NEET Exam > NEET Questions > Which of the following on reaction with nitro...
Start Learning for Free
Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.?
Most Upvoted Answer
Which of the following on reaction with nitrous acid followed by treat...
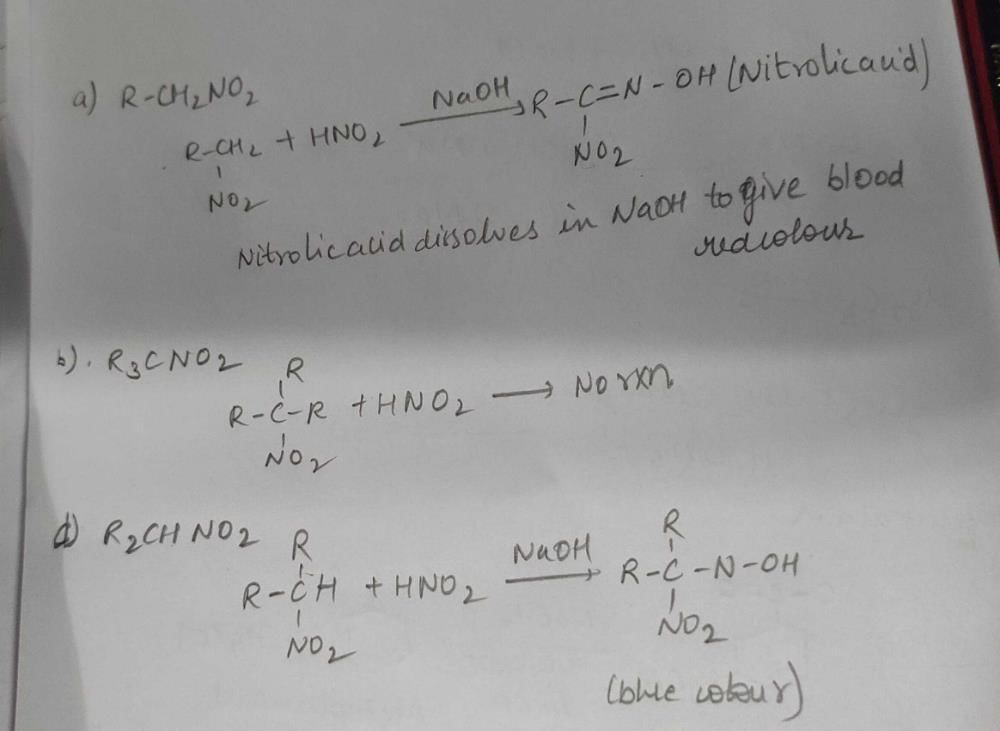
Community Answer
Which of the following on reaction with nitrous acid followed by treat...
Answer:
Nitrous acid (HNO2) can react with various organic compounds to form different products. In this case, we need to determine which compound, when reacted with nitrous acid followed by treatment with NaOH, produces a blue color.
Here are the given options:
A) RCH2NO2
B) R3CNO2
C) C6H5NO2
D) R2CHNO2
To identify the compound that produces a blue color, we need to consider the reaction mechanism and the nature of the resulting products.
Reaction Mechanism:
The reaction of nitrous acid (HNO2) with an organic compound involves the formation of a diazonium salt. This reaction is known as diazotization. Diazonium salts are unstable and can undergo different reactions depending on the conditions.
When a diazonium salt is treated with NaOH, it undergoes a reaction called diazo coupling. During this process, the diazonium salt reacts with a phenol or an aromatic amine to form an azo compound. Azo compounds are known for their vibrant colors, including blue.
Analysis of Options:
A) RCH2NO2 - This compound does not contain any aromatic or phenolic groups, so it is unlikely to produce a blue color during diazo coupling.
B) R3CNO2 - This compound is a nitroalkane, which is not expected to undergo diazotization or diazo coupling reactions.
C) C6H5NO2 - This compound is nitrobenzene, which contains both a nitro group and an aromatic ring. Nitrobenzene can undergo diazotization and subsequent diazo coupling reactions. Therefore, it is a potential candidate for producing a blue color.
D) R2CHNO2 - This compound does not contain any aromatic or phenolic groups, so it is unlikely to produce a blue color during diazo coupling.
Conclusion:
Based on the analysis, the compound that is most likely to produce a blue color when reacted with nitrous acid followed by treatment with NaOH is C6H5NO2 (nitrobenzene).
Nitrous acid (HNO2) can react with various organic compounds to form different products. In this case, we need to determine which compound, when reacted with nitrous acid followed by treatment with NaOH, produces a blue color.
Here are the given options:
A) RCH2NO2
B) R3CNO2
C) C6H5NO2
D) R2CHNO2
To identify the compound that produces a blue color, we need to consider the reaction mechanism and the nature of the resulting products.
Reaction Mechanism:
The reaction of nitrous acid (HNO2) with an organic compound involves the formation of a diazonium salt. This reaction is known as diazotization. Diazonium salts are unstable and can undergo different reactions depending on the conditions.
When a diazonium salt is treated with NaOH, it undergoes a reaction called diazo coupling. During this process, the diazonium salt reacts with a phenol or an aromatic amine to form an azo compound. Azo compounds are known for their vibrant colors, including blue.
Analysis of Options:
A) RCH2NO2 - This compound does not contain any aromatic or phenolic groups, so it is unlikely to produce a blue color during diazo coupling.
B) R3CNO2 - This compound is a nitroalkane, which is not expected to undergo diazotization or diazo coupling reactions.
C) C6H5NO2 - This compound is nitrobenzene, which contains both a nitro group and an aromatic ring. Nitrobenzene can undergo diazotization and subsequent diazo coupling reactions. Therefore, it is a potential candidate for producing a blue color.
D) R2CHNO2 - This compound does not contain any aromatic or phenolic groups, so it is unlikely to produce a blue color during diazo coupling.
Conclusion:
Based on the analysis, the compound that is most likely to produce a blue color when reacted with nitrous acid followed by treatment with NaOH is C6H5NO2 (nitrobenzene).
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.?
Question Description
Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.?.
Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.?.
Solutions for Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.?, a detailed solution for Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? has been provided alongside types of Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? theory, EduRev gives you an
ample number of questions to practice Which of the following on reaction with nitrous acid followed by treatment with NaOH produces a blue colour? A)RCH2NO2 B)R3CNO2 C)C6H5NO2 D)R2CHNO2 Answer with explanation pls.? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























