of gas = 0.25 kg
Internal energy = 200kg
Additional gas = 300kg
Enthalpy entering the tank = 400kg
To Find: Internal energy of gas in the tank
Solution:
U = u1m1 + ( 1-m1 )h
U = 200 . 0.25 + ( 1 - 0.25) . 400 = 350
U = 350
Answer: The final internal energy is 350 kJ.
Internal energy = 200kg
Additional gas = 300kg
Enthalpy entering the tank = 400kg
To Find: Internal energy of gas in the tank
Solution:
U = u1m1 + ( 1-m1 )h
U = 200 . 0.25 + ( 1 - 0.25) . 400 = 350
U = 350
Answer: The final internal energy is 350 kJ.
An ideal cycle is shown in the figure. Its thermal efficiency is given by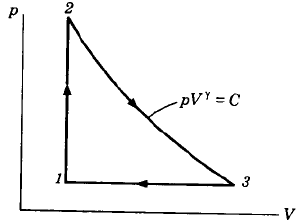
- a)
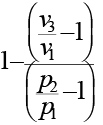
- b)
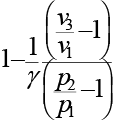
- c)
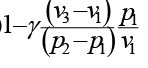
- d)
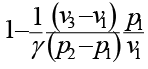
Correct answer is option 'C'. Can you explain this answer?
An ideal cycle is shown in the figure. Its thermal efficiency is given by
a)
b)
c)
d)
|
|
Janani Bajaj answered |
Total heat addition is constant volume heat addition 
Total heat rejection is constant pressure heat rejection, 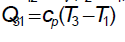
Now from equation of state
85 kJ of heat is supplied to a closed system at constant volume. During the next process, the system rejects 90 kJ of heat at constant pressure while 20 kJ of work is done on it. The system is brought to the original state by an adiabatic process. The initial internal energy is 100 kJ. Then what is the quantity of work transfer during the process?- a)30 kJ
- b)25 kJ
- c)20 kJ
- d)15 kJ
Correct answer is option 'D'. Can you explain this answer?
85 kJ of heat is supplied to a closed system at constant volume. During the next process, the system rejects 90 kJ of heat at constant pressure while 20 kJ of work is done on it. The system is brought to the original state by an adiabatic process. The initial internal energy is 100 kJ. Then what is the quantity of work transfer during the process?
a)
30 kJ
b)
25 kJ
c)
20 kJ
d)
15 kJ
|
|
Gopal Chatterjee answered |
Chapter doubts & questions for Thermodynamics - GATE Mechanical (ME) Mock Test Series 2026 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - GATE Mechanical (ME) Mock Test Series 2026 in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
GATE Mechanical (ME) Mock Test Series 2026
30 docs|220 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









