All Exams >
Class 10 >
Science Class 10 >
All Questions
All questions of Chemical Reactions and Equations for Class 10 Exam
Which of the following is a combination reaction?
- a)CaCO3 → CaO + CO2
- b)H2 + Cl2 → 2HCl
- c)H2CO3 → H2O + CO2
- d)2KClO3 → 2KCl + 3O2
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a combination reaction?
a)
CaCO3 → CaO + CO2
b)
H2 + Cl2 → 2HCl
c)
H2CO3 → H2O + CO2
d)
2KClO3 → 2KCl + 3O2
|
|
Roshni chauhan answered |
Balanced Equation:
H2 + Cl2 → 2HCl2
H2 + Cl2 → 2HCl2
The above reaction is an example of combination reaction because two different elements are combining to form a single compound.
Which of the following is a decomposition reaction?- a)NaOH + HCl → NaCl + H2O
- b)NH4CNO → H2NCONH2
- c)2KClO3 → 2KCl + 3O2
- d)H2 + I2 → 2HI
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a decomposition reaction?
a)
NaOH + HCl → NaCl + H2O
b)
NH4CNO → H2NCONH2
c)
2KClO3 → 2KCl + 3O2
d)
H2 + I2 → 2HI
|
|
Meera Rana answered |
A decomposition reaction occurs when one reactant breaks down into two or more products. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
2KClO3 → 2KCl + 3O2
2KClO3 → 2KCl + 3O2
The reaction C + O2 → CO2 + Heat is a:
- a)Combination reaction
- b)Oxidation reaction
- c)Exothermic reaction
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
The reaction C + O2 → CO2 + Heat is a:
a)
Combination reaction
b)
Oxidation reaction
c)
Exothermic reaction
d)
All of the above
|
|
Amit Sharma answered |
C + O2 → CO2 + Heat
- This is a Combination reaction because C and O2 are combining to produce one single compound CO2.
- This is also an Oxidation reaction because carbon is getting oxidized.
- This is also an exothermic reaction because in this reaction heat is getting released.
So Option D is correct
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?- a)Red litmus solution
- b)Lime water
- c)A burning splinter
- d)Blue litmus solution
Correct answer is option 'C'. Can you explain this answer?
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?
a)
Red litmus solution
b)
Lime water
c)
A burning splinter
d)
Blue litmus solution
|
|
Kiran Mehta answered |
A burning splinter since hydrogen gas burns with a pop sound.
Zinc being an active metal readily reacts with hydrochloric acid at room temperature to form soluble zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2 + H2.
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:- a)Decomposition reaction
- b)Combination reaction
- c)Displacement reaction
- d)Single displacement reaction
Correct answer is option 'C'. Can you explain this answer?
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
The above reaction is an example of a:
a)
Decomposition reaction
b)
Combination reaction
c)
Displacement reaction
d)
Single displacement reaction

|
Anushka Chopra answered |
The given equation is a displacement reaction in which Fe of Fe2O3 has been displaced by Al. Hence, (c) is the correct option.
Can you explain the answer of this question below:ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
- A:
A = 1, B = 1, C = 2, D = 1
- B:
A = 2, B = 2, C = 2, D = 1
- C:
A = 2, B = 1, C = 2, D = 1
- D:
none of these
The answer is b.
ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
A = 1, B = 1, C = 2, D = 1
A = 2, B = 2, C = 2, D = 1
A = 2, B = 1, C = 2, D = 1
none of these
|
|
Shweta singh answered |
2 Na + 2H2O gives 2NaOH + H2
So option B is Correct answer.
Which of the following is a displacement reaction?- a)CaCO3 → CaO + CO2
- b)CaO + 2HCl → CaCl2 + H2O
- c)Fe + CuSO4 → FeSO4 + Cu
- d)NaOH + HCl → NaCl + H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a displacement reaction?
a)
CaCO3 → CaO + CO2
b)
CaO + 2HCl → CaCl2 + H2O
c)
Fe + CuSO4 → FeSO4 + Cu
d)
NaOH + HCl → NaCl + H2O
|
|
Gaurav Kumar answered |
Fe + CuSo4 → FeSO4 + Cu
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
(a) is a decomposition reaction.
(b) and (d) are neutralisation reactions.
(b) and (d) are neutralisation reactions.
Which of the following is an example of displacement reaction?- a)4 Na+ O2 → 2 Na2O
- b)2 Cu + O2 → 2 CuO
- c)Mg + 2 HCl → MgCl2 + H2
- d)N2 + 3 H2 → 2 NH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of displacement reaction?
a)
4 Na+ O2 → 2 Na2O
b)
2 Cu + O2 → 2 CuO
c)
Mg + 2 HCl → MgCl2 + H2
d)
N2 + 3 H2 → 2 NH3

|
Orion Classes answered |
It is a single replacement reaction.
Mg + 2HCl → MgCl2 + H2
Mg + 2HCl → MgCl2 + H2
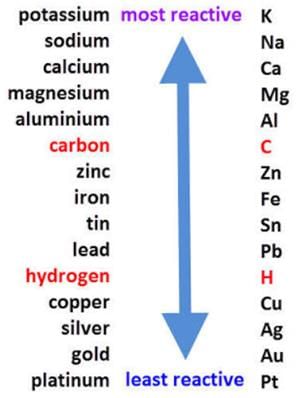
Which of the following metals comes above zinc in reactivity series?
- a)Silver
- b)Copper
- c)Aluminium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals comes above zinc in reactivity series?
a)
Silver
b)
Copper
c)
Aluminium
d)
Iron
|
|
Karthik murthy answered |
Reactivity Series:
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
The reaction H2 + Cl2 → 2HCl is a –- a)Decomposition reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Displacement reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction H2 + Cl2 → 2HCl is a –
a)
Decomposition reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Displacement reaction
|
|
Anjana Khatri answered |
It is a reaction in which two substances react with each other to make a single substance. Therefore, H2 + CL2 = 2HCL is an COMBINATION REACTION. It is a special case of addition reaction known as synthesis.
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
- a)SO2
- b)H2S
- c)H2O
- d)S
Correct answer is option 'B'. Can you explain this answer?
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
a)
SO2
b)
H2S
c)
H2O
d)
S
|
|
Raghav Bansal answered |
- In the reaction SO2 is getting reduced to S as oxygen is being removed.
- Similarly, H2S is oxidised to S as hydrogen is removed.
- The species getting oxidised facilitates the reduction process and is the reducing agent.
- Hence, H2S is the reducing agent and Oxidising agent is SO2.
So, option B is correct
Which of the following represent a double displacement reaction?- a)2H2 + O2 → 2H2O
- b)2Mg + O2 → 2MgO
- c)AgNO3 + NaCl → AgCl
 + NaNO3
+ NaNO3 - d)H2 + Cl2 → 2HCl
Correct answer is option 'C'. Can you explain this answer?
Which of the following represent a double displacement reaction?
a)
2H2 + O2 → 2H2O
b)
2Mg + O2 → 2MgO
c)
AgNO3 + NaCl → AgCl + NaNO3
+ NaNO3
d)
H2 + Cl2 → 2HCl

|
Environment Lover answered |
Yah the correct answer is c as it follow the definition of displacement reaction
The colour of zinc metal is
- a)reddish-brown
- b)grey
- c)Blue
- d)silvery-white
Correct answer is option 'D'. Can you explain this answer?
The colour of zinc metal is
a)
reddish-brown
b)
grey
c)
Blue
d)
silvery-white
|
|
Vikas Kumar answered |
The correct option is D
silvery-white
Explanation for correct option
(D) The color of Zinc metal is silvery.
- An electron gets excited to a higher energy orbital from a lower energy d orbital. The frequency of light absorbed is proportional to the excitation energy.
- This frequency is usually in the visible range.
- The color seen corresponds to the complementary color of the light absorbed.
- Zinc (Zn) is a silvery-white metal.
Hence, option (D) is correct. The color of Zinc metal is silvery.
Which of the following is not an example of chemical change?- a)rusting of iron
- b)milk changes to curd
- c)digestion of food in our body
- d)changing of water to water vapour
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an example of chemical change?
a)
rusting of iron
b)
milk changes to curd
c)
digestion of food in our body
d)
changing of water to water vapour
|
|
Amit Sharma answered |
The process of changing water vapour into water is called condensation. It is not a chemical reaction.
When milk converts in curd the process is irreversible and a new substance with different properties is formed hence it is an example of a chemical change.
Rusting of iron is also a chemical change.
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:- a)Displacement reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Decomposition reaction
Correct answer is option 'A'. Can you explain this answer?
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:
a)
Displacement reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Decomposition reaction
|
|
Neha Patel answered |
This is a metal/acid reaction if carried out in aqueous solution.
It is also a displacement reaction where 1 atom of Zinc displaces 2 H+ ions
and it is a redox reaction as the Zn atom loses electrons (it is OXIDISED) and the 2 H+ ions gain electrons (they are REDUCED)
What happens when dil hydrochloric acid is added to iron fillings?- a)Hydrogen gas and Iron chloride are produced
- b)Chlorine gas and Iron hydroxide are produced
- c)NO reaction takes place
- d)Iron salt and water are produced
Correct answer is option 'A'. Can you explain this answer?
What happens when dil hydrochloric acid is added to iron fillings?
a)
Hydrogen gas and Iron chloride are produced
b)
Chlorine gas and Iron hydroxide are produced
c)
NO reaction takes place
d)
Iron salt and water are produced
|
|
Amit Sharma answered |
- Hydrogen gas and iron chloride are produced.
Fe + HCl → FeCl2 + H2
- The iron displaces hydrogen from hydrochloric acid to form iron (II) chloride & hydrogen gas. This is a single displacement reaction.
- Thus the answer is option (A) Hydrogen gas and iron chloride are produced.
An element X on exposure to moist air turns reddish-brown and a new compound Y is formed. The substance X and Y are- a)X = Fe, Y = Fe2O3
- b)X = Ag, Y = Ag2S
- c)X = Cu, Y = CuO
- d)X = Al, Y = Al2O3
Correct answer is option 'A'. Can you explain this answer?
An element X on exposure to moist air turns reddish-brown and a new compound Y is formed. The substance X and Y are
a)
X = Fe, Y = Fe2O3
b)
X = Ag, Y = Ag2S
c)
X = Cu, Y = CuO
d)
X = Al, Y = Al2O3
|
|
Suresh Reddy answered |
An element X exposure to moist air, forms a reddish brown and a new compound Y is formed.
Fe + H2O +O2 → Fe2 O3
This Fe2O3 is the reddish brown compound which is called rust.
So , X → Fe
Y → Fe2O3
Fe + H2O +O2 → Fe2 O3
This Fe2O3 is the reddish brown compound which is called rust.
So , X → Fe
Y → Fe2O3
So Option A is correct
The reaction between lead nitrate and potassium iodide is an example of _______ .- a)decomposition reaction
- b)double displacement reaction
- c)combustion reaction
- d)combination reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction between lead nitrate and potassium iodide is an example of _______ .
a)
decomposition reaction
b)
double displacement reaction
c)
combustion reaction
d)
combination reaction
|
|
Subset Academy answered |
Lead nitrate reacts with Potassium iodide to form Lead iodide and Potassium nitrate.
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
In reaction SO2 + 2H2S → 2H2O + 3S the reducing agent is –- a)SO2
- b)H2S
- c)H2O
- d)S
Correct answer is option 'B'. Can you explain this answer?
In reaction SO2 + 2H2S → 2H2O + 3S the reducing agent is –
a)
SO2
b)
H2S
c)
H2O
d)
S

|
Navya Patel answered |
In the reaction SO2 is reduced to S as oxygen is removed.
H2S is oxidised to S as hydrogen is removed.
H2S is oxidised to S as hydrogen is removed.
The species getting oxidised facilitates the reduction process and is the reducing agent.
Hence, H2S is the reducing agent and Oxidising agent is SO2.
The given reaction indicates that:
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag- a)Copper is more reactive than silver.
- b)Silver is more reactive than copper.
- c)Both are equally reactive.
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
The given reaction indicates that:
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
a)
Copper is more reactive than silver.
b)
Silver is more reactive than copper.
c)
Both are equally reactive.
d)
None of these
|
|
Malini sharma answered |
Explanation:
The given reaction is: Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
This reaction involves the displacement of silver from its compound (AgNO3) by copper. This type of reaction is known as a single displacement reaction or a substitution reaction.
To determine which metal is more reactive, we need to look at the activity series of metals. This series arranges metals in order of their reactivity, with the most reactive metals at the top and the least reactive at the bottom.
Copper is placed below silver in the activity series, which means that silver is more reactive than copper. Therefore, when copper is added to silver nitrate solution, it displaces the silver from its compound and forms copper nitrate. The displaced silver ions combine with copper atoms to form solid silver, which is observed as a brownish precipitate.
Hence, the correct option is A - Copper is more reactive than silver.
The given reaction is: Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
This reaction involves the displacement of silver from its compound (AgNO3) by copper. This type of reaction is known as a single displacement reaction or a substitution reaction.
To determine which metal is more reactive, we need to look at the activity series of metals. This series arranges metals in order of their reactivity, with the most reactive metals at the top and the least reactive at the bottom.
Copper is placed below silver in the activity series, which means that silver is more reactive than copper. Therefore, when copper is added to silver nitrate solution, it displaces the silver from its compound and forms copper nitrate. The displaced silver ions combine with copper atoms to form solid silver, which is observed as a brownish precipitate.
Hence, the correct option is A - Copper is more reactive than silver.
Which of the following statement is incorrect?- a)In oxidation, oxygen in added to a substance.
- b)In reduction, Hydrogen is added to a substance.
- c)Oxidizing agent is oxidized.
- d)Reducing agent is oxidized
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is incorrect?
a)
In oxidation, oxygen in added to a substance.
b)
In reduction, Hydrogen is added to a substance.
c)
Oxidizing agent is oxidized.
d)
Reducing agent is oxidized

|
Nk Classes answered |
- a) In oxidation, oxygen is added to a substance: This is correct. Oxidation involves the addition of oxygen or the loss of electrons.
- b) In reduction, hydrogen is added to a substance: This is correct. Reduction typically involves the addition of hydrogen or the gain of electrons.
- c) Oxidizing agent is oxidized: This is incorrect. The oxidizing agent is actually reduced as it gains electrons during the reaction, thereby oxidising the other substance.
- d) Reducing agent is oxidized: This is correct. The reducing agent loses electrons (is oxidized) while it reduces the other substance by donating electrons.
A balanced chemical equation is in accordance with which one of the following laws given below- a)Law of constant proportion
- b)Law of conservation of energy
- c)Law of conservation of mass
- d)Low of multiple proportion
Correct answer is option 'C'. Can you explain this answer?
A balanced chemical equation is in accordance with which one of the following laws given below
a)
Law of constant proportion
b)
Law of conservation of energy
c)
Law of conservation of mass
d)
Low of multiple proportion
|
|
Maitri Daga answered |
Answer C
its absolutely correct
its absolutely correct
Choose the correctly matched pair:
- a)CaO + H₂O → Ca(OH)₂ + Heat - Combination Reaction
- b)Fe + 4H₂O → Fe₃O₄ + 4H₂ - Decomposition Reaction
- c)3Fe + 4H₂O → Fe₃O₄ + 4H₂ - Combination Reaction
- d)CO + 2H₂ → CH₃OH - Displacement Reaction
Correct answer is option 'A'. Can you explain this answer?
Choose the correctly matched pair:
a)
CaO + H₂O → Ca(OH)₂ + Heat - Combination Reaction
b)
Fe + 4H₂O → Fe₃O₄ + 4H₂ - Decomposition Reaction
c)
3Fe + 4H₂O → Fe₃O₄ + 4H₂ - Combination Reaction
d)
CO + 2H₂ → CH₃OH - Displacement Reaction

|
Lakesway Classes answered |
- Option A: CaO + H₂O → Ca(OH)₂ + Heat - This is correctly matched as a Combination Reaction. In this reaction, calcium oxide (CaO) reacts with water (H₂O) to form calcium hydroxide (Ca(OH)₂) and releases heat. This is a classic example of a combination reaction where two or more substances combine to form a single product.
- Option B: Fe + 4H₂O → Fe₃O₄ + 4H₂ - This reaction is incorrectly labeled as a Decomposition Reaction. It is actually a redox reaction involving the oxidation of iron and the reduction of water.
- Option C: 3Fe + 4H₂O → Fe₃O₄ + 4H₂ - This is also incorrectly labeled. Although there is a combination of elements involved, it is best described as a redox reaction rather than a simple Decomposition reaction.
- Option D: CO + 2H₂ → CH₃OH - This is incorrectly matched as a Displacement Reaction. It is actually a synthesis reaction where carbon monoxide (CO) and hydrogen (H₂) combine to form methanol (CH₃OH).
Which of the following is a balanced chemical equation?- a)H₂ + O₂ → H₂O
- b)2H₂ + O₂ → H₂O
- c)H₂ + 2O₂ → H₂O
- d) 2H₂ + O₂ → 2H₂O
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a balanced chemical equation?
a)
H₂ + O₂ → H₂O
b)
2H₂ + O₂ → H₂O
c)
H₂ + 2O₂ → H₂O
d)
2H₂ + O₂ → 2H₂O

|
Lakesway Classes answered |
2H2 + O2 → 2H2O
The balanced chemical equation is:
- 2 molecules of hydrogen gas (H2) react with 1 molecule of oxygen gas (O2).
- This produces 2 molecules of water (H2O).
- Each side of the equation has 4 hydrogen atoms and 2 oxygen atoms, ensuring mass conservation.
What type of reaction is characterized by the formation of a single product from two or more reactants?- a)Combination Reaction
- b)Decomposition Reaction
- c)Displacement Reaction
- d)Double Displacement Reaction
Correct answer is option 'A'. Can you explain this answer?
What type of reaction is characterized by the formation of a single product from two or more reactants?
a)
Combination Reaction
b)
Decomposition Reaction
c)
Displacement Reaction
d)
Double Displacement Reaction

|
Lakesway Classes answered |
In a combination reaction, multiple reactants combine to form a single product. An example is the reaction between calcium oxide and water to produce calcium hydroxide. This type of reaction involves the synthesis of a new compound from the combining of elements or compounds.
Choose the correctly matched pair.
- a)Burning of coal - C(s) + O2(g) → CO(g)
- b)Formation of water - 2H2(g) + O2(g) → 2H2O2(l)
- c)Respiration - C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O2(l) + energy
- d)Whitewashing reaction - Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
Correct answer is option 'D'. Can you explain this answer?
Choose the correctly matched pair.
a)
Burning of coal - C(s) + O2(g) → CO(g)
b)
Formation of water - 2H2(g) + O2(g) → 2H2O2(l)
c)
Respiration - C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O2(l) + energy
d)
Whitewashing reaction - Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)

|
Lakesway Classes answered |
- Let's analyze each option to determine the correctly matched pair:
- Option A: (a) Burning of coal - C(s) + O2(g) → CO2(g)
- This is a correct chemical equation for the burning of coal, which is a combination reaction. However, let's verify the other options before concluding.
- Option B: (b) Formation of water - 2H2(g) + O2(g) → 2H2O(l)
- This is also a correct chemical equation for the formation of water from hydrogen and oxygen, which is another example of a combination reaction. We need to check the remaining options as well.
- Option C: (c) Respiration - C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy
- This equation correctly represents the process of respiration, an exothermic reaction where glucose reacts with oxygen to produce carbon dioxide, water, and energy.
- Option D: (d) Whitewashing reaction - Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
- This is the correct chemical equation for the whitewashing reaction. When calcium hydroxide reacts with carbon dioxide, it forms calcium carbonate (which gives a shiny finish on walls) and water.
- All options are correctly matched pairs, but the question asks for one correctly matched pair. Therefore, Option D is chosen as it represents a familiar and specific application that students are likely to recognize easily.
Which of the following is not a physical change ?- a)Boiling of water to give water vapour
- b)Melting of ice to give water
- c)Dissolution of salt in water
- d)Combustion of liquefied petroleum Gas (LPG)
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a physical change ?
a)
Boiling of water to give water vapour
b)
Melting of ice to give water
c)
Dissolution of salt in water
d)
Combustion of liquefied petroleum Gas (LPG)
|
|
Aarav agarwal answered |
Physical and Chemical Changes
Physical changes are reversible changes that do not involve a change in the chemical composition of a substance. Examples of physical changes include changes in state (solid, liquid, gas), changes in shape or size, and changes in color or texture. In contrast, chemical changes involve a change in the chemical composition of a substance, resulting in the formation of a new substance(s) with different properties.
Explanation
Of the given options, option 'D' - combustion of liquefied petroleum gas (LPG) is not a physical change. Combustion is a chemical change that involves the reaction of a fuel (LPG in this case) with oxygen in the air, producing heat and light energy. The products of the combustion of LPG are carbon dioxide and water vapor, which are different from the original fuel.
On the other hand, boiling of water to give water vapor, melting of ice to give water, and dissolution of salt in water are all physical changes. In these processes, no new substances are formed, and the original substance(s) retain their chemical composition.
Conclusion
In summary, physical changes are characterized by the absence of a chemical reaction, and the original substance(s) retain their chemical composition. Option 'D' is the correct answer as it represents combustion, which is a chemical change that involves the formation of new substances with different properties.
Physical changes are reversible changes that do not involve a change in the chemical composition of a substance. Examples of physical changes include changes in state (solid, liquid, gas), changes in shape or size, and changes in color or texture. In contrast, chemical changes involve a change in the chemical composition of a substance, resulting in the formation of a new substance(s) with different properties.
Explanation
Of the given options, option 'D' - combustion of liquefied petroleum gas (LPG) is not a physical change. Combustion is a chemical change that involves the reaction of a fuel (LPG in this case) with oxygen in the air, producing heat and light energy. The products of the combustion of LPG are carbon dioxide and water vapor, which are different from the original fuel.
On the other hand, boiling of water to give water vapor, melting of ice to give water, and dissolution of salt in water are all physical changes. In these processes, no new substances are formed, and the original substance(s) retain their chemical composition.
Conclusion
In summary, physical changes are characterized by the absence of a chemical reaction, and the original substance(s) retain their chemical composition. Option 'D' is the correct answer as it represents combustion, which is a chemical change that involves the formation of new substances with different properties.
A dilute ferrous sulphate solution was added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?- a)KMnO4 is an oxidising agent, it oxidises FeSO4
- b)FeSO4 acts an oxidising agent and oxidises KMnO4
- c)KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound
- d)The colour disappears due to the dilution : no reaction is involved
Correct answer is option 'A'. Can you explain this answer?
A dilute ferrous sulphate solution was added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
a)
KMnO4 is an oxidising agent, it oxidises FeSO4
b)
FeSO4 acts an oxidising agent and oxidises KMnO4
c)
KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound
d)
The colour disappears due to the dilution : no reaction is involved
|
|
Priyanka Kapoor answered |
In this reaction, potassium permanganate (KMnO4) is an oxidizing agent. It oxidises ferrous sulphate to ferric sulphate in the presence of dilute H2SO4.
2KMNO4 + 10FeSO4 + 8H2SO4 → K2SO4 + 2MnSO4 + 5Fe(SO4)3 + 8H2O
The solution is coloured purple because of the KMnO4 and it eventually disappears when all the KMnO4 in the solution is utilized.
2KMNO4 + 10FeSO4 + 8H2SO4 → K2SO4 + 2MnSO4 + 5Fe(SO4)3 + 8H2O
The solution is coloured purple because of the KMnO4 and it eventually disappears when all the KMnO4 in the solution is utilized.
Which form of energy is responsible for the decomposition reactions ?- a)Heat
- b)All of these
- c)Electricity
- d)light
Correct answer is option 'B'. Can you explain this answer?
Which form of energy is responsible for the decomposition reactions ?
a)
Heat
b)
All of these
c)
Electricity
d)
light

|
Upsc Toppers answered |
- Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants.
Assertion (A): The iron nail becomes brownish in color and the blue color of copper sulfate solution fades.Reason (R): In the reaction Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s), iron displaces copper in the copper sulfate solution.- a)If both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)If Assertion is true but Reason is false
- d)If both Assertion and Reason are false
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): The iron nail becomes brownish in color and the blue color of copper sulfate solution fades.
Reason (R): In the reaction Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s), iron displaces copper in the copper sulfate solution.
a)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion
b)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
c)
If Assertion is true but Reason is false
d)
If both Assertion and Reason are false

|
Lakesway Classes answered |
- The Assertion is true as the iron nail indeed becomes brownish and the blue color of the copper sulfate solution fades due to the displacement reaction described.
- The Reason is also true as iron displaces copper from the copper sulfate solution.
- The Reason is the correct explanation of the Assertion because the observed color changes are a direct result of the displacement reaction where iron replaces copper in the solution.
Which of the following gases can be used for storage of fresh sample of an oil for a long time?- a)Carbon dioxide or oxygen
- b)Carbon dioxide or helium
- c)Nitrogen or oxygen
- d)Nitrogen or helium
Correct answer is option 'D'. Can you explain this answer?
Which of the following gases can be used for storage of fresh sample of an oil for a long time?
a)
Carbon dioxide or oxygen
b)
Carbon dioxide or helium
c)
Nitrogen or oxygen
d)
Nitrogen or helium
|
|
Vidhi dubey answered |
Answer:
Introduction:
When storing a fresh sample of oil for a long time, it is important to prevent its degradation or oxidation. This can be achieved by using an appropriate gas to create an inert atmosphere that minimizes contact with oxygen, moisture, and other reactive substances. Among the options given, nitrogen or helium are the most suitable gases for this purpose.
Explanation:
Nitrogen:
- Nitrogen is an inert gas, meaning it is chemically unreactive and does not readily react with other substances.
- It is abundantly available in the atmosphere and is commonly used for various industrial and laboratory applications.
- When used for storing oil samples, nitrogen replaces the oxygen in the storage container, thus creating an oxygen-free environment.
- This prevents oxidation and slows down the degradation of the oil, helping to maintain its freshness and quality over a long period of time.
Helium:
- Helium is another inert gas that is chemically stable and does not react with other substances.
- It is lighter than air and has low solubility in most liquids, including oil.
- When used as a storage gas, helium creates a protective blanket over the oil sample, preventing oxygen and moisture from coming into contact with the oil.
- This helps to maintain the oil's freshness and prevents degradation or oxidation.
Comparison:
Both nitrogen and helium are suitable for storing oil samples for a long time. However, there are a few key differences between the two gases:
- Availability: Nitrogen is more readily available and less expensive compared to helium, which is relatively rare and more expensive.
- Density: Helium is lighter than air and has low solubility in oil, making it more effective in creating a protective blanket over the oil sample.
- Cost: Nitrogen is generally more cost-effective and practical for most applications. Helium is typically reserved for specialized or high-value samples due to its higher cost.
Conclusion:
In conclusion, nitrogen or helium can be used for the storage of fresh samples of oil for a long time. Both gases create an inert atmosphere that prevents oxidation and degradation of the oil. Nitrogen is more commonly used due to its availability and cost-effectiveness, while helium is preferred for specialized or high-value samples.
Introduction:
When storing a fresh sample of oil for a long time, it is important to prevent its degradation or oxidation. This can be achieved by using an appropriate gas to create an inert atmosphere that minimizes contact with oxygen, moisture, and other reactive substances. Among the options given, nitrogen or helium are the most suitable gases for this purpose.
Explanation:
Nitrogen:
- Nitrogen is an inert gas, meaning it is chemically unreactive and does not readily react with other substances.
- It is abundantly available in the atmosphere and is commonly used for various industrial and laboratory applications.
- When used for storing oil samples, nitrogen replaces the oxygen in the storage container, thus creating an oxygen-free environment.
- This prevents oxidation and slows down the degradation of the oil, helping to maintain its freshness and quality over a long period of time.
Helium:
- Helium is another inert gas that is chemically stable and does not react with other substances.
- It is lighter than air and has low solubility in most liquids, including oil.
- When used as a storage gas, helium creates a protective blanket over the oil sample, preventing oxygen and moisture from coming into contact with the oil.
- This helps to maintain the oil's freshness and prevents degradation or oxidation.
Comparison:
Both nitrogen and helium are suitable for storing oil samples for a long time. However, there are a few key differences between the two gases:
- Availability: Nitrogen is more readily available and less expensive compared to helium, which is relatively rare and more expensive.
- Density: Helium is lighter than air and has low solubility in oil, making it more effective in creating a protective blanket over the oil sample.
- Cost: Nitrogen is generally more cost-effective and practical for most applications. Helium is typically reserved for specialized or high-value samples due to its higher cost.
Conclusion:
In conclusion, nitrogen or helium can be used for the storage of fresh samples of oil for a long time. Both gases create an inert atmosphere that prevents oxidation and degradation of the oil. Nitrogen is more commonly used due to its availability and cost-effectiveness, while helium is preferred for specialized or high-value samples.
When a black and white photographic film is exposed to light, the grey colour on the photographic film is due to presence of- a)Ag2O
- b)Ag
- c)Br2
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
When a black and white photographic film is exposed to light, the grey colour on the photographic film is due to presence of
a)
Ag2O
b)
Ag
c)
Br2
d)
All of these
|
|
Raghav Choudhury answered |
Explanation:
When a black and white photographic film is exposed to light, the grey color on the film is due to the presence of silver (Ag).
Formation of Image on Photographic Film:
1. The photographic film consists of a layer of gelatin that contains tiny silver bromide (AgBr) crystals.
2. When light falls on the film, it interacts with the silver bromide crystals.
3. The light energy causes the silver bromide crystals to get activated and undergo a chemical reaction.
4. The activated silver bromide crystals break down into silver (Ag) and bromine (Br2) ions.
5. The silver ions combine with the electrons present in the gelatin layer of the film to form metallic silver (Ag).
6. The metallic silver particles form clusters, which are responsible for the grey color on the film.
Role of Silver:
Silver plays a crucial role in the formation of the image on the photographic film. The silver ions produced during the reaction with light interact with the gelatin layer and form metallic silver. These silver clusters are responsible for the grey color observed on the film.
Role of Bromine:
Bromine (Br2) is also involved in the reaction, but it does not contribute directly to the grey color on the film. Bromine ions are released when the silver bromide crystals are activated by light. However, it is the silver ions that interact with the gelatin layer and form metallic silver, resulting in the grey color.
Conclusion:
In conclusion, the grey color observed on a black and white photographic film when exposed to light is due to the presence of silver (Ag). The silver ions produced during the reaction with light combine with the gelatin layer to form metallic silver clusters, which give the film its characteristic grey color. Bromine (Br2) is also involved in the reaction as it is released when the silver bromide crystals are activated, but it does not directly contribute to the grey color on the film.
When a black and white photographic film is exposed to light, the grey color on the film is due to the presence of silver (Ag).
Formation of Image on Photographic Film:
1. The photographic film consists of a layer of gelatin that contains tiny silver bromide (AgBr) crystals.
2. When light falls on the film, it interacts with the silver bromide crystals.
3. The light energy causes the silver bromide crystals to get activated and undergo a chemical reaction.
4. The activated silver bromide crystals break down into silver (Ag) and bromine (Br2) ions.
5. The silver ions combine with the electrons present in the gelatin layer of the film to form metallic silver (Ag).
6. The metallic silver particles form clusters, which are responsible for the grey color on the film.
Role of Silver:
Silver plays a crucial role in the formation of the image on the photographic film. The silver ions produced during the reaction with light interact with the gelatin layer and form metallic silver. These silver clusters are responsible for the grey color observed on the film.
Role of Bromine:
Bromine (Br2) is also involved in the reaction, but it does not contribute directly to the grey color on the film. Bromine ions are released when the silver bromide crystals are activated by light. However, it is the silver ions that interact with the gelatin layer and form metallic silver, resulting in the grey color.
Conclusion:
In conclusion, the grey color observed on a black and white photographic film when exposed to light is due to the presence of silver (Ag). The silver ions produced during the reaction with light combine with the gelatin layer to form metallic silver clusters, which give the film its characteristic grey color. Bromine (Br2) is also involved in the reaction as it is released when the silver bromide crystals are activated, but it does not directly contribute to the grey color on the film.
Which one of the following is not a chemical change?- a)Cooking of food
- b)Evaporation of water
- c)Burning of candle wax
- d)Digestion of food in our body
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is not a chemical change?
a)
Cooking of food
b)
Evaporation of water
c)
Burning of candle wax
d)
Digestion of food in our body
|
|
Sarangi rao answered |
Chemical and Physical Changes
Chemical changes involve the formation of new substances with different chemical properties, while physical changes involve changes in the physical state or appearance of a substance without changing its chemical composition.
Cooking of Food
Cooking of food involves chemical changes, as the heat and other ingredients cause chemical reactions that alter the taste, texture, and nutritional value of the food.
Evaporation of Water
Evaporation of water is a physical change, as it involves the change of water from a liquid to a gas without any change in its chemical composition.
Burning of Candle Wax
Burning of candle wax is a chemical change, as the heat and oxygen cause the wax to react and form new compounds such as carbon dioxide and water.
Digestion of Food in our Body
Digestion of food in our body involves chemical changes, as the enzymes in our digestive system break down the food into simpler substances that can be used by our body for energy and growth.
Conclusion
Therefore, the correct answer is option B - Evaporation of water, as it is a physical change and does not involve any chemical reactions or the formation of new substances.
Chemical changes involve the formation of new substances with different chemical properties, while physical changes involve changes in the physical state or appearance of a substance without changing its chemical composition.
Cooking of Food
Cooking of food involves chemical changes, as the heat and other ingredients cause chemical reactions that alter the taste, texture, and nutritional value of the food.
Evaporation of Water
Evaporation of water is a physical change, as it involves the change of water from a liquid to a gas without any change in its chemical composition.
Burning of Candle Wax
Burning of candle wax is a chemical change, as the heat and oxygen cause the wax to react and form new compounds such as carbon dioxide and water.
Digestion of Food in our Body
Digestion of food in our body involves chemical changes, as the enzymes in our digestive system break down the food into simpler substances that can be used by our body for energy and growth.
Conclusion
Therefore, the correct answer is option B - Evaporation of water, as it is a physical change and does not involve any chemical reactions or the formation of new substances.
When ferrous sulphate crystals are strongly heated, the gas/vapour not evolved are of- a)SO2
- b)SO3
- c)O2
- d)H2O
Correct answer is option 'C'. Can you explain this answer?
When ferrous sulphate crystals are strongly heated, the gas/vapour not evolved are of
a)
SO2
b)
SO3
c)
O2
d)
H2O
|
|
Manish iyer answered |
Heating Ferrous Sulphate Crystals
Ferrous sulphate crystals have the chemical formula FeSO4·7H2O. When these crystals are strongly heated, they undergo a decomposition reaction, breaking down into different compounds.
Decomposition Reaction
The decomposition reaction of ferrous sulphate crystals is as follows:
FeSO4·7H2O(s) → Fe2O3(s) + SO3(g) + H2O(g)
According to this reaction, the ferrous sulphate crystals break down into solid iron(III) oxide (Fe2O3), gaseous sulphur trioxide (SO3), and water vapor (H2O).
Evolved Gas/Vapour
The question asks us to identify the gas/vapour that is not evolved during the heating process. From the above reaction, we can see that three different compounds are produced when ferrous sulphate crystals are strongly heated. These are:
- Iron(III) oxide (Fe2O3)
- Sulphur trioxide (SO3)
- Water vapor (H2O)
Out of these three compounds, only one is not a gas/vapour. That compound is iron(III) oxide (Fe2O3).
Therefore, the correct answer to the question is option 'C', which is O2. This is not one of the compounds produced during the heating of ferrous sulphate crystals, and hence it is not the gas/vapour that is not evolved.
Ferrous sulphate crystals have the chemical formula FeSO4·7H2O. When these crystals are strongly heated, they undergo a decomposition reaction, breaking down into different compounds.
Decomposition Reaction
The decomposition reaction of ferrous sulphate crystals is as follows:
FeSO4·7H2O(s) → Fe2O3(s) + SO3(g) + H2O(g)
According to this reaction, the ferrous sulphate crystals break down into solid iron(III) oxide (Fe2O3), gaseous sulphur trioxide (SO3), and water vapor (H2O).
Evolved Gas/Vapour
The question asks us to identify the gas/vapour that is not evolved during the heating process. From the above reaction, we can see that three different compounds are produced when ferrous sulphate crystals are strongly heated. These are:
- Iron(III) oxide (Fe2O3)
- Sulphur trioxide (SO3)
- Water vapor (H2O)
Out of these three compounds, only one is not a gas/vapour. That compound is iron(III) oxide (Fe2O3).
Therefore, the correct answer to the question is option 'C', which is O2. This is not one of the compounds produced during the heating of ferrous sulphate crystals, and hence it is not the gas/vapour that is not evolved.
Identify the substances that are oxidised and the substances that are reduced in the following reaction.Fe2O3 + 2Al → Al2O3 + 2Fe- a)Fe2O3
- b)Al
- c)Fe
- d)Both A and B
Correct answer is option 'D'. Can you explain this answer?
Identify the substances that are oxidised and the substances that are reduced in the following reaction.
Fe2O3 + 2Al → Al2O3 + 2Fe
a)
Fe2O3
b)
Al
c)
Fe
d)
Both A and B
|
|
Sagar Shah answered |
Understanding Oxidation and Reduction
In a chemical reaction, oxidation refers to the loss of electrons, while reduction involves the gain of electrons. These processes are often coupled, meaning that one substance is oxidized while another is reduced.
Analyzing the Reaction
The reaction given is:
Fe2O3 + 2Al → Al2O3 + 2Fe
To determine which substances are oxidized and reduced, we need to analyze the changes in oxidation states.
Oxidation State Changes
- Fe2O3 (Iron(III) oxide):
- Iron (Fe) has an oxidation state of +3 in Fe2O3.
- After the reaction, iron is reduced to elemental iron (Fe), which has an oxidation state of 0.
- Al (Aluminum):
- Aluminum (Al) in this reaction starts with an oxidation state of 0 (elemental form).
- After the reaction, it forms Al2O3, where aluminum has an oxidation state of +3.
Identifying Oxidized and Reduced Substances
- Oxidation:
- Aluminum (Al) is oxidized from 0 to +3, meaning it loses electrons.
- Reduction:
- Iron (Fe) in Fe2O3 is reduced from +3 to 0, meaning it gains electrons.
Conclusion
In this reaction:
- Fe2O3 is reduced (iron is reduced).
- Al is oxidized (aluminum is oxidized).
Therefore, the correct answer is option ‘D’: both Fe2O3 and Al participate in oxidation and reduction processes.
In a chemical reaction, oxidation refers to the loss of electrons, while reduction involves the gain of electrons. These processes are often coupled, meaning that one substance is oxidized while another is reduced.
Analyzing the Reaction
The reaction given is:
Fe2O3 + 2Al → Al2O3 + 2Fe
To determine which substances are oxidized and reduced, we need to analyze the changes in oxidation states.
Oxidation State Changes
- Fe2O3 (Iron(III) oxide):
- Iron (Fe) has an oxidation state of +3 in Fe2O3.
- After the reaction, iron is reduced to elemental iron (Fe), which has an oxidation state of 0.
- Al (Aluminum):
- Aluminum (Al) in this reaction starts with an oxidation state of 0 (elemental form).
- After the reaction, it forms Al2O3, where aluminum has an oxidation state of +3.
Identifying Oxidized and Reduced Substances
- Oxidation:
- Aluminum (Al) is oxidized from 0 to +3, meaning it loses electrons.
- Reduction:
- Iron (Fe) in Fe2O3 is reduced from +3 to 0, meaning it gains electrons.
Conclusion
In this reaction:
- Fe2O3 is reduced (iron is reduced).
- Al is oxidized (aluminum is oxidized).
Therefore, the correct answer is option ‘D’: both Fe2O3 and Al participate in oxidation and reduction processes.
Identify the substance oxidised and the substance reduced in the following reactions.MnO2 + 4HCl —–> MnCl2 + 2H20 + Cl2- a)MnO2 is getting reduced and H20 is getting oxidised.
- b)MnO2 is getting reduced and HCl is getting oxidised.
- c)MnCl2 is getting reduced and HCl is getting oxidised.
- d)HCl is getting reduced and Cl2 is getting oxidised.
Correct answer is option 'B'. Can you explain this answer?
Identify the substance oxidised and the substance reduced in the following reactions.
MnO2 + 4HCl —–> MnCl2 + 2H20 + Cl2
a)
MnO2 is getting reduced and H20 is getting oxidised.
b)
MnO2 is getting reduced and HCl is getting oxidised.
c)
MnCl2 is getting reduced and HCl is getting oxidised.
d)
HCl is getting reduced and Cl2 is getting oxidised.

|
EduRev Class 10 answered |
MnO2 is getting reduced and HCl is getting oxidised.
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
- a)2 H2 (g) + O2 (l) → 2 H2O (l)
- b)2 H2 (l) + O2 (l) → 2 H2O (g)
- c)2 H2 (g) + O2 (g) → 2 H2O (l)
- d)2 H2 (g) + O2 (g) → 2 H2O (g)
Correct answer is option 'C'. Can you explain this answer?
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
a)
2 H2 (g) + O2 (l) → 2 H2O (l)
b)
2 H2 (l) + O2 (l) → 2 H2O (g)
c)
2 H2 (g) + O2 (g) → 2 H2O (l)
d)
2 H2 (g) + O2 (g) → 2 H2O (g)
|
|
Ritu Saxena answered |
At the reaction temperature, H2 gas combine with O2 gas to form H2O vapour.
Fe2 O3 + 2 Al → Al2O3 + 2 Fe
The above reaction is an example of a- a)Combination reaction
- b)Double displacement reaction
- c)Displacement reaction
- d)Decomposition reaction
Correct answer is option 'C'. Can you explain this answer?
Fe2 O3 + 2 Al → Al2O3 + 2 Fe
The above reaction is an example of a
The above reaction is an example of a
a)
Combination reaction
b)
Double displacement reaction
c)
Displacement reaction
d)
Decomposition reaction
|
|
Rohit Sharma answered |
It is an example of displacement reaction. Aluminium being more reactive than iron displaces iron and results in the formation of aluminium oxide.

What is the substance gaining oxygen and being oxidized during the described reaction?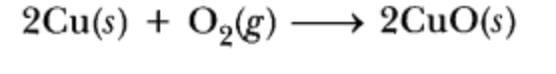
- a)Cu
- b)O
- c)CuO
- d)Not a redox reaction
Correct answer is option 'A'. Can you explain this answer?
What is the substance gaining oxygen and being oxidized during the described reaction?
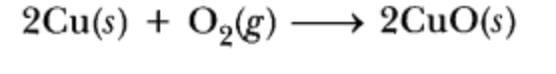
a)
Cu
b)
O
c)
CuO
d)
Not a redox reaction

|
Nk Classes answered |
- In a redox reaction, one substance is oxidized (loses electrons) and another is reduced (gains electrons).
- Oxidation involves the gain of oxygen.
- Copper (Cu) gains oxygen to form CuO, thus it is oxidized.
- Therefore, the substance gaining oxygen and being oxidized is copper (Cu).
- Correct answer: A: Cu
- Oxidation involves the gain of oxygen.
- Copper (Cu) gains oxygen to form CuO, thus it is oxidized.
- Therefore, the substance gaining oxygen and being oxidized is copper (Cu).
- Correct answer: A: Cu
Assertion (A): Burning of natural gas is an example of an exothermic reaction.Reason (R): Exothermic reactions release heat energy along with the formation of products.- a)If Assertion is true but Reason is false
- b)If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)If both Assertion and Reason are true and Reason is the correct explanation of Assertion
- d)If both Assertion and Reason are false
Correct answer is option 'C'. Can you explain this answer?
Assertion (A): Burning of natural gas is an example of an exothermic reaction.
Reason (R): Exothermic reactions release heat energy along with the formation of products.
a)
If Assertion is true but Reason is false
b)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
c)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion
d)
If both Assertion and Reason are false

|
Kamna Science Academy answered |
Let's analyze the statements:
Assertion (A): Burning of natural gas is an example of an exothermic reaction.
- This is true because burning natural gas (methane, CH4) releases heat energy.
Reason (R): Exothermic reactions release heat energy along with the formation of products.
- This is also true because exothermic reactions are characterized by the release of heat energy during the reaction process.
Since both statements are true and the Reason correctly explains the Assertion: If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
Which of the following statements about the given reaction are correct ?
3 Fe(s) + 4 H2 O(g) → Fe3O4 (s) + 4 H2(g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent.- a)(i), (ii) and (iii)
- b)(iii) and (iv)
- c)(i), (ii) and (iv)
- d)(ii) and (iv)
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about the given reaction are correct ?
3 Fe(s) + 4 H2 O(g) → Fe3O4 (s) + 4 H2(g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent.
3 Fe(s) + 4 H2 O(g) → Fe3O4 (s) + 4 H2(g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent.
a)
(i), (ii) and (iii)
b)
(iii) and (iv)
c)
(i), (ii) and (iv)
d)
(ii) and (iv)
|
|
Vivek Bansal answered |
Here, oxygen combines with water to get oxidized. Oxygen is removed from water hence, it is getting reduced. Water is providing oxygen and it acts as an oxidizing agent.
- On introducing two molecules to each other, one molecule loses an electron. This reaction is called oxidation.
- On introducing two molecules to each other, one molecule gains an electron. This reaction is called reduction.
- The oxidized substance is called a reduction agent. The reduced substance is called an oxidizing agent.
Which of the following is (are) on endothermic process(es)?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water- a)(i) and (ii)
- b)(ii) and (iv)
- c)(ii) only
- d)(iii) only
Correct answer is option 'B'. Can you explain this answer?
Which of the following is (are) on endothermic process(es)?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
a)
(i) and (ii)
b)
(ii) and (iv)
c)
(ii) only
d)
(iii) only
|
|
Khusboo jain answered |
Endothermic Processes
Dilution of sulphuric acid
- Dilution of sulphuric acid is an exothermic process as it releases heat during the reaction.
Sublimation of dry ice
- Sublimation of dry ice is an endothermic process as it requires heat input to change the solid directly into a gas without passing through the liquid phase.
Condensation of water vapours
- Condensation of water vapours is an exothermic process as it releases heat when water vapours change into liquid water.
Evaporation of water
- Evaporation of water is an endothermic process as it requires heat input to change liquid water into water vapour.
Therefore, the endothermic processes in the given options are (ii) Sublimation of dry ice and (iv) Evaporation of water. So, the correct answer is option B - (ii) and (iv).
Dilution of sulphuric acid
- Dilution of sulphuric acid is an exothermic process as it releases heat during the reaction.
Sublimation of dry ice
- Sublimation of dry ice is an endothermic process as it requires heat input to change the solid directly into a gas without passing through the liquid phase.
Condensation of water vapours
- Condensation of water vapours is an exothermic process as it releases heat when water vapours change into liquid water.
Evaporation of water
- Evaporation of water is an endothermic process as it requires heat input to change liquid water into water vapour.
Therefore, the endothermic processes in the given options are (ii) Sublimation of dry ice and (iv) Evaporation of water. So, the correct answer is option B - (ii) and (iv).
Which one of the following does not result in the evolution of H2 gas?- a)Zinc and hydrochloric acid
- b)Iron and sulphuric acid
- c)Magnesium and very dilute nitric acid
- d)Aluminium and nitric acid
Correct answer is option 'D'. Can you explain this answer?
Which one of the following does not result in the evolution of H2 gas?
a)
Zinc and hydrochloric acid
b)
Iron and sulphuric acid
c)
Magnesium and very dilute nitric acid
d)
Aluminium and nitric acid
|
|
Rohini chauhan answered |
Explanation:
The reaction between a metal and an acid usually results in the evolution of hydrogen gas. However, there are certain conditions under which this reaction may not occur. In this case, we are given four different reactions and we have to identify which one does not result in the evolution of H2 gas.
Zinc and hydrochloric acid:
When zinc reacts with hydrochloric acid, it undergoes a displacement reaction and produces zinc chloride and hydrogen gas. The balanced chemical equation for this reaction is:
Zn + 2HCl → ZnCl2 + H2
Iron and sulphuric acid:
When iron reacts with sulfuric acid, it also undergoes a displacement reaction and produces iron(II) sulfate and hydrogen gas. The balanced chemical equation for this reaction is:
Fe + H2SO4 → FeSO4 + H2
Magnesium and very dilute nitric acid:
When magnesium reacts with nitric acid, it undergoes a redox reaction and produces magnesium nitrate, nitrogen dioxide gas, and water. The balanced chemical equation for this reaction is:
3Mg + 8HNO3 → 3Mg(NO3)2 + 2NO + 4H2O
Aluminium and nitric acid:
When aluminium reacts with nitric acid, it also undergoes a redox reaction and produces aluminium nitrate, nitrogen dioxide gas, and water. The balanced chemical equation for this reaction is:
2Al + 6HNO3 → 2Al(NO3)3 + 3H2O + 3NO2
From the above equations, we can see that all the reactions except the one involving aluminium and nitric acid result in the evolution of hydrogen gas. Therefore, the correct answer is option 'D' - Aluminium and nitric acid.
The reaction between a metal and an acid usually results in the evolution of hydrogen gas. However, there are certain conditions under which this reaction may not occur. In this case, we are given four different reactions and we have to identify which one does not result in the evolution of H2 gas.
Zinc and hydrochloric acid:
When zinc reacts with hydrochloric acid, it undergoes a displacement reaction and produces zinc chloride and hydrogen gas. The balanced chemical equation for this reaction is:
Zn + 2HCl → ZnCl2 + H2
Iron and sulphuric acid:
When iron reacts with sulfuric acid, it also undergoes a displacement reaction and produces iron(II) sulfate and hydrogen gas. The balanced chemical equation for this reaction is:
Fe + H2SO4 → FeSO4 + H2
Magnesium and very dilute nitric acid:
When magnesium reacts with nitric acid, it undergoes a redox reaction and produces magnesium nitrate, nitrogen dioxide gas, and water. The balanced chemical equation for this reaction is:
3Mg + 8HNO3 → 3Mg(NO3)2 + 2NO + 4H2O
Aluminium and nitric acid:
When aluminium reacts with nitric acid, it also undergoes a redox reaction and produces aluminium nitrate, nitrogen dioxide gas, and water. The balanced chemical equation for this reaction is:
2Al + 6HNO3 → 2Al(NO3)3 + 3H2O + 3NO2
From the above equations, we can see that all the reactions except the one involving aluminium and nitric acid result in the evolution of hydrogen gas. Therefore, the correct answer is option 'D' - Aluminium and nitric acid.
What is a reduction reaction?- a)addition of hydrogen to a substance
- b)addition of oxygen to a substance
- c)removal of hydrogen from a substance
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?
What is a reduction reaction?
a)
addition of hydrogen to a substance
b)
addition of oxygen to a substance
c)
removal of hydrogen from a substance
d)
none of the above
|
|
Sonam dasgupta answered |
Understanding Reduction Reactions
Reduction reactions are a fundamental concept in chemistry, particularly in the context of redox (reduction-oxidation) reactions.
Definition of Reduction
- Reduction is defined as the process where a substance gains electrons, leading to a decrease in its oxidation state.
- This process often involves the addition of hydrogen or the removal of oxygen.
Why Option A is Correct
- Addition of Hydrogen: When hydrogen is added to a substance, it effectively reduces the oxidation state of that substance. For example, adding hydrogen to a metal oxide can convert it into a metal, a clear example of reduction.
- Contrast with Oxidation: In contrast, oxidation involves the addition of oxygen or the removal of hydrogen. Therefore, when we talk about reduction, we specifically refer to the addition of hydrogen as a primary way to achieve it.
Examples of Reduction Reactions
- Hydrogenation: This is a common reduction reaction in organic chemistry where unsaturated hydrocarbons (like alkenes) are converted to saturated hydrocarbons by the addition of hydrogen.
- Metal Reduction: In metallurgy, metal oxides are reduced to their elemental forms by using hydrogen gas, showcasing the importance of reduction in extracting metals.
Conclusion
In summary, option A is the correct answer as it directly relates to the definition of reduction in terms of chemical reactions. The addition of hydrogen plays a crucial role in reducing various substances, demonstrating the essential nature of reduction in chemical processes.
Reduction reactions are a fundamental concept in chemistry, particularly in the context of redox (reduction-oxidation) reactions.
Definition of Reduction
- Reduction is defined as the process where a substance gains electrons, leading to a decrease in its oxidation state.
- This process often involves the addition of hydrogen or the removal of oxygen.
Why Option A is Correct
- Addition of Hydrogen: When hydrogen is added to a substance, it effectively reduces the oxidation state of that substance. For example, adding hydrogen to a metal oxide can convert it into a metal, a clear example of reduction.
- Contrast with Oxidation: In contrast, oxidation involves the addition of oxygen or the removal of hydrogen. Therefore, when we talk about reduction, we specifically refer to the addition of hydrogen as a primary way to achieve it.
Examples of Reduction Reactions
- Hydrogenation: This is a common reduction reaction in organic chemistry where unsaturated hydrocarbons (like alkenes) are converted to saturated hydrocarbons by the addition of hydrogen.
- Metal Reduction: In metallurgy, metal oxides are reduced to their elemental forms by using hydrogen gas, showcasing the importance of reduction in extracting metals.
Conclusion
In summary, option A is the correct answer as it directly relates to the definition of reduction in terms of chemical reactions. The addition of hydrogen plays a crucial role in reducing various substances, demonstrating the essential nature of reduction in chemical processes.
Which of the following substance is reduced in the given reaction below?
PbS(s) + 4H2O2 (aq) → PbSO4(s) + 4H2O- a)Lead sulphide
- b)Hydrogen peroxide
- c)Both lead sulphide and Hydrogen peroxide
- d)Water
Correct answer is option 'B'. Can you explain this answer?
Which of the following substance is reduced in the given reaction below?
PbS(s) + 4H2O2 (aq) → PbSO4(s) + 4H2O
PbS(s) + 4H2O2 (aq) → PbSO4(s) + 4H2O
a)
Lead sulphide
b)
Hydrogen peroxide
c)
Both lead sulphide and Hydrogen peroxide
d)
Water
|
|
Rahul agarwal answered |
Understanding the Reaction
In the reaction:
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O,
we need to identify which substance is reduced.
Reduction Process
- Definition of Reduction: Reduction is a chemical reaction that involves the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Analyzing Hydrogen Peroxide
- Hydrogen Peroxide (H2O2): In this reaction, H2O2 acts as an oxidizing agent. It is known to undergo reduction during the reaction.
- Oxidation State Change: The oxidation state of oxygen in H2O2 is -1, and in water (H2O), it is -2. This change indicates that H2O2 is gaining electrons and being reduced.
Analyzing Lead Sulfide
- Lead Sulfide (PbS): In this reaction, PbS is converted to PbSO4.
- Oxidation State Change: Lead (Pb) in PbS has an oxidation state of +2 in PbSO4, but the lead does not gain electrons. Instead, it is oxidized.
Conclusion
- Final Identification: Since H2O2 is reduced (gaining electrons) and PbS is oxidized (losing electrons), the correct answer is indeed b) Hydrogen peroxide.
Thus, understanding the changes in oxidation states helps clarify the reduction process in this reaction.
In the reaction:
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O,
we need to identify which substance is reduced.
Reduction Process
- Definition of Reduction: Reduction is a chemical reaction that involves the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Analyzing Hydrogen Peroxide
- Hydrogen Peroxide (H2O2): In this reaction, H2O2 acts as an oxidizing agent. It is known to undergo reduction during the reaction.
- Oxidation State Change: The oxidation state of oxygen in H2O2 is -1, and in water (H2O), it is -2. This change indicates that H2O2 is gaining electrons and being reduced.
Analyzing Lead Sulfide
- Lead Sulfide (PbS): In this reaction, PbS is converted to PbSO4.
- Oxidation State Change: Lead (Pb) in PbS has an oxidation state of +2 in PbSO4, but the lead does not gain electrons. Instead, it is oxidized.
Conclusion
- Final Identification: Since H2O2 is reduced (gaining electrons) and PbS is oxidized (losing electrons), the correct answer is indeed b) Hydrogen peroxide.
Thus, understanding the changes in oxidation states helps clarify the reduction process in this reaction.
Which among the following is called double displacement reaction (s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2 NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2 O2 → CO2 + 2H2O- a)(i) and (iv)
- b)(ii) and (i)
- c)(ii) only
- d)(iii) and (iv)
Correct answer is option 'C'. Can you explain this answer?
Which among the following is called double displacement reaction (s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2 NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2 O2 → CO2 + 2H2O
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2 NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2 O2 → CO2 + 2H2O
a)
(i) and (iv)
b)
(ii) and (i)
c)
(ii) only
d)
(iii) and (iv)
|
|
Radha Iyer answered |
The reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. In double replacement reactions, the positive ions exchange negative ion partners. A double replacement reaction is represented by the general equation.
AB + CD → AD + CB
In a double displacement reaction, atoms from two different compounds switch places. The reactants are two compounds and the products are two different compounds.
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
AB + CD → AD + CB
In a double displacement reaction, atoms from two different compounds switch places. The reactants are two compounds and the products are two different compounds.
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
Identify the substance that is oxidised and reduced in the following reaction.

- a)CuO-reduced and Zn-oxidised
- b)Zn- reduced and CuO oxidised
- c)Cu- reduced and Zn-oxidised
- d)ZnO- reduced, Zn-oxidised
Correct answer is option 'A'. Can you explain this answer?
Identify the substance that is oxidised and reduced in the following reaction.


a)
CuO-reduced and Zn-oxidised
b)
Zn- reduced and CuO oxidised
c)
Cu- reduced and Zn-oxidised
d)
ZnO- reduced, Zn-oxidised

|
Nk Classes answered |
Zn is getting oxidised, CuO is getting reduced.
Which of the following metal will react with dilute hydrochloric acid to give out hydrogen gas- a)Cu
- b)Pb
- c)Ag
- d)Hg
Correct answer is option 'B'. Can you explain this answer?
Which of the following metal will react with dilute hydrochloric acid to give out hydrogen gas
a)
Cu
b)
Pb
c)
Ag
d)
Hg
|
|
Niyati kapoor answered |
**Answer:**
**Introduction:**
When a metal reacts with an acid, it displaces hydrogen from the acid, resulting in the formation of a salt and the evolution of hydrogen gas. This type of reaction is known as a displacement reaction.
**Explanation:**
Among the given options, the metal that will react with dilute hydrochloric acid to give out hydrogen gas is Pb (lead). The reaction between lead and hydrochloric acid can be represented by the following equation:
Pb + 2HCl → PbCl2 + H2
**Reasoning:**
To determine which metal will react with hydrochloric acid, we need to consider the reactivity series of metals. The reactivity series is a list of metals in order of their reactivity, with the most reactive metal at the top and the least reactive metal at the bottom.
The reactivity series of metals is as follows:
Potassium (K) > Sodium (Na) > Calcium (Ca) > Magnesium (Mg) > Aluminum (Al) > Zinc (Zn) > Iron (Fe) > Lead (Pb) > Hydrogen (H) > Copper (Cu) > Silver (Ag) > Gold (Au)
According to the reactivity series, metals higher in the series are more reactive than metals lower in the series. This means that metals higher in the series can displace metals lower in the series from their compounds.
In the given options, lead (Pb) is below hydrogen (H) in the reactivity series. This means that lead can displace hydrogen from hydrochloric acid to form lead chloride (PbCl2) and release hydrogen gas (H2).
Therefore, the correct answer is option B - Pb.
**Introduction:**
When a metal reacts with an acid, it displaces hydrogen from the acid, resulting in the formation of a salt and the evolution of hydrogen gas. This type of reaction is known as a displacement reaction.
**Explanation:**
Among the given options, the metal that will react with dilute hydrochloric acid to give out hydrogen gas is Pb (lead). The reaction between lead and hydrochloric acid can be represented by the following equation:
Pb + 2HCl → PbCl2 + H2
**Reasoning:**
To determine which metal will react with hydrochloric acid, we need to consider the reactivity series of metals. The reactivity series is a list of metals in order of their reactivity, with the most reactive metal at the top and the least reactive metal at the bottom.
The reactivity series of metals is as follows:
Potassium (K) > Sodium (Na) > Calcium (Ca) > Magnesium (Mg) > Aluminum (Al) > Zinc (Zn) > Iron (Fe) > Lead (Pb) > Hydrogen (H) > Copper (Cu) > Silver (Ag) > Gold (Au)
According to the reactivity series, metals higher in the series are more reactive than metals lower in the series. This means that metals higher in the series can displace metals lower in the series from their compounds.
In the given options, lead (Pb) is below hydrogen (H) in the reactivity series. This means that lead can displace hydrogen from hydrochloric acid to form lead chloride (PbCl2) and release hydrogen gas (H2).
Therefore, the correct answer is option B - Pb.
Which one of the following solution on mixing will not form a precipitate?- a)Lead acetate and potassium iodide
- b)Lead nitrate and sulphuric acid
- c)Iron sulphide and dilute sulphuric acid
- d)Potassium bromide and silver nitrate
Correct answer is option 'C'. Can you explain this answer?
Which one of the following solution on mixing will not form a precipitate?
a)
Lead acetate and potassium iodide
b)
Lead nitrate and sulphuric acid
c)
Iron sulphide and dilute sulphuric acid
d)
Potassium bromide and silver nitrate
|
|
Tanishq Iyer answered |
Explanation:
Iron sulphide and dilute sulphuric acid:
- When iron sulphide reacts with dilute sulphuric acid, it forms hydrogen sulfide gas and iron(II) sulfate.
- Since hydrogen sulfide gas is a gas and not a solid, no precipitate is formed in this reaction.
- Therefore, this combination will not form a precipitate.
Lead acetate and potassium iodide:
- When lead acetate reacts with potassium iodide, it forms lead iodide which is insoluble in water and hence, a yellow precipitate of lead iodide is formed.
Lead nitrate and sulphuric acid:
- When lead nitrate reacts with sulphuric acid, it forms lead sulfate which is insoluble in water and hence, a white precipitate of lead sulfate is formed.
Potassium bromide and silver nitrate:
- When potassium bromide reacts with silver nitrate, it forms silver bromide which is insoluble in water and hence, a cream precipitate of silver bromide is formed.
Therefore, out of the given options, only the combination of iron sulphide and dilute sulphuric acid will not form a precipitate.
Iron sulphide and dilute sulphuric acid:
- When iron sulphide reacts with dilute sulphuric acid, it forms hydrogen sulfide gas and iron(II) sulfate.
- Since hydrogen sulfide gas is a gas and not a solid, no precipitate is formed in this reaction.
- Therefore, this combination will not form a precipitate.
Lead acetate and potassium iodide:
- When lead acetate reacts with potassium iodide, it forms lead iodide which is insoluble in water and hence, a yellow precipitate of lead iodide is formed.
Lead nitrate and sulphuric acid:
- When lead nitrate reacts with sulphuric acid, it forms lead sulfate which is insoluble in water and hence, a white precipitate of lead sulfate is formed.
Potassium bromide and silver nitrate:
- When potassium bromide reacts with silver nitrate, it forms silver bromide which is insoluble in water and hence, a cream precipitate of silver bromide is formed.
Therefore, out of the given options, only the combination of iron sulphide and dilute sulphuric acid will not form a precipitate.
A chemical equation is said to be balanced if number of- a)Compounds are same in both sides
- b)Molecules are same in both sides
- c)Number of atoms is same in both sides
- d)Number of electrons are same in both sides
Correct answer is option 'C'. Can you explain this answer?
A chemical equation is said to be balanced if number of
a)
Compounds are same in both sides
b)
Molecules are same in both sides
c)
Number of atoms is same in both sides
d)
Number of electrons are same in both sides
|
|
Debolina Nair answered |
Balanced chemical equation: A balanced equation is a chemical equation in which number of atoms of each element is equal on both sides of the equation i.e number of atoms of an element on reactant side = number of atoms of that element on the product side.
Chapter doubts & questions for Chemical Reactions and Equations - Science Class 10 2025 is part of Class 10 exam preparation. The chapters have been prepared according to the Class 10 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 10 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Reactions and Equations - Science Class 10 in English & Hindi are available as part of Class 10 exam.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









