GATE Exam > GATE Questions > Metallic copper is know to form cubic crystal...
Start Learning for Free
Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude that
- a)There is insufficient data to distinguish between the option below
- b)The crystals are a mixture of f.c.c and b.c.c types
- c)The crystals are of b.c.c type
- d)The crystals are of f.c.c type
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Metallic copper is know to form cubic crystals and the lattice constan...
Atomic weight of copper = 63.5
mass of one copper atom =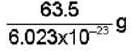
= 1.054 x 10-22 g
Volume of unit cell V = a3
=(0.36x10-7)3 cm3
= 4.6656 x 10-23 cm3
mass of one unit cell = 4.6656 x 10-23 x 8.96
= 4.18x10-22 g
Number of Cu atom in one unit cell
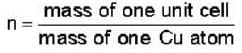
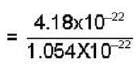 = 3.96= 4 So, crystal is f.c.c.
= 3.96= 4 So, crystal is f.c.c.
mass of one copper atom =
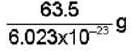
= 1.054 x 10-22 g
Volume of unit cell V = a3
=(0.36x10-7)3 cm3
= 4.6656 x 10-23 cm3
mass of one unit cell = 4.6656 x 10-23 x 8.96
= 4.18x10-22 g
Number of Cu atom in one unit cell
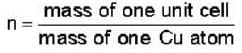
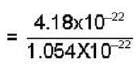 = 3.96= 4 So, crystal is f.c.c.
= 3.96= 4 So, crystal is f.c.c.Most Upvoted Answer
Metallic copper is know to form cubic crystals and the lattice constan...
Explanation:
Copper is a metallic element that forms crystals with a cubic structure. The lattice constant of copper is measured from X-ray diffraction studies to be about 0.36 nm. The specific gravity of copper is 8.96, and its atomic weight is 63.5. Based on these values, we can conclude that the crystals are of f.c.c type.
Reasoning:
To understand why the crystals are of f.c.c type, we need to consider the following factors:
- Lattice constant: The lattice constant is a measure of the distance between atoms in a crystal lattice. For copper, the measured lattice constant is 0.36 nm.
- Specific gravity: Specific gravity is a measure of the density of a substance relative to the density of water. For copper, the specific gravity is 8.96.
- Atomic weight: Atomic weight is the average mass of an element's atoms, taking into account the relative abundance of each isotope. For copper, the atomic weight is 63.5.
Using this information, we can make the following observations:
- The lattice constant of copper (0.36 nm) is consistent with the lattice constant of f.c.c metals like aluminum, which have a lattice constant of 0.4049 nm. This suggests that copper also has an f.c.c structure.
- The specific gravity of copper (8.96) is higher than that of b.c.c metals like iron (7.87) and tungsten (19.25). This suggests that copper is unlikely to have a b.c.c structure.
- The atomic weight of copper (63.5) is consistent with the atomic weight of f.c.c metals like silver (107.9) and gold (196.9). This further supports the idea that copper has an f.c.c structure.
Therefore, based on these observations, we can conclude that the crystals of copper are of f.c.c type.
Copper is a metallic element that forms crystals with a cubic structure. The lattice constant of copper is measured from X-ray diffraction studies to be about 0.36 nm. The specific gravity of copper is 8.96, and its atomic weight is 63.5. Based on these values, we can conclude that the crystals are of f.c.c type.
Reasoning:
To understand why the crystals are of f.c.c type, we need to consider the following factors:
- Lattice constant: The lattice constant is a measure of the distance between atoms in a crystal lattice. For copper, the measured lattice constant is 0.36 nm.
- Specific gravity: Specific gravity is a measure of the density of a substance relative to the density of water. For copper, the specific gravity is 8.96.
- Atomic weight: Atomic weight is the average mass of an element's atoms, taking into account the relative abundance of each isotope. For copper, the atomic weight is 63.5.
Using this information, we can make the following observations:
- The lattice constant of copper (0.36 nm) is consistent with the lattice constant of f.c.c metals like aluminum, which have a lattice constant of 0.4049 nm. This suggests that copper also has an f.c.c structure.
- The specific gravity of copper (8.96) is higher than that of b.c.c metals like iron (7.87) and tungsten (19.25). This suggests that copper is unlikely to have a b.c.c structure.
- The atomic weight of copper (63.5) is consistent with the atomic weight of f.c.c metals like silver (107.9) and gold (196.9). This further supports the idea that copper has an f.c.c structure.
Therefore, based on these observations, we can conclude that the crystals of copper are of f.c.c type.

|
Explore Courses for GATE exam
|

|
Similar GATE Doubts
Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer?
Question Description
Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer?.
Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Metallic copper is know to form cubic crystals and the lattice constant is measured from X-ray diffraction studies to be about 0.36 nm. If the specific gravity of copper is 8.96 and its atomic weight is 63.5, one can conclude thata)There is insufficient data to distinguish between the option belowb)The crystals are a mixture of f.c.c and b.c.c typesc)The crystals are of b.c.c typed)The crystals are of f.c.c typeCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















