GATE Exam > GATE Questions > Moist air is treated as an ideal gas mixture ...
Start Learning for Free
Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.
Correct answer is between '14.7,15.1'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
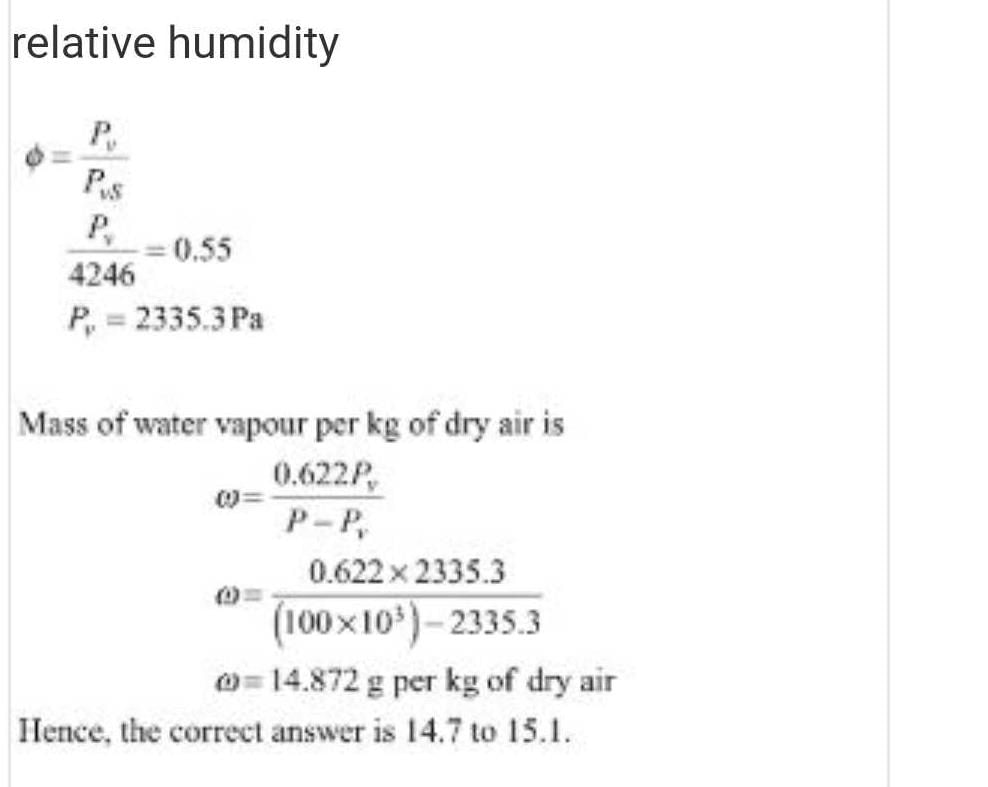
Moist air is treated as an ideal gas mixture of water vapor and dry ai...
Total pressure p=100kpa
temperature t=30 degree c
relative humidity


Most Upvoted Answer
Moist air is treated as an ideal gas mixture of water vapor and dry ai...
Free Test
FREE
| Start Free Test |
Community Answer
Moist air is treated as an ideal gas mixture of water vapor and dry ai...
°C, and the relative humidity is 50%. Calculate the partial pressure of water vapor and dry air in the mixture.
To calculate the partial pressure of water vapor and dry air in the mixture, we need to use the concept of partial pressure and the ideal gas law.
First, let's convert the temperature from Celsius to Kelvin:
T = 30°C + 273.15 = 303.15 K
Next, we need to calculate the vapor pressure of water at this temperature. We can use the Antoine equation to estimate it:
ln(P) = A - (B / (T + C))
Where P is the vapor pressure in kPa and T is the temperature in Kelvin. For water, the Antoine coefficients are:
A = 8.07131
B = 1730.63
C = 233.426
Using these values, we can calculate the vapor pressure of water:
ln(P) = 8.07131 - (1730.63 / (303.15 + 233.426))
ln(P) = 8.07131 - (1730.63 / 536.576)
ln(P) = 8.07131 - 3.2252
ln(P) = 4.84611
P = e^4.84611 = 127.58 kPa
Now, let's calculate the mole fraction of water vapor in the mixture:
X_water = P_water / P_total
X_water = 127.58 kPa / 100 kPa
X_water = 1.2758
The mole fraction of dry air can be calculated as:
X_air = 1 - X_water
X_air = 1 - 1.2758
X_air = -0.2758
However, we cannot have a negative mole fraction. This indicates an error in the calculation or assumptions made. It is likely due to the fact that the relative humidity value provided (50%) is not consistent with the temperature and total pressure given. A relative humidity of 50% at 30°C typically corresponds to a vapor pressure of water around 4.24 kPa, which is much lower than the value we calculated.
Therefore, we cannot accurately calculate the partial pressure of water vapor and dry air in the mixture using the given information.
To calculate the partial pressure of water vapor and dry air in the mixture, we need to use the concept of partial pressure and the ideal gas law.
First, let's convert the temperature from Celsius to Kelvin:
T = 30°C + 273.15 = 303.15 K
Next, we need to calculate the vapor pressure of water at this temperature. We can use the Antoine equation to estimate it:
ln(P) = A - (B / (T + C))
Where P is the vapor pressure in kPa and T is the temperature in Kelvin. For water, the Antoine coefficients are:
A = 8.07131
B = 1730.63
C = 233.426
Using these values, we can calculate the vapor pressure of water:
ln(P) = 8.07131 - (1730.63 / (303.15 + 233.426))
ln(P) = 8.07131 - (1730.63 / 536.576)
ln(P) = 8.07131 - 3.2252
ln(P) = 4.84611
P = e^4.84611 = 127.58 kPa
Now, let's calculate the mole fraction of water vapor in the mixture:
X_water = P_water / P_total
X_water = 127.58 kPa / 100 kPa
X_water = 1.2758
The mole fraction of dry air can be calculated as:
X_air = 1 - X_water
X_air = 1 - 1.2758
X_air = -0.2758
However, we cannot have a negative mole fraction. This indicates an error in the calculation or assumptions made. It is likely due to the fact that the relative humidity value provided (50%) is not consistent with the temperature and total pressure given. A relative humidity of 50% at 30°C typically corresponds to a vapor pressure of water around 4.24 kPa, which is much lower than the value we calculated.
Therefore, we cannot accurately calculate the partial pressure of water vapor and dry air in the mixture using the given information.

|
Explore Courses for GATE exam
|

|
Similar GATE Doubts
Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer?
Question Description
Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer?.
Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer?.
Solutions for Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer?, a detailed solution for Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? has been provided alongside types of Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Moist air is treated as an ideal gas mixture of water vapor and dry air (molecular weight of air = 28.84 and molecular weight of water = 18). At a location, the total pressure is 100 kPa, the temperature is 30°C and the relative humidity is 55%. Given that the saturation pressure of water at 30°C is 4246 Pa, the mass of water vapor per kg of dry air is _____________ grams.Correct answer is between '14.7,15.1'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















