GATE Exam > GATE Questions > A steel part with surface area of 125 cm2 is ...
Start Learning for Free
A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:
I = 12 + 0.2t
where, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).
I = 12 + 0.2t
where, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).
Correct answer is '5.0'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
A steel part with surface area of 125 cm2 is to be chrome coaled throu...
I = 12 + 0.2t
After time, ‘t’, Next infinitly small time ‘dt’ let heat deposited ‘dQ’.
∴ dQ = 2.50 × 10–2 (mm3/A.s) × 12 + 0.2t × dt As we have to convert this ‘s’ to ‘min’
∴ dQ = 2.50 × 10–2 (mm3/A × min) × 12 + 0.2t × dt
Considering cathode efficiency of 15%
dQ = 2.50 × 10–2 × 60 × (12 + 0.2t )dt × 0.15 mm3
∴ In 20 min,
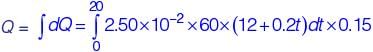 = 63 mm3
= 63 mm3
As area os 125 cm2
Plating thickness, t =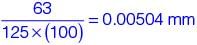 = 5.04 μm
= 5.04 μm
(As 1 cm2 = 100 mm2)
After time, ‘t’, Next infinitly small time ‘dt’ let heat deposited ‘dQ’.
∴ dQ = 2.50 × 10–2 (mm3/A.s) × 12 + 0.2t × dt As we have to convert this ‘s’ to ‘min’
∴ dQ = 2.50 × 10–2 (mm3/A × min) × 12 + 0.2t × dt
Considering cathode efficiency of 15%
dQ = 2.50 × 10–2 × 60 × (12 + 0.2t )dt × 0.15 mm3
∴ In 20 min,
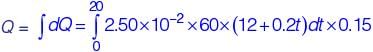 = 63 mm3
= 63 mm3As area os 125 cm2
Plating thickness, t =
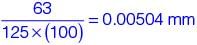 = 5.04 μm
= 5.04 μm(As 1 cm2 = 100 mm2)
Most Upvoted Answer
A steel part with surface area of 125 cm2 is to be chrome coaled throu...
First, let's calculate the total charge needed to plate the steel part.
The current (I) is given by I = 12 + 0.2t.
Since the part is submerged in the plating solution for 20 minutes, we can substitute t=20 into the equation to find the current at that time:
I = 12 + 0.2(20) = 12 + 4 = 16 A.
To calculate the total charge (Q) needed, we use the equation Q = I * t.
Q = 16 A * 20 min = 320 Coulombs.
Next, let's calculate the amount of chromium plated on the steel part.
The cathode efficiency of chromium is given as 15%, which means only 15% of the charge is used for plating chromium. Therefore, the actual charge used for plating chromium is:
Actual charge = 0.15 * 320 Coulombs = 48 Coulombs.
The plating constant of chromium acid sulphate is given as 2.50. This constant represents the amount of chromium plated per unit charge (Coulombs).
Amount of chromium plated = Actual charge * plating constant.
Amount of chromium plated = 48 Coulombs * 2.50 = 120 g.
Therefore, the steel part will be chrome coated with 120 grams of chromium.
The current (I) is given by I = 12 + 0.2t.
Since the part is submerged in the plating solution for 20 minutes, we can substitute t=20 into the equation to find the current at that time:
I = 12 + 0.2(20) = 12 + 4 = 16 A.
To calculate the total charge (Q) needed, we use the equation Q = I * t.
Q = 16 A * 20 min = 320 Coulombs.
Next, let's calculate the amount of chromium plated on the steel part.
The cathode efficiency of chromium is given as 15%, which means only 15% of the charge is used for plating chromium. Therefore, the actual charge used for plating chromium is:
Actual charge = 0.15 * 320 Coulombs = 48 Coulombs.
The plating constant of chromium acid sulphate is given as 2.50. This constant represents the amount of chromium plated per unit charge (Coulombs).
Amount of chromium plated = Actual charge * plating constant.
Amount of chromium plated = 48 Coulombs * 2.50 = 120 g.
Therefore, the steel part will be chrome coated with 120 grams of chromium.

|
Explore Courses for GATE exam
|

|
Similar GATE Doubts
A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer?
Question Description
A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer?.
A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer?.
Solutions for A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer?, a detailed solution for A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? has been provided alongside types of A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A steel part with surface area of 125 cm2 is to be chrome coaled through an electroplating process using chromium acid sulphate as an electrolyte. An increasing current is applied to the part according to the following current time relation:I = 12 + 0.2twhere, I = current (A) and t = time (minutes). The part is submerged in the plating solution for a duration of 20 minutes for plating purpose. Assuming the cathode efficiency of chromium to be 15% and the plating constant of chromium acid sulphate to be 2.50 × 10–2 mm3/A·s, the resulting coating thickness on the part surface is _________ μm (round off to one decimal place).Correct answer is '5.0'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















