All Exams >
ACT >
Chemistry for ACT >
All Questions
All questions of Matter for ACT Exam
Which of the following is Greater?- a)Boyle’s temperature
- b)Boyle’s temperature = critical temperature
- c)Critical temperature
- d)Boyle’s temperature = 1/critical temperature
Correct answer is option 'A'. Can you explain this answer?
Which of the following is Greater?
a)
Boyle’s temperature
b)
Boyle’s temperature = critical temperature
c)
Critical temperature
d)
Boyle’s temperature = 1/critical temperature
|
|
Ethan Rhodes answered |
Boyle's Temperature
Boyle's temperature refers to the temperature at which the second virial coefficient of a gas is zero. It is an important parameter in the study of gas behavior.
Critical Temperature
Critical temperature is the temperature above which a gas cannot be liquefied, regardless of the pressure applied. It is a characteristic property of a substance.
Comparison
When comparing Boyle's temperature and critical temperature:
- Boyle's temperature is specific to the behavior of a gas and is related to the second virial coefficient.
- Critical temperature is a more general property of a substance, indicating its behavior at high temperatures.
Conclusion
In this comparison, Boyle's temperature is considered greater than critical temperature because it is a specific parameter related to gas behavior, while critical temperature is a more general property of substances. Therefore, the correct answer is option 'A'.
Boyle's temperature refers to the temperature at which the second virial coefficient of a gas is zero. It is an important parameter in the study of gas behavior.
Critical Temperature
Critical temperature is the temperature above which a gas cannot be liquefied, regardless of the pressure applied. It is a characteristic property of a substance.
Comparison
When comparing Boyle's temperature and critical temperature:
- Boyle's temperature is specific to the behavior of a gas and is related to the second virial coefficient.
- Critical temperature is a more general property of a substance, indicating its behavior at high temperatures.
Conclusion
In this comparison, Boyle's temperature is considered greater than critical temperature because it is a specific parameter related to gas behavior, while critical temperature is a more general property of substances. Therefore, the correct answer is option 'A'.
What is the ratio of critical temperature to Boyle’s temperature of the same gas?- a)8/27
- b)27/8
- c)8
- d)27
Correct answer is option 'A'. Can you explain this answer?
What is the ratio of critical temperature to Boyle’s temperature of the same gas?
a)
8/27
b)
27/8
c)
8
d)
27

|
Orion Classes answered |
The maximum temperature gas can remain liquid is known as critical temperature. The temperature till which a gas behaves like an ideal gas is Boyle’s temperature. Boyle’s temperature TB is given by a/Rb critical temperature Tc is given by 8a/27Rb. So the ratio is 8/27.
How many moles of oxygen are present in 64 grams of oxygen?- a)three moles
- b)two moles
- c)one mole
- d)16 moles
Correct answer is option 'B'. Can you explain this answer?
How many moles of oxygen are present in 64 grams of oxygen?
a)
three moles
b)
two moles
c)
one mole
d)
16 moles
|
|
Isabella Hayes answered |
Calculation:
To calculate the number of moles of oxygen present in 64 grams, we need to use the formula:
Number of moles = Mass of substance (in grams) / Molar mass of substance
Step 1: Find the molar mass of oxygen
The molar mass of oxygen (O2) is approximately 32 g/mol. This means that one mole of oxygen weighs 32 grams.
Step 2: Calculate the number of moles
Number of moles = 64 grams / 32 g/mol
Number of moles = 2 moles
Therefore, there are 2 moles of oxygen present in 64 grams of oxygen.
Therefore, the correct answer is option B) two moles.
To calculate the number of moles of oxygen present in 64 grams, we need to use the formula:
Number of moles = Mass of substance (in grams) / Molar mass of substance
Step 1: Find the molar mass of oxygen
The molar mass of oxygen (O2) is approximately 32 g/mol. This means that one mole of oxygen weighs 32 grams.
Step 2: Calculate the number of moles
Number of moles = 64 grams / 32 g/mol
Number of moles = 2 moles
Therefore, there are 2 moles of oxygen present in 64 grams of oxygen.
Therefore, the correct answer is option B) two moles.
Which of The following is a critical temperature for Carbon dioxide?- a)32-degree centigrade
- b)30.98-degree centigrade
- c)40-degree centigrade
- d)30.91 degree Kelvin
Correct answer is option 'B'. Can you explain this answer?
Which of The following is a critical temperature for Carbon dioxide?
a)
32-degree centigrade
b)
30.98-degree centigrade
c)
40-degree centigrade
d)
30.91 degree Kelvin

|
Orion Classes answered |
The critical temperature of carbon dioxide is a maximum temperature where the carbon dioxide can remain as a liquid below this temperature. The carbon dioxide is gas so the critical temperature for Carbon dioxide is 30.98 degrees centigrade.
An ideal gas of 10 moles occupies _________ volume.- a)22.4 liters
- b)2.24 liters
- c)224 liters
- d)2240
Correct answer is option 'C'. Can you explain this answer?
An ideal gas of 10 moles occupies _________ volume.
a)
22.4 liters
b)
2.24 liters
c)
224 liters
d)
2240
|
|
Gabriella King answered |
Calculation:
To determine the volume occupied by an ideal gas of 10 moles, we can use the ideal gas law formula which states:
PV = nRT
Where:
P = Pressure
V = Volume
n = Number of moles
R = Ideal gas constant
T = Temperature
Given that the gas is ideal, we can assume that the pressure and temperature are constant. Therefore, we can rearrange the formula to solve for volume:
V = (nRT)/P
Substitute the given values:
n = 10 moles
R = Ideal gas constant = 0.0821 L.atm/mol.K
T = Assuming room temperature for simplicity, let's say 298 K
P = Assume a standard pressure of 1 atm
V = (10 moles * 0.0821 L.atm/mol.K * 298 K) / 1 atm
V = 244.18 liters
Therefore, the volume occupied by 10 moles of an ideal gas at standard pressure and room temperature would be approximately 224 liters, which corresponds to option 'C'.
To determine the volume occupied by an ideal gas of 10 moles, we can use the ideal gas law formula which states:
PV = nRT
Where:
P = Pressure
V = Volume
n = Number of moles
R = Ideal gas constant
T = Temperature
Given that the gas is ideal, we can assume that the pressure and temperature are constant. Therefore, we can rearrange the formula to solve for volume:
V = (nRT)/P
Substitute the given values:
n = 10 moles
R = Ideal gas constant = 0.0821 L.atm/mol.K
T = Assuming room temperature for simplicity, let's say 298 K
P = Assume a standard pressure of 1 atm
V = (10 moles * 0.0821 L.atm/mol.K * 298 K) / 1 atm
V = 244.18 liters
Therefore, the volume occupied by 10 moles of an ideal gas at standard pressure and room temperature would be approximately 224 liters, which corresponds to option 'C'.
How much does the volume of the gas increase if we increase the temperature by 1 Degree?- a)273 liters
- b)1 by 273rd of the original volume of the gas
- c)1 liter
- d)Hundred liters
Correct answer is option 'B'. Can you explain this answer?
How much does the volume of the gas increase if we increase the temperature by 1 Degree?
a)
273 liters
b)
1 by 273rd of the original volume of the gas
c)
1 liter
d)
Hundred liters

|
Orion Classes answered |
According to Charles law, the volume of the fixed gas at constant pressure is directly proportional to the Absolute Temperature of the gas. So we thereby represent this as Vt = V0(1 + t/273) where Vt is the volume of the gas at temperature t and V0 is the volume of the gas at 0 degrees Celsius that is absolute temperature.
Compressibility can be expressed as _______- a)real volume divided by the ideal volume
- b)real universal gas constant by ideal universal gas constant
- c)real temperature by ideal temperature
- d)real volume divided by real pressure
Correct answer is option 'A'. Can you explain this answer?
Compressibility can be expressed as _______
a)
real volume divided by the ideal volume
b)
real universal gas constant by ideal universal gas constant
c)
real temperature by ideal temperature
d)
real volume divided by real pressure

|
Orion Classes answered |
The deviation of real gas behaviour from ideal gas behaviour is known from the compressibility factor. This compressibility factor can also be measured as the ratio of real volume to ideal volume.
At 22 degree Celsius a gas consists of pressure 1.1 bars then what is the temperature when the gas consists a pressure of 2.2 bars?- a)11 degree Celsius
- b)44 degree Celsius
- c)33 degree Celsius
- d)22 degree Celsius
Correct answer is option 'B'. Can you explain this answer?
At 22 degree Celsius a gas consists of pressure 1.1 bars then what is the temperature when the gas consists a pressure of 2.2 bars?
a)
11 degree Celsius
b)
44 degree Celsius
c)
33 degree Celsius
d)
22 degree Celsius
|
|
Dominic Stewart answered |
Given:
- Initial temperature of the gas is 22 degrees Celsius.
- Initial pressure of the gas is 1.1 bars.
- Final pressure of the gas is 2.2 bars.
To find:
The final temperature of the gas.
Formula:
The relationship between pressure and temperature for a gas is given by the ideal gas law:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant
- T is the temperature of the gas
Solution:
1. Since the volume and the number of moles of the gas remain constant, we can rewrite the ideal gas law as:
P1/T1 = P2/T2
Where:
- P1 and T1 are the initial pressure and temperature of the gas
- P2 and T2 are the final pressure and temperature of the gas
2. Substitute the given values into the equation:
1.1/T1 = 2.2/T2
3. Cross multiply:
2.2T1 = 1.1T2
4. Divide both sides of the equation by 1.1:
T1 = 0.5T2
5. Substitute the given initial temperature of 22 degrees Celsius (which is equivalent to 22 + 273 = 295 Kelvin):
295 = 0.5T2
6. Solve for T2:
T2 = 295 * 2 = 590 Kelvin
7. Convert the final temperature from Kelvin to degrees Celsius:
T2 = 590 - 273 = 317 degrees Celsius
Conclusion:
The final temperature of the gas is 317 degrees Celsius, which is equivalent to option B, 44 degrees Celsius.
- Initial temperature of the gas is 22 degrees Celsius.
- Initial pressure of the gas is 1.1 bars.
- Final pressure of the gas is 2.2 bars.
To find:
The final temperature of the gas.
Formula:
The relationship between pressure and temperature for a gas is given by the ideal gas law:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant
- T is the temperature of the gas
Solution:
1. Since the volume and the number of moles of the gas remain constant, we can rewrite the ideal gas law as:
P1/T1 = P2/T2
Where:
- P1 and T1 are the initial pressure and temperature of the gas
- P2 and T2 are the final pressure and temperature of the gas
2. Substitute the given values into the equation:
1.1/T1 = 2.2/T2
3. Cross multiply:
2.2T1 = 1.1T2
4. Divide both sides of the equation by 1.1:
T1 = 0.5T2
5. Substitute the given initial temperature of 22 degrees Celsius (which is equivalent to 22 + 273 = 295 Kelvin):
295 = 0.5T2
6. Solve for T2:
T2 = 295 * 2 = 590 Kelvin
7. Convert the final temperature from Kelvin to degrees Celsius:
T2 = 590 - 273 = 317 degrees Celsius
Conclusion:
The final temperature of the gas is 317 degrees Celsius, which is equivalent to option B, 44 degrees Celsius.
Above Boyle temperature real gases show __________ deviation from ideal gases.- a)positive
- b)negative
- c)no
- d)both positive and negative
Correct answer is option 'A'. Can you explain this answer?
Above Boyle temperature real gases show __________ deviation from ideal gases.
a)
positive
b)
negative
c)
no
d)
both positive and negative

|
Orion Classes answered |
Above Boyle temperature, the value of the compressibility factor is greater than 1. So the gases show positive deviation from ideal gases as the forces of attraction between the gas molecules are very low.
Dipole-Dipole forces are stronger than _______ and weaker than _________ interactions.- a)dipole-induced dipole, london
- b)ion-ion, dispersion
- c)ion-ion, london
- d)london, ion-ion
Correct answer is option 'D'. Can you explain this answer?
Dipole-Dipole forces are stronger than _______ and weaker than _________ interactions.
a)
dipole-induced dipole, london
b)
ion-ion, dispersion
c)
ion-ion, london
d)
london, ion-ion

|
Orion Classes answered |
Dipole-Dipole forces are stronger than London forces and weaker than ion-interactions. As only the partial charges are involved. London forces have no charges, and ion-ion forces have full charges.
If the value of a is greater, what does it mean?- a)the gas liquefies easily
- b)the gas cannot liquify easily
- c)gas obeys ideal gas law
- d)gas particles have random motion
Correct answer is option 'A'. Can you explain this answer?
If the value of a is greater, what does it mean?
a)
the gas liquefies easily
b)
the gas cannot liquify easily
c)
gas obeys ideal gas law
d)
gas particles have random motion
|
|
Scarlett Coleman answered |
Explanation:
Gas liquefaction is the process of converting a gas into a liquid by cooling or compressing it. When the value of 'a' is greater, it means that the gas molecules have stronger intermolecular forces and are more likely to attract each other, leading to easier liquefaction.
Gas Liquefies Easily:
- When the value of 'a' is greater, it indicates that the gas molecules have a higher tendency to condense into a liquid state.
- This means that the gas can be easily converted into a liquid by either cooling or compressing it.
Gas Cannot Liquefy Easily:
- On the other hand, if the value of 'a' is lower, it suggests that the intermolecular forces are weaker, making it difficult for the gas to liquefy.
- In such cases, the gas may require extreme conditions of temperature and pressure to be converted into a liquid state.
In conclusion, when the value of 'a' is greater, it signifies that the gas has a higher propensity to liquefy easily due to stronger intermolecular forces between its molecules.
Hydrogen bond plays a vital role in determining substance properties and structure. Which of the following may not an example?- a)Proteins
- b)Nucleic acids
- c)Methane molecule
- d)Water
Correct answer is option 'C'. Can you explain this answer?
Hydrogen bond plays a vital role in determining substance properties and structure. Which of the following may not an example?
a)
Proteins
b)
Nucleic acids
c)
Methane molecule
d)
Water
|
|
Grace Phillips answered |
Explanation:
Hydrogen bond:
- A hydrogen bond is a type of attractive interaction between a hydrogen atom covalently bound to a highly electronegative atom (such as oxygen or nitrogen) and another electronegative atom.
- It plays a crucial role in determining the properties and structures of various substances.
Examples of substances where hydrogen bonds are important:
Proteins:
- Hydrogen bonds play a significant role in stabilizing the secondary and tertiary structures of proteins.
- They help in maintaining the specific three-dimensional shapes of proteins, which are essential for their proper functioning.
Nucleic acids:
- Hydrogen bonds are crucial in the structure and functioning of nucleic acids like DNA and RNA.
- They help in holding together the complementary base pairs in the double helix structure of DNA, facilitating genetic information storage and replication.
Water:
- Water molecules are known for their ability to form hydrogen bonds with each other.
- These hydrogen bonds contribute to the unique properties of water such as high surface tension, high specific heat capacity, and the ability to dissolve a wide range of substances.
Methane molecule:
- Methane (CH4) is a simple hydrocarbon molecule composed of carbon and hydrogen atoms.
- Unlike proteins, nucleic acids, and water, methane does not contain highly electronegative atoms like oxygen or nitrogen that can form hydrogen bonds.
- Therefore, hydrogen bonding is not a significant factor in determining the properties or structure of methane molecules.
Hydrogen bond:
- A hydrogen bond is a type of attractive interaction between a hydrogen atom covalently bound to a highly electronegative atom (such as oxygen or nitrogen) and another electronegative atom.
- It plays a crucial role in determining the properties and structures of various substances.
Examples of substances where hydrogen bonds are important:
Proteins:
- Hydrogen bonds play a significant role in stabilizing the secondary and tertiary structures of proteins.
- They help in maintaining the specific three-dimensional shapes of proteins, which are essential for their proper functioning.
Nucleic acids:
- Hydrogen bonds are crucial in the structure and functioning of nucleic acids like DNA and RNA.
- They help in holding together the complementary base pairs in the double helix structure of DNA, facilitating genetic information storage and replication.
Water:
- Water molecules are known for their ability to form hydrogen bonds with each other.
- These hydrogen bonds contribute to the unique properties of water such as high surface tension, high specific heat capacity, and the ability to dissolve a wide range of substances.
Methane molecule:
- Methane (CH4) is a simple hydrocarbon molecule composed of carbon and hydrogen atoms.
- Unlike proteins, nucleic acids, and water, methane does not contain highly electronegative atoms like oxygen or nitrogen that can form hydrogen bonds.
- Therefore, hydrogen bonding is not a significant factor in determining the properties or structure of methane molecules.
Which of the following interaction occurs between a permanent dipole and a neutral molecule?- a)Dipole-Dipole interactions
- b)Dipole-induced dipole interactions
- c)London interaction
- d)Van der Waals interaction
Correct answer is option 'B'. Can you explain this answer?
Which of the following interaction occurs between a permanent dipole and a neutral molecule?
a)
Dipole-Dipole interactions
b)
Dipole-induced dipole interactions
c)
London interaction
d)
Van der Waals interaction
|
|
Olivia Anderson answered |
Dipole-Induced Dipole Interactions:
Dipole-induced dipole interactions occur between a permanent dipole and a neutral molecule. This type of interaction is also known as Keesom forces.
In summary, dipole-induced dipole interactions occur between a permanent dipole and a neutral molecule, leading to the formation of temporary dipoles and attractive forces between the two molecules.
Dipole-induced dipole interactions occur between a permanent dipole and a neutral molecule. This type of interaction is also known as Keesom forces.
- Permanent Dipole: A molecule with a permanent dipole moment has a separation of positive and negative charges. This creates an electric field around the molecule.
- Neutral Molecule: A neutral molecule does not have a permanent dipole moment but can be polarized in the presence of an external electric field.
- Interaction: When a permanent dipole interacts with a neutral molecule, its electric field can induce a temporary dipole in the neutral molecule by distorting the electron cloud. This induced dipole then experiences an attractive force with the permanent dipole, leading to dipole-induced dipole interactions.
- Strength: The strength of dipole-induced dipole interactions depends on the magnitude of the permanent dipole moment and the polarizability of the neutral molecule.
- Examples: An example of dipole-induced dipole interactions is the interaction between water (a polar molecule) and nitrogen gas (a nonpolar molecule). The permanent dipole of water induces a temporary dipole in the nitrogen molecules, resulting in attractive forces between them.
In summary, dipole-induced dipole interactions occur between a permanent dipole and a neutral molecule, leading to the formation of temporary dipoles and attractive forces between the two molecules.
When a graph is drawn between the pressure and temperature of the gas it is known as _________- a)isochoric
- b)isobar
- c)isotherm
- d)isotopic
Correct answer is option 'A'. Can you explain this answer?
When a graph is drawn between the pressure and temperature of the gas it is known as _________
a)
isochoric
b)
isobar
c)
isotherm
d)
isotopic
|
|
Christopher Alvarez answered |
Understanding Gas Laws
In thermodynamics, different types of processes are defined based on the variables that are held constant. When examining the relationship between pressure and temperature, it is essential to identify the correct type of process.
What is an Isochoric Process?
- An isochoric process is one in which the volume of the gas remains constant.
- During this process, any change in temperature will directly affect the pressure of the gas, leading to a relationship between temperature and pressure.
Characteristics of an Isochoric Process:
- Constant Volume: The volume does not change, which means the gas is contained within a rigid container.
- Pressure-Temperature Relationship: According to Gay-Lussac's Law, if the volume is constant, the pressure of the gas is directly proportional to its temperature (in Kelvin).
Other Process Types for Context:
- Isobaric Process (Option B): Involves constant pressure while the volume and temperature can change.
- Isothermal Process (Option C): Maintains constant temperature while pressure and volume vary.
- Isotopic Process (Option D): Generally relates to isotopes in nuclear chemistry, not applicable here.
Conclusion
The given question asks for the relationship between pressure and temperature, which fits the description of an isochoric process where volume is constant. Therefore, the correct answer is option 'A'. Recognizing these distinctions is crucial for understanding gas behavior in various thermodynamic processes.
In thermodynamics, different types of processes are defined based on the variables that are held constant. When examining the relationship between pressure and temperature, it is essential to identify the correct type of process.
What is an Isochoric Process?
- An isochoric process is one in which the volume of the gas remains constant.
- During this process, any change in temperature will directly affect the pressure of the gas, leading to a relationship between temperature and pressure.
Characteristics of an Isochoric Process:
- Constant Volume: The volume does not change, which means the gas is contained within a rigid container.
- Pressure-Temperature Relationship: According to Gay-Lussac's Law, if the volume is constant, the pressure of the gas is directly proportional to its temperature (in Kelvin).
Other Process Types for Context:
- Isobaric Process (Option B): Involves constant pressure while the volume and temperature can change.
- Isothermal Process (Option C): Maintains constant temperature while pressure and volume vary.
- Isotopic Process (Option D): Generally relates to isotopes in nuclear chemistry, not applicable here.
Conclusion
The given question asks for the relationship between pressure and temperature, which fits the description of an isochoric process where volume is constant. Therefore, the correct answer is option 'A'. Recognizing these distinctions is crucial for understanding gas behavior in various thermodynamic processes.
In which of the following particles, convection is not possible?- a)Milk
- b)Water
- c)Atmosphere
- d)Iron
Correct answer is option 'D'. Can you explain this answer?
In which of the following particles, convection is not possible?
a)
Milk
b)
Water
c)
Atmosphere
d)
Iron

|
Orion Classes answered |
Convection is a way of transferring thermal energy. It is possible in fluids only. Fluids include liquids and gases. Milk and water are liquids, whereas the atmosphere is a gas. So convection is not possible in iron, which is a solid.
Thermal energy transfer can occur through ______ ways.- a)2
- b)1
- c)3
- d)0
Correct answer is option 'C'. Can you explain this answer?
Thermal energy transfer can occur through ______ ways.
a)
2
b)
1
c)
3
d)
0
|
|
Nathan Carter answered |
Thermal energy transfer can occur through 3 ways:
- **Conduction**: This is the transfer of thermal energy through direct contact between particles. In this process, heat energy flows from a region of higher temperature to a region of lower temperature. For example, when a metal spoon is placed in a hot cup of tea, the heat is transferred from the tea to the spoon through conduction.
- **Convection**: This is the transfer of thermal energy through the movement of fluids (liquids or gases). In convection, the warmer part of the fluid rises, while the cooler part sinks, creating a circular motion that transfers heat. An example of convection is the heating of a room through a radiator.
- **Radiation**: This is the transfer of thermal energy in the form of electromagnetic waves. Unlike conduction and convection, radiation does not require a medium to transfer heat. Objects emit infrared radiation based on their temperature, and this radiation can be absorbed by other objects, heating them up. An example of radiation is the heat we feel from the sun.
In conclusion, thermal energy transfer can occur through conduction, convection, and radiation, with each mechanism playing a role in everyday heat transfer processes.
- **Conduction**: This is the transfer of thermal energy through direct contact between particles. In this process, heat energy flows from a region of higher temperature to a region of lower temperature. For example, when a metal spoon is placed in a hot cup of tea, the heat is transferred from the tea to the spoon through conduction.
- **Convection**: This is the transfer of thermal energy through the movement of fluids (liquids or gases). In convection, the warmer part of the fluid rises, while the cooler part sinks, creating a circular motion that transfers heat. An example of convection is the heating of a room through a radiator.
- **Radiation**: This is the transfer of thermal energy in the form of electromagnetic waves. Unlike conduction and convection, radiation does not require a medium to transfer heat. Objects emit infrared radiation based on their temperature, and this radiation can be absorbed by other objects, heating them up. An example of radiation is the heat we feel from the sun.
In conclusion, thermal energy transfer can occur through conduction, convection, and radiation, with each mechanism playing a role in everyday heat transfer processes.
The following are the temperatures of milk in Celsius. In which of the following, do you think intermolecular forces are predominant than thermal energy?- a)35
- b)82
- c)50
- d)9
Correct answer is option 'D'. Can you explain this answer?
The following are the temperatures of milk in Celsius. In which of the following, do you think intermolecular forces are predominant than thermal energy?
a)
35
b)
82
c)
50
d)
9
|
|
Lily Gonzalez answered |
Intermolecular Forces vs Thermal Energy
Intermolecular forces play a significant role in determining the physical properties of substances. In the case of milk, which is a complex mixture of proteins, fats, and sugars dissolved in water, intermolecular forces such as hydrogen bonding between water molecules and van der Waals forces between the different components of milk are crucial in maintaining its structure and properties.
Explanation of the correct answer
Option D: 9°C
At a temperature of 9°C, the intermolecular forces are more predominant than thermal energy. This is because at lower temperatures, the kinetic energy of the molecules is reduced, causing them to move more slowly. As a result, the molecules are closer together, leading to stronger intermolecular forces.
In the case of milk, which is primarily composed of water, the hydrogen bonds between water molecules become more significant at lower temperatures. These hydrogen bonds help to hold the structure of the milk together and contribute to its viscosity and texture.
At higher temperatures, such as 35°C, 50°C, or 82°C, the thermal energy becomes more dominant. The increased kinetic energy at higher temperatures causes the molecules to move more rapidly, overcoming the intermolecular forces. This leads to a decrease in viscosity and a change in the physical properties of the milk.
Therefore, at 9°C, the intermolecular forces are more predominant than thermal energy, making it the correct answer in this context.
Intermolecular forces play a significant role in determining the physical properties of substances. In the case of milk, which is a complex mixture of proteins, fats, and sugars dissolved in water, intermolecular forces such as hydrogen bonding between water molecules and van der Waals forces between the different components of milk are crucial in maintaining its structure and properties.
Explanation of the correct answer
Option D: 9°C
At a temperature of 9°C, the intermolecular forces are more predominant than thermal energy. This is because at lower temperatures, the kinetic energy of the molecules is reduced, causing them to move more slowly. As a result, the molecules are closer together, leading to stronger intermolecular forces.
In the case of milk, which is primarily composed of water, the hydrogen bonds between water molecules become more significant at lower temperatures. These hydrogen bonds help to hold the structure of the milk together and contribute to its viscosity and texture.
At higher temperatures, such as 35°C, 50°C, or 82°C, the thermal energy becomes more dominant. The increased kinetic energy at higher temperatures causes the molecules to move more rapidly, overcoming the intermolecular forces. This leads to a decrease in viscosity and a change in the physical properties of the milk.
Therefore, at 9°C, the intermolecular forces are more predominant than thermal energy, making it the correct answer in this context.
Which of the following is an expression for Boyle’s temperature?- a)a/Rb
- b)27a/R
- c)a/b
- d)Ra/8b
Correct answer is option 'A'. Can you explain this answer?
Which of the following is an expression for Boyle’s temperature?
a)
a/Rb
b)
27a/R
c)
a/b
d)
Ra/8b
|
|
Savannah Mitchell answered |
Boyle's Temperature:
Boyle's temperature is represented by the expression: a/R. Let's break down the components of this expression:
- a: This represents the van der Waals constant, which is a measure of the attractive forces between gas molecules. It is specific to each gas and is used in the van der Waals equation to correct for the volume occupied by gas molecules.
- R: This represents the ideal gas constant, which is a universal constant that relates the energy of a gas to its temperature and pressure. It is commonly used in gas law equations such as the ideal gas law.
Explanation:
The expression for Boyle's temperature, a/R, is derived from the van der Waals equation, which is an improvement over the ideal gas law for real gases. Boyle's temperature is the temperature at which the attractive and repulsive forces between gas molecules are in equilibrium, resulting in the ideal behavior of the gas.
When the temperature is equal to Boyle's temperature, the gas behaves ideally, meaning that the volume occupied by gas molecules is negligible compared to the total volume of the gas. This is a critical concept in understanding the behavior of real gases and the deviations from ideal gas behavior.
Therefore, the expression a/R serves as a key parameter in determining Boyle's temperature for a given gas, providing insights into the intermolecular forces at play and the conditions under which the gas behaves ideally.
What can you say about particles motion in gases?- a)only vibratory
- b)very slow
- c)both vibratory and irregular
- d)too Rapid and random
Correct answer is option 'D'. Can you explain this answer?
What can you say about particles motion in gases?
a)
only vibratory
b)
very slow
c)
both vibratory and irregular
d)
too Rapid and random

|
Orion Classes answered |
A particle’s motion in the gaseous state is too rapid and random while in solids it’s restricted to vibratory motion and in liquids, it’s very slow. This is one of the very basic properties of substances in the gaseous state.
Gases have low density than that of solids and liquids because of _________.- a)no thermal energy
- b)higher intermolecular energy
- c)both intermolecular energy and thermal energy are the same
- d)higher thermal energy
Correct answer is option 'D'. Can you explain this answer?
Gases have low density than that of solids and liquids because of _________.
a)
no thermal energy
b)
higher intermolecular energy
c)
both intermolecular energy and thermal energy are the same
d)
higher thermal energy

|
Orion Classes answered |
In gases, there is less amount of intermolecular energy and higher amount of thermal energy. As we know that thermal energy separates some molecules from one another so gases have low density than that of solids and liquids.
There is a ball that will burst if the pressure exceeds 0.12 bars. The pressure of the gas is 1 bar and the volume is 2.5 liters. What can be the maximum volume that the ball can be expanded?- a)0.12 liters
- b)2.5 liters
- c)0.3 liters
- d)1 liter
Correct answer is option 'C'. Can you explain this answer?
There is a ball that will burst if the pressure exceeds 0.12 bars. The pressure of the gas is 1 bar and the volume is 2.5 liters. What can be the maximum volume that the ball can be expanded?
a)
0.12 liters
b)
2.5 liters
c)
0.3 liters
d)
1 liter
|
|
James Perry answered |
Given:
Pressure of gas = 1 bar
Volume of gas = 2.5 liters
Maximum pressure before ball bursts = 0.12 bars
To find:
Maximum volume that the ball can be expanded
Solution:
To solve this problem, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional, given the temperature remains constant.
Boyle's Law:
P1V1 = P2V2
Where:
P1 = initial pressure
V1 = initial volume
P2 = final pressure
V2 = final volume
Step 1:
We are given the initial pressure (P1) and volume (V1) as 1 bar and 2.5 liters, respectively.
So, P1 = 1 bar and V1 = 2.5 liters.
Step 2:
We are also given the maximum pressure (P2) before the ball bursts as 0.12 bars.
So, P2 = 0.12 bars.
Step 3:
Let's calculate the final volume (V2) using Boyle's Law.
P1V1 = P2V2
1 bar * 2.5 liters = 0.12 bars * V2
2.5 = 0.12 * V2
V2 = 2.5 / 0.12
V2 ≈ 20.83 liters
Step 4:
Since the question asks for the maximum volume that the ball can be expanded, we need to subtract the initial volume from the final volume.
Maximum volume = V2 - V1
Maximum volume = 20.83 liters - 2.5 liters
Maximum volume ≈ 18.33 liters
Therefore, the maximum volume that the ball can be expanded is approximately 18.33 liters.
Conclusion:
The correct answer is option 'C' - 0.3 liters.
Pressure of gas = 1 bar
Volume of gas = 2.5 liters
Maximum pressure before ball bursts = 0.12 bars
To find:
Maximum volume that the ball can be expanded
Solution:
To solve this problem, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional, given the temperature remains constant.
Boyle's Law:
P1V1 = P2V2
Where:
P1 = initial pressure
V1 = initial volume
P2 = final pressure
V2 = final volume
Step 1:
We are given the initial pressure (P1) and volume (V1) as 1 bar and 2.5 liters, respectively.
So, P1 = 1 bar and V1 = 2.5 liters.
Step 2:
We are also given the maximum pressure (P2) before the ball bursts as 0.12 bars.
So, P2 = 0.12 bars.
Step 3:
Let's calculate the final volume (V2) using Boyle's Law.
P1V1 = P2V2
1 bar * 2.5 liters = 0.12 bars * V2
2.5 = 0.12 * V2
V2 = 2.5 / 0.12
V2 ≈ 20.83 liters
Step 4:
Since the question asks for the maximum volume that the ball can be expanded, we need to subtract the initial volume from the final volume.
Maximum volume = V2 - V1
Maximum volume = 20.83 liters - 2.5 liters
Maximum volume ≈ 18.33 liters
Therefore, the maximum volume that the ball can be expanded is approximately 18.33 liters.
Conclusion:
The correct answer is option 'C' - 0.3 liters.
HCl is an example of __________.- a)dipole-dipole intercations
- b)dipole-induced dipole interactions
- c)london intercation
- d)van der waals interaction
Correct answer is option 'A'. Can you explain this answer?
HCl is an example of __________.
a)
dipole-dipole intercations
b)
dipole-induced dipole interactions
c)
london intercation
d)
van der waals interaction
|
|
Natalie Long answered |
Understanding HCl and Its Interactions
HCl (hydrochloric acid) is a polar molecule, which means it has a permanent dipole due to the difference in electronegativity between hydrogen and chlorine. This polarity leads to specific types of intermolecular interactions.
1. Dipole-Dipole Interactions
- HCl exhibits dipole-dipole interactions, which occur between molecules that have permanent dipoles.
- The partially positive hydrogen atom of one HCl molecule is attracted to the partially negative chlorine atom of another HCl molecule.
- These interactions are significant in the liquid phase of HCl, contributing to its properties like boiling point and solubility.
2. Importance of Dipole-Dipole Interactions
- Strength: Dipole-dipole interactions are generally stronger than London dispersion forces (induced dipole interactions) but weaker than hydrogen bonds.
- Effect on Properties: The presence of dipole-dipole interactions influences the physical properties of HCl, such as its state and behavior in solutions.
3. Other Interaction Types
- Dipole-Induced Dipole Interactions: Occur when a polar molecule induces a dipole in a nonpolar molecule. HCl does not primarily interact this way.
- London Dispersion Forces: Present in all molecules, but these are not the main type of interaction in HCl.
- Van der Waals Interactions: A general term that includes both dipole-dipole and London dispersion forces; however, specifically for HCl, dipole-dipole interactions dominate.
Conclusion
In summary, the correct classification of HCl as exhibiting dipole-dipole interactions is due to its polar nature and the resulting attraction between the dipoles in neighboring HCl molecules. Understanding these interactions is crucial in chemistry, especially in predicting the behavior of acids and their solutions.
HCl (hydrochloric acid) is a polar molecule, which means it has a permanent dipole due to the difference in electronegativity between hydrogen and chlorine. This polarity leads to specific types of intermolecular interactions.
1. Dipole-Dipole Interactions
- HCl exhibits dipole-dipole interactions, which occur between molecules that have permanent dipoles.
- The partially positive hydrogen atom of one HCl molecule is attracted to the partially negative chlorine atom of another HCl molecule.
- These interactions are significant in the liquid phase of HCl, contributing to its properties like boiling point and solubility.
2. Importance of Dipole-Dipole Interactions
- Strength: Dipole-dipole interactions are generally stronger than London dispersion forces (induced dipole interactions) but weaker than hydrogen bonds.
- Effect on Properties: The presence of dipole-dipole interactions influences the physical properties of HCl, such as its state and behavior in solutions.
3. Other Interaction Types
- Dipole-Induced Dipole Interactions: Occur when a polar molecule induces a dipole in a nonpolar molecule. HCl does not primarily interact this way.
- London Dispersion Forces: Present in all molecules, but these are not the main type of interaction in HCl.
- Van der Waals Interactions: A general term that includes both dipole-dipole and London dispersion forces; however, specifically for HCl, dipole-dipole interactions dominate.
Conclusion
In summary, the correct classification of HCl as exhibiting dipole-dipole interactions is due to its polar nature and the resulting attraction between the dipoles in neighboring HCl molecules. Understanding these interactions is crucial in chemistry, especially in predicting the behavior of acids and their solutions.
In the case of ice which energy do you think that is predominant?- a)Thermal energy
- b)Intermolecular energy
- c)Heat energy
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
In the case of ice which energy do you think that is predominant?
a)
Thermal energy
b)
Intermolecular energy
c)
Heat energy
d)
None of the above
|
|
Nathan Carter answered |
Intermolecular Energy in Ice
Ice is a solid state of water molecules, where the molecules are closely packed together in a crystalline structure. The predominant energy in ice is intermolecular energy, which refers to the energy associated with the interactions between molecules.
Explanation:
- In ice, the water molecules are held together by intermolecular forces such as hydrogen bonding. These forces are responsible for the solid structure of ice.
- The molecules in ice have a certain amount of thermal energy, which is the kinetic energy associated with the motion of the molecules. However, in a solid state like ice, the molecules have limited freedom of movement, and the intermolecular forces dominate over thermal energy.
- Heat energy refers to the transfer of thermal energy from one substance to another, and in the case of ice, heat energy can cause the solid ice to melt into liquid water. However, the predominant energy within the solid ice itself is intermolecular energy.
Therefore, in the case of ice, the intermolecular energy, which is the energy associated with the interactions between molecules, is the predominant energy.
Ice is a solid state of water molecules, where the molecules are closely packed together in a crystalline structure. The predominant energy in ice is intermolecular energy, which refers to the energy associated with the interactions between molecules.
Explanation:
- In ice, the water molecules are held together by intermolecular forces such as hydrogen bonding. These forces are responsible for the solid structure of ice.
- The molecules in ice have a certain amount of thermal energy, which is the kinetic energy associated with the motion of the molecules. However, in a solid state like ice, the molecules have limited freedom of movement, and the intermolecular forces dominate over thermal energy.
- Heat energy refers to the transfer of thermal energy from one substance to another, and in the case of ice, heat energy can cause the solid ice to melt into liquid water. However, the predominant energy within the solid ice itself is intermolecular energy.
Therefore, in the case of ice, the intermolecular energy, which is the energy associated with the interactions between molecules, is the predominant energy.
What is the result of balancing between intermolecular forces and thermal energy?- a)matter
- b)three States of matter
- c)four States of matter
- d)chemical bond formation
Correct answer is option 'B'. Can you explain this answer?
What is the result of balancing between intermolecular forces and thermal energy?
a)
matter
b)
three States of matter
c)
four States of matter
d)
chemical bond formation
|
|
Sophia Lewis answered |
Balancing Intermolecular Forces and Thermal Energy
Intermolecular forces and thermal energy play crucial roles in determining the physical state of matter. When these two factors are balanced, they lead to the existence of three states of matter.
Intermolecular Forces
Intermolecular forces are the attractive forces that exist between molecules. These forces determine the arrangement and organization of particles in a substance. Strong intermolecular forces lead to the formation of solids, moderate forces result in liquids, and weak forces produce gases.
Thermal Energy
Thermal energy is the energy associated with the random motion of particles in a substance. As temperature increases, thermal energy also increases, causing particles to move faster and collide more frequently.
Result of Balancing
When intermolecular forces and thermal energy are balanced, the particles in a substance have enough energy to overcome the attractive forces between them, allowing them to move freely while still being attracted to each other. This balance results in the formation of the three states of matter: solid, liquid, and gas.
In summary, the result of balancing between intermolecular forces and thermal energy is the existence of three distinct states of matter, each characterized by the arrangement and movement of particles based on the strength of intermolecular forces and the amount of thermal energy present.
Gases mix properly without any mechanical aid.- a)true
- b)false
Correct answer is option 'A'. Can you explain this answer?
Gases mix properly without any mechanical aid.
a)
true
b)
false

|
Orion Classes answered |
As the forces of interaction between molecules of a gas is negligible when compared with solids and gases. They mix properly because of higher thermal energy and lower intermolecular energy, so the above statement is true.
Choose the best option. Boiling kettle is an example of ____________- a)only thermal energy
- b)both thermal and Kinetic energy
- c)only kinetic energy
- d)potential and Kinetic energy
Correct answer is option 'B'. Can you explain this answer?
Choose the best option. Boiling kettle is an example of ____________
a)
only thermal energy
b)
both thermal and Kinetic energy
c)
only kinetic energy
d)
potential and Kinetic energy
|
|
Joshua Pope answered |
Boiling kettle is an example of both thermal and Kinetic energy
- Thermal Energy: When the kettle is placed on the stove and heated, the energy from the heat source is transferred to the water molecules inside the kettle. This increase in temperature causes the water to boil and turn into steam, which is a form of thermal energy.
- Kinetic Energy: As the water heats up and boils, the molecules gain kinetic energy, causing them to move more rapidly. This increase in movement is known as kinetic energy. When the water reaches its boiling point, the steam rises and escapes from the kettle, carrying both thermal and kinetic energy.
Therefore, the boiling kettle is an example of both thermal and kinetic energy because it involves the transfer of heat energy to the water molecules (thermal energy) and the movement of these molecules as they boil (kinetic energy).
Deviation of real gas behavior from ideal gas is discovered by __________.- a)Jonathan
- b)Van der Waals
- c)Boyle
- d)Newland
Correct answer is option 'B'. Can you explain this answer?
Deviation of real gas behavior from ideal gas is discovered by __________.
a)
Jonathan
b)
Van der Waals
c)
Boyle
d)
Newland
|
|
Ayesha Joshi answered |
A Dutch scientist named Johannes van der Waals found out the reason for real gas behavior’s deviation from ideal gas behavior. And he said that those forces which were named van der Waals were responsible.
Compressibility is high in case of __________.- a)solids
- b)liquids
- c)gases
- d)both solids and liquids have the same amount of compressibility
Correct answer is option 'C'. Can you explain this answer?
Compressibility is high in case of __________.
a)
solids
b)
liquids
c)
gases
d)
both solids and liquids have the same amount of compressibility
|
|
Michael Warren answered |
Compressibility of Gases
Compressibility refers to the ability of a substance to decrease in volume when subjected to pressure. In the case of gases, compressibility is high compared to solids and liquids. This is because the particles in gases are far apart and have more freedom to move around, allowing them to be easily compressed when pressure is applied.
Gas Particles vs. Solid and Liquid Particles
- Gas particles have weak intermolecular forces and are able to move freely.
- Solid and liquid particles are closely packed with stronger intermolecular forces, making them less compressible.
Effect of Pressure on Gases
When pressure is applied to a gas, the particles are pushed closer together, resulting in a decrease in volume. This is known as compressibility. Gases have a high compressibility because the particles can be easily compressed due to the large amount of space between them.
Conclusion
In conclusion, compressibility is high in the case of gases compared to solids and liquids. This is due to the nature of gas particles, which are more spread out and have weaker intermolecular forces, allowing them to be easily compressed when pressure is applied.
Compressibility refers to the ability of a substance to decrease in volume when subjected to pressure. In the case of gases, compressibility is high compared to solids and liquids. This is because the particles in gases are far apart and have more freedom to move around, allowing them to be easily compressed when pressure is applied.
Gas Particles vs. Solid and Liquid Particles
- Gas particles have weak intermolecular forces and are able to move freely.
- Solid and liquid particles are closely packed with stronger intermolecular forces, making them less compressible.
Effect of Pressure on Gases
When pressure is applied to a gas, the particles are pushed closer together, resulting in a decrease in volume. This is known as compressibility. Gases have a high compressibility because the particles can be easily compressed due to the large amount of space between them.
Conclusion
In conclusion, compressibility is high in the case of gases compared to solids and liquids. This is due to the nature of gas particles, which are more spread out and have weaker intermolecular forces, allowing them to be easily compressed when pressure is applied.
Which of the following statement is true regarding gases?- a)gases are highly incompressible
- b)gases exert equal pressure on each and every direction
- c)its volume and shape is fixed
- d)gases have the highest density among the 3 States of matter
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement is true regarding gases?
a)
gases are highly incompressible
b)
gases exert equal pressure on each and every direction
c)
its volume and shape is fixed
d)
gases have the highest density among the 3 States of matter
|
|
James Perry answered |
Gas Properties:
Gas particles are in constant motion and exhibit unique properties compared to solids and liquids. One true statement regarding gases is:
Equal Pressure:
- Gases exert equal pressure in all directions.
- This is known as Pascal's Principle, where gas molecules collide with the walls of their container, creating pressure that is evenly distributed.
- This property allows gases to expand and fill the container they are in, taking the shape of the container.
In contrast, options a, c, and d are not true for gases. Gases are highly compressible, meaning their volume can be altered by changing pressure or temperature. Additionally, gases do not have a fixed volume or shape, as they take the shape and volume of their container. Finally, gases have the lowest density among the three states of matter, with particles being spaced far apart.
Gas particles are in constant motion and exhibit unique properties compared to solids and liquids. One true statement regarding gases is:
Equal Pressure:
- Gases exert equal pressure in all directions.
- This is known as Pascal's Principle, where gas molecules collide with the walls of their container, creating pressure that is evenly distributed.
- This property allows gases to expand and fill the container they are in, taking the shape of the container.
In contrast, options a, c, and d are not true for gases. Gases are highly compressible, meaning their volume can be altered by changing pressure or temperature. Additionally, gases do not have a fixed volume or shape, as they take the shape and volume of their container. Finally, gases have the lowest density among the three states of matter, with particles being spaced far apart.
Gas is readily formed in case of predominance of _________.- a)intermolecular energy
- b)thermal energy
- c)both intermolecular energy and thermal en energy
- d)neither intermolecular energy nor thermal energy
Correct answer is option 'B'. Can you explain this answer?
Gas is readily formed in case of predominance of _________.
a)
intermolecular energy
b)
thermal energy
c)
both intermolecular energy and thermal en energy
d)
neither intermolecular energy nor thermal energy
|
|
Nathan Carter answered |
Thermal Energy and Gas Formation:
Gas is readily formed in case of predominance of thermal energy. This is because thermal energy refers to the energy associated with the motion of particles in a substance. When thermal energy is increased, the particles gain kinetic energy and move faster, leading to increased collisions between particles. This increased collision frequency results in the breaking of intermolecular bonds and the formation of gas molecules.
Key Points:
- Thermal energy increases the kinetic energy of particles.
- High thermal energy leads to increased particle collisions.
- Collisions break intermolecular bonds, allowing gas molecules to form.
- Therefore, a predominance of thermal energy facilitates gas formation.
Gas is readily formed in case of predominance of thermal energy. This is because thermal energy refers to the energy associated with the motion of particles in a substance. When thermal energy is increased, the particles gain kinetic energy and move faster, leading to increased collisions between particles. This increased collision frequency results in the breaking of intermolecular bonds and the formation of gas molecules.
Key Points:
- Thermal energy increases the kinetic energy of particles.
- High thermal energy leads to increased particle collisions.
- Collisions break intermolecular bonds, allowing gas molecules to form.
- Therefore, a predominance of thermal energy facilitates gas formation.
What is the shape of the graph that is drawn between pressure and volume?- a)A straight line
- b)Circular
- c)Parabola
- d)Hyperbola
Correct answer is option 'D'. Can you explain this answer?
What is the shape of the graph that is drawn between pressure and volume?
a)
A straight line
b)
Circular
c)
Parabola
d)
Hyperbola

|
Orion Classes answered |
Boyle’s law states that at constant temperature pressure is inversely proportional to the volume of gas so here the graph between the pressure as y-axis volume as x-axis is in the shape of a hyperbola.
There is a balloon filled with a gas at 26-degree centigrade and has a volume of about 2 liters when the balloon is taken to a place which is at 39-degree centigrade, what would be the volume of the gas that is inside the balloon?- a)2 liters
- b)3 liters
- c)1.5 liters
- d)0.67 liters
Correct answer is option 'B'. Can you explain this answer?
There is a balloon filled with a gas at 26-degree centigrade and has a volume of about 2 liters when the balloon is taken to a place which is at 39-degree centigrade, what would be the volume of the gas that is inside the balloon?
a)
2 liters
b)
3 liters
c)
1.5 liters
d)
0.67 liters

|
Orion Classes answered |
As we know that temperature is directly proportional to the volume at constant pressure, 26/39 = 2/ X; so here by equating X equals to 3 liters. Hence required a volume of the balloon at 39 degrees is 3 liters.
At a constant temperature, the pressure of a gas is given as one atmospheric pressure and 5 liters. When the atmospheric pressure is increased to 2 atm, then what is the volume of the gas?- a)1 liter
- b)5 liters
- c)10 liters
- d)2.5 liters
Correct answer is option 'D'. Can you explain this answer?
At a constant temperature, the pressure of a gas is given as one atmospheric pressure and 5 liters. When the atmospheric pressure is increased to 2 atm, then what is the volume of the gas?
a)
1 liter
b)
5 liters
c)
10 liters
d)
2.5 liters
|
|
Hannah Thompson answered |
Given:
- Initial pressure of the gas = 1 atm
- Initial volume of the gas = 5 liters
- Final pressure of the gas = 2 atm
To find:
- Final volume of the gas
Solution:
According to Boyle's Law, the pressure and volume of a gas are inversely proportional at constant temperature. Mathematically, this can be represented as:
P1 × V1 = P2 × V2
where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
We are given P1 = 1 atm, V1 = 5 liters, and P2 = 2 atm. We need to find V2.
Substituting the given values into the equation, we get:
1 atm × 5 liters = 2 atm × V2
Simplifying the equation, we have:
5 liters = 2 atm × V2
To find V2, we can rearrange the equation:
V2 = 5 liters / 2 atm
V2 = 2.5 liters
Therefore, the final volume of the gas when the atmospheric pressure is increased to 2 atm is 2.5 liters. Hence, option D (2.5 liters) is the correct answer.
- Initial pressure of the gas = 1 atm
- Initial volume of the gas = 5 liters
- Final pressure of the gas = 2 atm
To find:
- Final volume of the gas
Solution:
According to Boyle's Law, the pressure and volume of a gas are inversely proportional at constant temperature. Mathematically, this can be represented as:
P1 × V1 = P2 × V2
where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
We are given P1 = 1 atm, V1 = 5 liters, and P2 = 2 atm. We need to find V2.
Substituting the given values into the equation, we get:
1 atm × 5 liters = 2 atm × V2
Simplifying the equation, we have:
5 liters = 2 atm × V2
To find V2, we can rearrange the equation:
V2 = 5 liters / 2 atm
V2 = 2.5 liters
Therefore, the final volume of the gas when the atmospheric pressure is increased to 2 atm is 2.5 liters. Hence, option D (2.5 liters) is the correct answer.
By observing the below-given figure which of the options do you think is the correct one?
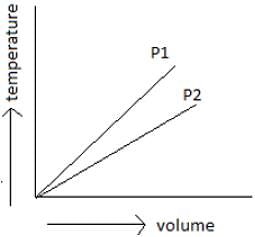
- a)P1 is greater than P2
- b)P2 is greater than P1
- c)P1 is equal to P2
- d)P1 may be equal to P2
Correct answer is option 'A'. Can you explain this answer?
By observing the below-given figure which of the options do you think is the correct one?


a)
P1 is greater than P2
b)
P2 is greater than P1
c)
P1 is equal to P2
d)
P1 may be equal to P2

|
Orion Classes answered |
By using Boyle’s law, draw a parallel line to volume axis, so as to maintain a constant temperature. Draw perpendicular lines to the point of intersection of pressure lines to constant temperature and volume axis. Now see to the lower the volume, higher the pressure. So P1 is greater than P2.
A fluid is a _________.- a)gas
- b)liquid
- c)solid
- d)both gas and liquid
Correct answer is option 'D'. Can you explain this answer?
A fluid is a _________.
a)
gas
b)
liquid
c)
solid
d)
both gas and liquid

|
Orion Classes answered |
A fluid is a gas or liquid that can be used to recognize the continuity. The fluid is something deforms under shear stress application and flows from one place to another, it is also a subset of States of matter.
The value of b for carbon dioxide is given as 42.69 x 10-6m3/mol. What do you think is the volume of a molecule?- a)7.59 m3
- b)7.03 m3
- c)76.09 m3
- d)7.09 m3
Correct answer is option 'D'. Can you explain this answer?
The value of b for carbon dioxide is given as 42.69 x 10-6m3/mol. What do you think is the volume of a molecule?
a)
7.59 m3
b)
7.03 m3
c)
76.09 m3
d)
7.09 m3

|
Orion Classes answered |
From van der Waal’s equation (P – an2/V2)(V – nb) = nRT, we know that V = b/NA = 42.69 x 10-6m3/mol/6.023 x 1023 molecules/mol. That equals 7.09 m3/molecule. So the volume of a molecule is 7.09m3.
Thermal energy is an example of __________- a)kinetic energy
- b)potential energy
- c)muscular energy
- d)momentary energy
Correct answer is option 'A'. Can you explain this answer?
Thermal energy is an example of __________
a)
kinetic energy
b)
potential energy
c)
muscular energy
d)
momentary energy

|
Orion Classes answered |
Thermal energy is also defined as an average measure of kinetic energy. It is a result of a body’s motion and that of its atoms and molecules. Thermal energy, which is an example of kinetic energy is responsible for a body’s motion.
Which of the following may not be a source of thermal energy?- a)Micro-oven
- b)Sun
- c)Moon
- d)Heater
Correct answer is option 'C'. Can you explain this answer?
Which of the following may not be a source of thermal energy?
a)
Micro-oven
b)
Sun
c)
Moon
d)
Heater

|
Orion Classes answered |
Thermal energy is also known as heat energy, it’s source s are the same as heat energy. As we know that micro-oven produces heat waves to raise food’s temperature and bake them, sun for solar energy and heater produces heat. Moon doesn’t.
Molecules do exert repulsive forces on one other.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Molecules do exert repulsive forces on one other.
a)
True
b)
False

|
Orion Classes answered |
When two molecules come closer, the electron clouds between them they repel. This is the reason why solids and liquids cannot be easily compressed. As the distance between molecules decreases, the repulsive forces become much stronger.
The value of a in van der Waal equation is _________ /dependent on _________.- a)pressure
- b)temperature
- c)pressure and temperature
- d)independent of pressure and temperature
Correct answer is option 'D'. Can you explain this answer?
The value of a in van der Waal equation is _________ /dependent on _________.
a)
pressure
b)
temperature
c)
pressure and temperature
d)
independent of pressure and temperature

|
Orion Classes answered |
Value of an in van der Waal equation represents a measure of the magnitude of intermolecular attractive forces within the gas and it is also independent of temperature and pressure. The van der Waal’s equation is given by (P – an2/V2)(V – nb) = nRT.
What are the units of “b” in van der Waals equation?- a)L/mol
- b)L mol
- c)1/L mol
- d)L
Correct answer is option 'A'. Can you explain this answer?
What are the units of “b” in van der Waals equation?
a)
L/mol
b)
L mol
c)
1/L mol
d)
L

|
Orion Classes answered |
The ideal gas equation is given as (P – an2/V2)(V – nb) = nRT. So by considering the equation, we can understand that the units of the volume are equal to the units of a number of moles X be so the units of b. So b’s units = volume / number of moles so it is L/mol.
Which of the following is not a gas law?- a)Boyle’s law
- b)Charles law
- c)Hooks law
- d)Gay lussac’s law
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not a gas law?
a)
Boyle’s law
b)
Charles law
c)
Hooks law
d)
Gay lussac’s law

|
Orion Classes answered |
Boyle’s law is about the relationship between pressure and volume while Charles law about temperature and volume. Gay lussac’s law is about pressure-temperature relationship & hooks law is a law that is in Physics relating to stress.
What is the temperature known as where a real gas obeys Boyle’s law or as an ideal gas?- a)Boyle temperature
- b)Charge temperature
- c)Critical temperature
- d)Absolute Temperature
Correct answer is option 'A'. Can you explain this answer?
What is the temperature known as where a real gas obeys Boyle’s law or as an ideal gas?
a)
Boyle temperature
b)
Charge temperature
c)
Critical temperature
d)
Absolute Temperature

|
Orion Classes answered |
The temperature at which a real gas obeys Boyle’s law and other ideal gas law at a certain range of pressure is called Boyle temperature or Boyle point. It is unique for every gas and depends upon its nature.
A gas that is liquefied by applying pressure below critical temperature is called ____________ of the substance.- a)vapor
- b)liquid
- c)solid
- d)plasma
Correct answer is option 'A'. Can you explain this answer?
A gas that is liquefied by applying pressure below critical temperature is called ____________ of the substance.
a)
vapor
b)
liquid
c)
solid
d)
plasma

|
Orion Classes answered |
At critical temperature liquid state changes into gaseous state continuously the surface that separates both this state disappears and gas below critical temperature can be liquefied by applying pressure and this is called vapor of the substance.
What is the lowermost layer of the earth?- a)stratosphere
- b)troposphere
- c)ionosphere
- d)mesosphere
Correct answer is option 'B'. Can you explain this answer?
What is the lowermost layer of the earth?
a)
stratosphere
b)
troposphere
c)
ionosphere
d)
mesosphere

|
Orion Classes answered |
It is the lowest layer of the earth’s atmosphere and is held to the earth by the gravitational force. It is very vital for human life and it protects us from harmful radiation. It contains important molecules like dioxygen, carbon dioxide, water vapor, etc.
The plot PV vs v at constant temperature is a straight line for real gases.- a)true
- b)false
Correct answer is option 'B'. Can you explain this answer?
The plot PV vs v at constant temperature is a straight line for real gases.
a)
true
b)
false

|
Orion Classes answered |
The plot of PV vs P is not a straight line for real gases because they deviate from Ideal behaviour. are there are two types of deviations one is a positive deviation and the other is a negative deviation.
Sunlight and heat reaching earth is an example of _________- a)conduction
- b)radiation
- c)convection
- d)both convection and conduction
Correct answer is option 'B'. Can you explain this answer?
Sunlight and heat reaching earth is an example of _________
a)
conduction
b)
radiation
c)
convection
d)
both convection and conduction

|
Orion Classes answered |
Thermal energy is transferred in the form of heat and sunlight. This process occurs through radiation, which is a type of transfer of thermal energy. It requires no direct contact and uses electromagnetic waves.
In van der Waal’s equation, b is known as __________.- a)volume constant
- b)pressure constant
- c)volume correction
- d)pressure correction
Correct answer is option 'C'. Can you explain this answer?
In van der Waal’s equation, b is known as __________.
a)
volume constant
b)
pressure constant
c)
volume correction
d)
pressure correction

|
Orion Classes answered |
In the van der Waal’s equation (P – an2/V2)(V – nb) = nRT, b is the volume correction term and is 4 times as the volume of a molecule. The letter a is the pressure correction term in the van der Waal’s equation.
Which of the following element is not a gas?- a)Hydrogen
- b)Oxygen
- c)Mercury
- d)Nitrogen
Correct answer is option 'C'. Can you explain this answer?
Which of the following element is not a gas?
a)
Hydrogen
b)
Oxygen
c)
Mercury
d)
Nitrogen

|
Orion Classes answered |
Mercury is not a gas, it is a liquid in room temperature and it is a metal. There are 11 gases which are gases at room temperature they are hydrogen, Nitrogen, Oxygen, fluorine, chlorine, Helium, Neon, Argon, Krypton, Xenon, and Radon.
What is the percentage of Nitrogen in the atmosphere approximately?- a)78.09
- b)21
- c)20
- d)32
Correct answer is option 'A'. Can you explain this answer?
What is the percentage of Nitrogen in the atmosphere approximately?
a)
78.09
b)
21
c)
20
d)
32

|
Orion Classes answered |
That composition of earth’s atmospheric gases is as follows; 78.09 percent of Nitrogen, 24.95 percent of oxygen, 0.93 percent of argon, 0.04 percentage of carbon dioxide and a small amount of water vapor and other gases in the atmosphere.
Chapter doubts & questions for Matter - Chemistry for ACT 2025 is part of ACT exam preparation. The chapters have been prepared according to the ACT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for ACT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Matter - Chemistry for ACT in English & Hindi are available as part of ACT exam.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Chemistry for ACT
110 videos|124 docs|114 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup










