NEET Exam > NEET Questions > The ratio of charge to mass of an electron in...
Start Learning for Free
The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?
- a)-1.76 x 108 coulombs/g
- b)1.76 x 10-8 coulombs/g
- c)-1.76 x 1010 coulombs/g
- d)- 1.76 x 10-10 coulombs/g
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The ratio of charge to mass of an electron in coulombs per gram was de...
Charge of an electron is
= −1.6 × 10−19C
Mass of electron is
9.1 × 10−31kg = 9.1 × 10−28g
Thus, Charge/mass ratio is
= −1.6 × 10−19C
Mass of electron is
9.1 × 10−31kg = 9.1 × 10−28g
Thus, Charge/mass ratio is
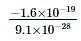
- ve sign is only a convention used by us
= - 1.76 × 108 coulombs/g
Most Upvoted Answer
The ratio of charge to mass of an electron in coulombs per gram was de...
J.J. Thomson's Experiment to Determine Charge to Mass Ratio of Electron
J.J. Thomson conducted an experiment to determine the charge to mass ratio of an electron. He used a cathode ray tube in which a high voltage was applied across a cathode and an anode. The cathode ray was produced due to the emission of negatively charged particles from the cathode, which then moved towards the anode in a straight line.
Determination of Charge to Mass Ratio
Thomson used two types of fields - electric and magnetic - to measure the charge to mass ratio of an electron. He observed that when an electric field was applied perpendicular to the direction of motion of the cathode rays, the rays were deflected towards the positive plate. Similarly, when a magnetic field was applied perpendicular to the direction of motion of the cathode rays, the rays were deflected towards the negative pole.
Thomson used the following formula to calculate the charge to mass ratio:
e/m = E/(B^2d^2)
where e/m is the charge to mass ratio of an electron, E is the electric field strength, B is the magnetic field strength, and d is the distance between the plates.
Value of Charge to Mass Ratio
J.J. Thomson's experiment yielded a value of -1.76 x 10^8 coulombs/gram for the charge to mass ratio of an electron. This value was significant because it provided evidence for the existence of subatomic particles, specifically electrons, and their properties.
Conclusion
J.J. Thomson's experiment was a groundbreaking discovery that led to the development of modern atomic theory. The charge to mass ratio of an electron is a fundamental constant that has been used in many significant scientific discoveries, including the development of the television and the electron microscope.
J.J. Thomson conducted an experiment to determine the charge to mass ratio of an electron. He used a cathode ray tube in which a high voltage was applied across a cathode and an anode. The cathode ray was produced due to the emission of negatively charged particles from the cathode, which then moved towards the anode in a straight line.
Determination of Charge to Mass Ratio
Thomson used two types of fields - electric and magnetic - to measure the charge to mass ratio of an electron. He observed that when an electric field was applied perpendicular to the direction of motion of the cathode rays, the rays were deflected towards the positive plate. Similarly, when a magnetic field was applied perpendicular to the direction of motion of the cathode rays, the rays were deflected towards the negative pole.
Thomson used the following formula to calculate the charge to mass ratio:
e/m = E/(B^2d^2)
where e/m is the charge to mass ratio of an electron, E is the electric field strength, B is the magnetic field strength, and d is the distance between the plates.
Value of Charge to Mass Ratio
J.J. Thomson's experiment yielded a value of -1.76 x 10^8 coulombs/gram for the charge to mass ratio of an electron. This value was significant because it provided evidence for the existence of subatomic particles, specifically electrons, and their properties.
Conclusion
J.J. Thomson's experiment was a groundbreaking discovery that led to the development of modern atomic theory. The charge to mass ratio of an electron is a fundamental constant that has been used in many significant scientific discoveries, including the development of the television and the electron microscope.
Free Test
FREE
| Start Free Test |
Community Answer
The ratio of charge to mass of an electron in coulombs per gram was de...
Charge /mass= -1.6×10^-19C/9.1×10^-28g=-1.75×10^8 C/g
therefore option A is correct
therefore option A is correct
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer?
Question Description
The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer?.
The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The ratio of charge to mass of an electron in coulombs per gram was determined by J.J. Thomson. He determined this ratio by measuring the deflection of cathode rays in electric and magnetic fields. What value did he find for this ratio?a)-1.76 x 108 coulombs/gb)1.76 x 10-8 coulombs/gc)-1.76 x 1010 coulombs/gd)- 1.76 x 10-10 coulombs/gCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























