All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Structure of Atoms for EmSAT Achieve Exam
Can you explain the answer of this question below:The number of radial nodes for 3p orbital is __________.A:3B:4C:2D:1The answer is d.
|
|
Krishna Iyer answered |
► Number of radial nodes = n - 1 – 1
► For 3p orbital, n = 3 – 1 – 1 = 1
► Number of radial nodes = 3 – 1 – 1 = 1.
► For 3p orbital, n = 3 – 1 – 1 = 1
► Number of radial nodes = 3 – 1 – 1 = 1.
The nucleus of a tritium atom, 3H, contains- a)three protons
- b)three neutrons
- c)two protons and one neutron
- d)two neutrons and one proton
Correct answer is option 'D'. Can you explain this answer?
The nucleus of a tritium atom, 3H, contains
a)
three protons
b)
three neutrons
c)
two protons and one neutron
d)
two neutrons and one proton
|
|
Hansa Sharma answered |
Tritium (3H) is a radioactive isotope of hydrogen. The nucleus decays (by emitting an electron and an antineutrino), changing from a triton (one proton and two neutrons) to a 3He nucleus (two protons and one neutron).
Thomson’s plum pudding model explained:
- a) Existence of electrons
- b)Electrical neutrality of an atom
- c)Existence of atoms
- d)Electrons move in fixed circular orbits
Correct answer is option 'B'. Can you explain this answer?
Thomson’s plum pudding model explained:
a)
Existence of electrons
b)
Electrical neutrality of an atom
c)
Existence of atoms
d)
Electrons move in fixed circular orbits
|
|
Raghav Bansal answered |
Postulates of Thomson’s atomic model
- An atom consists of a positively charged sphere with electrons filled into it. The negative and positive charge present inside an atom are equal and as a whole, an atom is electrically neutral.
- Thomson’s model of the atom was compared to plum pudding and watermelon. He compared the red edible part of the watermelon to positively charged sphere whereas the seeds of watermelon to negatively charged particles.
An element has 18 electrons, and 20 .neutrons. Its charge is —2. What is its mass number?- a)32
- b)38
- c)39
- d) 40
Correct answer is option 'C'. Can you explain this answer?
An element has 18 electrons, and 20 .neutrons. Its charge is —2. What is its mass number?
a)
32
b)
38
c)
39
d)
40

|
Knowledge Hub answered |
Number of orbitals in a shell = n2 = (5)2 = 25
Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom ?- a)n = 1 to n = 2
- b)n = 2 to n = 4
- c)n = 3 to n = 6
- d)n = 3 to n = ∞
Correct answer is option 'A'. Can you explain this answer?
Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom ?
a)
n = 1 to n = 2
b)
n = 2 to n = 4
c)
n = 3 to n = 6
d)
n = 3 to n = ∞
|
|
Naina Sharma answered |
Therefore, electronic transition (a) requires greatest quantity of energy.
Atomic mass of an element is equal to the sum of?- a) Electron and neutron
- b) Electron and proton
- c) Proton and neutron
- d) None of the above
Correct answer is option 'C'. Can you explain this answer?
Atomic mass of an element is equal to the sum of?
a)
Electron and neutron
b)
Electron and proton
c)
Proton and neutron
d)
None of the above
|
|
Neha Joshi answered |
Atomic Mass is the sum of no. of protons and neutrons.
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Which of the following is correct?- a)1H1 and 2He3 are isotopes
- b)6C14 and 7N14 are isotopes
- c)19K39 and 20Ca40 are isotones
- d)9F19 and 11Na24 are isodiaphers
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following is correct?
a)
1H1 and 2He3 are isotopes
b)
6C14 and 7N14 are isotopes
c)
19K39 and 20Ca40 are isotones
d)
9F19 and 11Na24 are isodiaphers
|
|
Raghav Bansal answered |
Because isotones means the same number of neutron, So, from the question, option c is right, number of neutron in k is,39-19= 20,and,the number of neutron in ca is also 40-20=20 so it is isotones.
In the absorption spectrum, the wavelengths which are absorbed, are missing and they appear as:- a)Bright lines
- b)Light bands
- c)Bright bands
- d)Dark lines
Correct answer is option 'D'. Can you explain this answer?
In the absorption spectrum, the wavelengths which are absorbed, are missing and they appear as:
a)
Bright lines
b)
Light bands
c)
Bright bands
d)
Dark lines
|
|
Riya Banerjee answered |
Light not absorbed by the sample will, as before, be separated (dispersed) into its component wavelengths (colors) by the prism. The appearance of the spectrum will resemble that obtained without the sample in place, with the exception that those wavelengths which have been absorbed are missing, and will appear as dark lines within the spectrum of colors. If a piece of the photographic film is used instead of the card, the absorption spectrum can be recorded.
The energy associated with the transition of an electron from the n=1 state to the n=3 state of H atoms is:- a)+1.74 x 10-17 Joules.
- b)-1.94 x 10-18 Joules.
- c)+1.94 x 10-18 Joules.
- d)-1.74 x 10-17 Joules.
Correct answer is option 'C'. Can you explain this answer?
The energy associated with the transition of an electron from the n=1 state to the n=3 state of H atoms is:
a)
+1.74 x 10-17 Joules.
b)
-1.94 x 10-18 Joules.
c)
+1.94 x 10-18 Joules.
d)
-1.74 x 10-17 Joules.
|
|
Lavanya Menon answered |
The formula to calculate the excitation energy is 13.6Z2(1/n12-1/n22), but this gives value in eV. To convert it in Joules we divide it by 6.24×1018 Here, Z=1,n1=1,n2=3 Putting these values in above formula we have, [13.6×1(1-1/9)]/6.24×1018 =(13.6×8×10-18)/(9×6.24) =1.94×10-18 Hence, the correct answer is C.
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of - a)V volt
- b)4V volt
- c)2V volt
- d)V/8 volt
Correct answer is option 'D'. Can you explain this answer?
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
a)
V volt
b)
4V volt
c)
2V volt
d)
V/8 volt
|
|
Sonal Dey answered |
Direction (Q. Nos. 16 - 19) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)Q. The energy of an electron in the first Bohr orbit of H-atom is -13.6 eV. What is the possible value of quantum number for the excited state to have energy -3.4 eV?
Correct answer is '2'. Can you explain this answer?
Direction (Q. Nos. 16 - 19) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. The energy of an electron in the first Bohr orbit of H-atom is -13.6 eV. What is the possible value of quantum number for the excited state to have energy -3.4 eV?
|
|
Pooja Shah answered |
For Bohr radius;
E = -13.6×Z2/ n2
For H atom, Z=1 and given energy = -3.4
So, we have, -3.4 = -13.6/n2
Or n = 2
E = -13.6×Z2/ n2
For H atom, Z=1 and given energy = -3.4
So, we have, -3.4 = -13.6/n2
Or n = 2
For Balmer series,the initial state n1 is :- a)4
- b)2
- c)3
- d)1
Correct answer is option 'B'. Can you explain this answer?
For Balmer series,the initial state n1 is :
a)
4
b)
2
c)
3
d)
1
|
|
Pooja Shah answered |
The Balmer series just sets n1 = 2, which means the value of the principal quantum number (n) is two for the transitions being considered. Balmer’s formula can therefore be written:
1/λ = RH ((1/22) − (1 / n22))
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Amrita Kumar answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
The nature of positive rays depends on?
- a)The nature of discharge tube.
- b)The nature of residual gas.
- c)The nature of electrode.
- d)All of above
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas.
c)
The nature of electrode.
d)
All of above
|
|
Om Desai answered |
- The nature of positive rays produced in a vacuum discharge tube depends upon the nature of the gas-filled.
- The positive rays consist of positive ions obtained by removing one or more electrons from gas molecules.
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?- a)13.6 eV
- b)10.2 eV
- c)3.4eV
- d)1 eV
Correct answer is option 'A'. Can you explain this answer?
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?
a)
13.6 eV
b)
10.2 eV
c)
3.4eV
d)
1 eV
|
|
Pooja Shah answered |
For hydrogen Z=1,
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
Most penetrating radiation of radioactive element is:- a) β — rays
- b) α — rays
- c) x — rays
- d) γ — rays
Correct answer is option 'D'. Can you explain this answer?
Most penetrating radiation of radioactive element is:
a)
β — rays
b)
α — rays
c)
x — rays
d)
γ — rays
|
|
Anjana Sharma answered |
Alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating.
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.- a)434 nm
- b)275 nm
- c)305 nm
- d)183 nm
Correct answer is option 'A'. Can you explain this answer?
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.
a)
434 nm
b)
275 nm
c)
305 nm
d)
183 nm
|
|
Hansa Sharma answered |
1/wavelength =RH x z2 x (1/22-1/52) =109677 x 1 x (1/4-1/25) =109677 x 21/100 =2303.2m wavelength=1/2303.2m =1/2303.2 x 107nm =434.1nm~434nm
Increasing order of magnetic moment among the following species is __________ .Na+, Fe+3, Co2+, Cr+2- a)Na+<Fe+3<Co2+ <Cr+2
- b)Na+<Co2+<Cr2+ <Fe+3
- c)Na+<Cr2+<Co2+ <Fe+3
- d)Na+<Fe3+<Cr2+ <Co+2
Correct answer is option 'B'. Can you explain this answer?
Increasing order of magnetic moment among the following species is __________ .
Na+, Fe+3, Co2+, Cr+2
a)
Na+<Fe+3<Co2+ <Cr+2
b)
Na+<Co2+<Cr2+ <Fe+3
c)
Na+<Cr2+<Co2+ <Fe+3
d)
Na+<Fe3+<Cr2+ <Co+2
|
|
Nitin Khanna answered |
Na+, Co+2, Cr2+, Fe+3
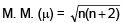
we get Na+, Co+2, Cr2+, Fe+3
we get Na+, Co+2, Cr2+, Fe+3
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?- a)Energy of electron in an atom is lower than energy of an electron far away from nucleus
- b)The electron has a negative charge.
- c)The electron is far away from nucleus
- d)Electrons have both wave and particle-like properties.
Correct answer is option 'A'. Can you explain this answer?
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
a)
Energy of electron in an atom is lower than energy of an electron far away from nucleus
b)
The electron has a negative charge.
c)
The electron is far away from nucleus
d)
Electrons have both wave and particle-like properties.
|
|
Gaurav Kumar answered |
The negative sign means that the energy of the electron in the atom is lower than the energy of a free electron at rest. A free electron at rest is an electron that is infinitely far away from the nucleus and is assigned the energy value zero.
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?- a)Rutherford Model
- b)Bohr’s Model
- c)J.J Thomson Model
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
a)
Rutherford Model
b)
Bohr’s Model
c)
J.J Thomson Model
d)
None of the above
|
|
Suresh Reddy answered |
Bohr Model of atom:
- An atom is made up of three particles: Electrons, neutrons and protons.
- The protons and neutrons are located in a small nucleus at the centre of the atom.
- The electrons revolve rapidly around the nucleus at the centre of the atom.
- There is a limit to the number of electrons that each energy level can hold.
- Each energy level is associated with a fixed amount of energy.
- There is no change in the energy of electrons as long as they keep revolving in the same energy level.
Bohr explained the stability through the concept of revolution of electrons in different energy levels.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The nature of positive rays depends on?- a) The nature of discharge tube.
- b) The nature of residual gas
- c) All of above.
- d) The nature of electrode
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas
c)
All of above.
d)
The nature of electrode

|
Abhiram Choudhary answered |
The positive charges in these rays, other than negative cathode rays (which are electrons), depend on the gas that is used because they are cations - atoms with mostly one electron missing and thus one positive charge. So, if you accelerate, argon cations and protons over the same electric potential, the particles in the rays will have the same kinetic energy, but the argon ions will be much slower, as they are much heavier than the protons.
Zeeman effect is the splitting of spectral line in presence of:- a)electricity
- b)magnetic effect
- c)molecule
- d)electric field
Correct answer is option 'B'. Can you explain this answer?
Zeeman effect is the splitting of spectral line in presence of:
a)
electricity
b)
magnetic effect
c)
molecule
d)
electric field
|
|
Lavanya Menon answered |
The Zeeman effect is the splitting of the spectral lines of an atom in the presence of a strong magnetic field. The effect is due to the distortion of the electron orbitals because of the magnetic field. The (normal) Zeeman effect can be understood classically, as Lorentz predicted.
The first use of quantum theory to explain the structure of atom was made by :- a)Heisenberg
- b)Bohr
- c)Planck
- d)Einstein
Correct answer is option 'B'. Can you explain this answer?
The first use of quantum theory to explain the structure of atom was made by :
a)
Heisenberg
b)
Bohr
c)
Planck
d)
Einstein
|
|
Hansa Sharma answered |
Bohr's theory was based upon some postulations of classical physics and some postulations of the newly proposed quantum theory of Planck.
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is option 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Swara Saha answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
Which of the following subatomic particles is responsible for the spectrum of radiation emitted by an element or compound?- a)neutron
- b)electron
- c)proton
- d)photon
Correct answer is option 'B'. Can you explain this answer?
Which of the following subatomic particles is responsible for the spectrum of radiation emitted by an element or compound?
a)
neutron
b)
electron
c)
proton
d)
photon
|
|
Om Desai answered |
Any radiation is emitted in the quantized form as photons. These photons are actually generated when an electron changes its energy level. This can happen if the atom gets the required work function. For e.g. The emission of X Ray. When the cathode ray hits the nuclei of heavier metals, two cases may occur -
1)Nuclei absorbs the electron from K shell and this process results in radiation of energy in form of X Ray photons.
2)Auger Effect: Sometimes during the collision, an electron from main atom can also be emitted, releasing energy in form of X Ray photons. So, Now it should be more clear that while photons are the product, the real culprit behind the crime scene. So correct option is B These things can be understood easily if you know the basics of Particle Physics.
1)Nuclei absorbs the electron from K shell and this process results in radiation of energy in form of X Ray photons.
2)Auger Effect: Sometimes during the collision, an electron from main atom can also be emitted, releasing energy in form of X Ray photons. So, Now it should be more clear that while photons are the product, the real culprit behind the crime scene. So correct option is B These things can be understood easily if you know the basics of Particle Physics.
The charge on electron was determined by- a)Crooks
- b)Bohr
- c)Milliken
- d)Schrodinger
Correct answer is option 'C'. Can you explain this answer?
The charge on electron was determined by
a)
Crooks
b)
Bohr
c)
Milliken
d)
Schrodinger
|
|
Naina Bansal answered |
Millikan
The experiment helped earn Millikan a Nobel prize in 1923 but has been a source of some controversy over the years. J. J. Thomson discovered the electron in 1897 when he measured the charge-to-mass ratio for electrons in a beam. But the value of the charge and whether it was fundamental remained open questions.
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
- a)n=3, l=0
- b)n=3, l=1
- c)n=2, l=0
- d)n=2, l=1
Correct answer is option 'D'. Can you explain this answer?
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
a)
n=3, l=0
b)
n=3, l=1
c)
n=2, l=0
d)
n=2, l=1
|
|
Om Desai answered |
Given a set of quantum numbers, n=2,l=0 for a multi-electron atom refers to 2s orbital.
The next higher allowed set of 'n' and 'l' quantum numbers for this atom in the ground state is n=2,l=1. This corresponds to 2p orbital.
Note: The orbital with a higher value of the sum (n+l) has higher energy.
For 2s orbital (n+l)=(2+0)=2
For 2p orbital (n+l)=(2+1)=3
If in the hydrogen atom P.E. at ∞ is choosen to be 13.6 eV then the ratio of T.E. to K.E. for 1st orbit of H-atom is __________ .- a)Zero
- b)1
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
If in the hydrogen atom P.E. at ∞ is choosen to be 13.6 eV then the ratio of T.E. to K.E. for 1st orbit of H-atom is __________ .
a)
Zero
b)
1
c)
2
d)
3
|
|
Rahul Kumar answered |
Zero
Refrence level is Ist orbit
itself T. E. = 0
Ratio becomes zero
Refrence level is Ist orbit
itself T. E. = 0
Ratio becomes zero
An electron falls from one energy level to another; it releases a certain amount of light with a frequency of 5.100 x 1014 Hz. What energy is associated with this electron?
a)1.3 13x 1022 Jb)3.379 x 10-20 kJc)33.8 x 10-20 Jd)1.79 0x 10-10kJCorrect answer is option 'C'. Can you explain this answer?
|
|
Preeti Iyer answered |
E = h.v
= 6.63 x 10^-34 x 5.1 x 10^14
= 33.8 x 10^-20 J
4000 A photon is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol. - a)8%
- b)12%
- c)17%
- d)25%
Correct answer is option 'C'. Can you explain this answer?
4000 A photon is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol.
a)
8%
b)
12%
c)
17%
d)
25%

|
EduRev JEE answered |
Energy of one photon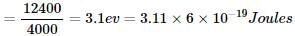
Energy supplied by one mole photon in KJ/mole = 3.11×6×10−19×6×1023×10−3=297KJ/mole
% of energy converted to K.E.=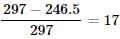
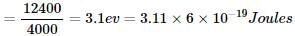
Energy supplied by one mole photon in KJ/mole = 3.11×6×10−19×6×1023×10−3=297KJ/mole
% of energy converted to K.E.=
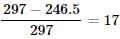
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?a) Most of the space in the atom is empty.b) The radius of the atom is about 10^–10 m while that of nucleus is 10^–15 m.c) Electrons move in a circular path of fixed energy called orbits.d) Electrons and the nucleus are held together by electrostatic forces of attraction.Correct answer is 'C'. Can you explain this answer?
|
|
Preeti Iyer answered |
Conclusions of Rutherford's scattering experiment:
1. Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
2. Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
3. A very small fraction of α-particles were deflected by very large angles, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
4. From the data, he also calculated that the radius of the nucleus is about 10^5times less than the radius of the atom.
5. Electrons and the nucleus are held together by electrostatic forces of attraction.
Can you explain the answer of this question below:Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which is a possible set of quantum numbers for a valence unpaired electrons in ground state atom of phosphorus (Z = 15)?
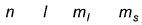
- A:
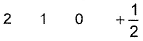
- B:
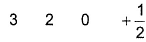
- C:
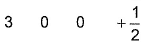
- D:
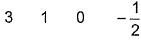
The answer is d.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which is a possible set of quantum numbers for a valence unpaired electrons in ground state atom of phosphorus (Z = 15)?
|
|
Dipika Singh answered |
Valence unpaired electrons are in 3p
Valence unpaired electrons are in 3p.
Thus, (d).
Which of the following is not true about Bohr’s model of the atom?- a)Electrons behave like waves and particles simultaneously.
- b)Electrons revolve in discrete, quantized orbits only.
- c)mvr = nh/ 2π
- d)Atoms radiate discrete Electromagnetic energies only.
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not true about Bohr’s model of the atom?
a)
Electrons behave like waves and particles simultaneously.
b)
Electrons revolve in discrete, quantized orbits only.
c)
mvr = nh/ 2π
d)
Atoms radiate discrete Electromagnetic energies only.
|
|
Suresh Reddy answered |
It was De broglie who proposed the dual nature of matter and that of electrons. Davisson and germer went on to experimentally verify his theory, regarding nature of electrons(they have both particle and wave nature).
Heisenberg further introduced the uncertainty principle, related to dual nature of matter.
Bohr never putforth any such ideals and considered electron as a particle.
Heisenberg further introduced the uncertainty principle, related to dual nature of matter.
Bohr never putforth any such ideals and considered electron as a particle.
Can you explain the answer of this question below:Radiation of λ = 155 nm was irradiated on Li (work function = 5eV) plate. The stopping potential (in eV) is.
- A:
3eV
- B:
4eV
- C:
0.3eV
- D:
0.5 eV
The answer is a.
Radiation of λ = 155 nm was irradiated on Li (work function = 5eV) plate. The stopping potential (in eV) is.
3eV
4eV
0.3eV
0.5 eV

|
Ayush Joshi answered |
K.E.= hv − hv0 = hc/λ − W.F.= 6.6 x 10^−34 x 3 x 10^8/λ − 5 x 1.6 x 10^−19 and
K.Emax = eV0 where V0 is stopping potential so
V0 = 3eV
Given ΔH for the process Li(g) → Li+3(g) + 3e- is 19800 kJ/mole & IE1 for Li is 520 then IE2 & IE3 of Li+ are respectively (approx, value) :- a)11775, 7505
- b)19280, 520
- c)11775, 19280
- d)Data insufficient
Correct answer is option 'A'. Can you explain this answer?
Given ΔH for the process Li(g) → Li+3(g) + 3e- is 19800 kJ/mole & IE1 for Li is 520 then IE2 & IE3 of Li+ are respectively (approx, value) :
a)
11775, 7505
b)
19280, 520
c)
11775, 19280
d)
Data insufficient
|
|
Muskaan Kumar answered |
IE1 + IE2 + IE3 = 19800
IE2 + IE3 = 19800 – 520
IE2 + IE3 = 19280
IE2 + IE3 = 19800 – 520
IE2 + IE3 = 19280
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
Correct answer is '3'. Can you explain this answer?
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
|
|
Neha Sharma answered |
Number of waves = n(n - 1)/2 where n = Principal quantum number or number of orbit number of waves = 3(3 - 1)/2 = 3 * 2/2 = 3
ALTERNATIVE SOLUTIONS :
In general, the number of waves made by a Bohr electron in an orbit is equal to its quantum number.
According to Bohr’s postulate of angular momentum, in the 3rd orbit
Mur = n h/2π
Mur = 3 (h/2π) …..(i) [n = 3]
According to de Broglie relationship
λ = h/mu ….(ii)
Substituting (ii) in (i), we get
(h/λ) r = 3 (h/2π) or 3λ = 2πr
[∵ mu = h/λ]
Thus the circumference of the 3rd orbit is equal to 3 times the wavelength of electron i.e. the electron makes three revolution around the 3rd orbit.
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1 (Mass of proton = 1.67×10-27kg and h = 6.63×10-34Js)
- a)2.5 nm
- b)0.40 nm
- c) 14.0 nm
- d)32 nm
Correct answer is option 'B'. Can you explain this answer?
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1 (Mass of proton = 1.67×10-27kg and h = 6.63×10-34Js)
a)
2.5 nm
b)
0.40 nm
c)
14.0 nm
d)
32 nm
|
|
Siddharth Yadav answered |
To calculate the wavelength associated with a proton moving at 1.0 MeV, we can use the de Broglie wavelength equation:
λ = h / p
where λ is the wavelength, h is the Planck's constant (6.626 x 10^-34 J*s), and p is the momentum of the proton.
First, let's convert 1.0 MeV to joules:
1 MeV = 1.6 x 10^-13 J
Next, we need to calculate the momentum of the proton. The momentum (p) is given by:
p = sqrt(2 * m * KE)
Where m is the mass of the proton (1.6726219 x 10^-27 kg) and KE is the kinetic energy of the proton (1.0 MeV = 1.6 x 10^-13 J).
Now, let's calculate the momentum:
p = sqrt(2 * (1.6726219 x 10^-27 kg) * (1.6 x 10^-13 J))
p ≈ 1.29 x 10^-19 kg*m/s
Finally, we can calculate the wavelength:
λ = (6.626 x 10^-34 J*s) / (1.29 x 10^-19 kg*m/s)
λ ≈ 5.13 x 10^-16 m
To convert this wavelength to nanometers, we multiply by 10^9:
λ ≈ 5.13 x 10^-16 m * 10^9 nm/m
λ ≈ 5.13 x 10^-7 nm
Therefore, the wavelength associated with a proton moving at 1.0 MeV is approximately 5.13 x 10^-7 nanometers.
λ = h / p
where λ is the wavelength, h is the Planck's constant (6.626 x 10^-34 J*s), and p is the momentum of the proton.
First, let's convert 1.0 MeV to joules:
1 MeV = 1.6 x 10^-13 J
Next, we need to calculate the momentum of the proton. The momentum (p) is given by:
p = sqrt(2 * m * KE)
Where m is the mass of the proton (1.6726219 x 10^-27 kg) and KE is the kinetic energy of the proton (1.0 MeV = 1.6 x 10^-13 J).
Now, let's calculate the momentum:
p = sqrt(2 * (1.6726219 x 10^-27 kg) * (1.6 x 10^-13 J))
p ≈ 1.29 x 10^-19 kg*m/s
Finally, we can calculate the wavelength:
λ = (6.626 x 10^-34 J*s) / (1.29 x 10^-19 kg*m/s)
λ ≈ 5.13 x 10^-16 m
To convert this wavelength to nanometers, we multiply by 10^9:
λ ≈ 5.13 x 10^-16 m * 10^9 nm/m
λ ≈ 5.13 x 10^-7 nm
Therefore, the wavelength associated with a proton moving at 1.0 MeV is approximately 5.13 x 10^-7 nanometers.
The third line in Balmer series corresponds to an electronic transition between which Bohr's orbits in hydrogen :- a)5 → 3
- b)5 → 2
- c)4 → 3
- d)4 → 2
Correct answer is option 'B'. Can you explain this answer?
The third line in Balmer series corresponds to an electronic transition between which Bohr's orbits in hydrogen :
a)
5 → 3
b)
5 → 2
c)
4 → 3
d)
4 → 2

|
Krithika Kulkarni answered |
Balmer means transition to n = 2
1. line → 3 to 2
2. line → 4 to 2
3. line → 5 to 2
1. line → 3 to 2
2. line → 4 to 2
3. line → 5 to 2
Chapter doubts & questions for Structure of Atoms - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Structure of Atoms - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily