NEET Exam > NEET Questions > The molecular weight of benzoic acid in benze...
Start Learning for Free
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds to
- a)ionization of benzoic acid
- b)dimerisation of benzoic acid
- c)trimerisation of benzoic acid
- d)solution of benzoic acid
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The molecular weight of benzoic acid in benzene as determined by depre...
Molecular weight of benzoic acid in benzene is determined by the depression in freezing point method. And comes out to be 244. The actual molecules mass is 122. Therefore, it corresponds to the dimers which are formed by hydrogen bonding.
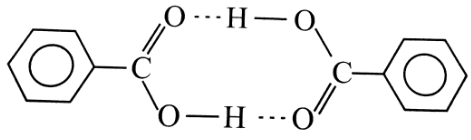
View all questions of this test
Most Upvoted Answer
The molecular weight of benzoic acid in benzene as determined by depre...
Depression in Freezing Point Method and Molecular Weight of Benzoic Acid in Benzene
Depression in Freezing Point Method:
The depression in freezing point method is a technique used to determine the molecular weight of a solute in a solvent. It is based on the principle that when a solute is dissolved in a solvent, it lowers the freezing point of the solvent. This lowering of freezing point is directly proportional to the concentration of the solute in the solvent.
Molecular Weight of Benzoic Acid in Benzene:
In this case, the molecular weight of benzoic acid in benzene is determined using the depression in freezing point method. When benzoic acid is dissolved in benzene, it forms dimers through intermolecular hydrogen bonding. Each dimer consists of two molecules of benzoic acid held together by hydrogen bonds.
Dimerization of Benzoic Acid:
The formation of dimers in the solution of benzoic acid in benzene affects the freezing point depression. The presence of dimers increases the effective concentration of the solute in the solution, leading to a greater depression in freezing point.
Explanation of Correct Answer:
The correct answer to the question is option B, which states that the depression in freezing point method corresponds to the dimerization of benzoic acid. This is because the formation of dimers in the solution of benzoic acid in benzene is responsible for the observed depression in freezing point.
The presence of dimers increases the effective concentration of benzoic acid in the solution, leading to a greater depression in freezing point. This can be explained by the fact that the formation of dimers reduces the number of free solute particles in the solution, resulting in a lower concentration of solute particles available to lower the freezing point.
Therefore, the depression in freezing point method measures the degree of dimerization of benzoic acid in benzene, which in turn provides information about the molecular weight of benzoic acid in benzene.
In conclusion, the depression in freezing point method is used to determine the molecular weight of benzoic acid in benzene, and the observed depression corresponds to the dimerization of benzoic acid in the solution.
Depression in Freezing Point Method:
The depression in freezing point method is a technique used to determine the molecular weight of a solute in a solvent. It is based on the principle that when a solute is dissolved in a solvent, it lowers the freezing point of the solvent. This lowering of freezing point is directly proportional to the concentration of the solute in the solvent.
Molecular Weight of Benzoic Acid in Benzene:
In this case, the molecular weight of benzoic acid in benzene is determined using the depression in freezing point method. When benzoic acid is dissolved in benzene, it forms dimers through intermolecular hydrogen bonding. Each dimer consists of two molecules of benzoic acid held together by hydrogen bonds.
Dimerization of Benzoic Acid:
The formation of dimers in the solution of benzoic acid in benzene affects the freezing point depression. The presence of dimers increases the effective concentration of the solute in the solution, leading to a greater depression in freezing point.
Explanation of Correct Answer:
The correct answer to the question is option B, which states that the depression in freezing point method corresponds to the dimerization of benzoic acid. This is because the formation of dimers in the solution of benzoic acid in benzene is responsible for the observed depression in freezing point.
The presence of dimers increases the effective concentration of benzoic acid in the solution, leading to a greater depression in freezing point. This can be explained by the fact that the formation of dimers reduces the number of free solute particles in the solution, resulting in a lower concentration of solute particles available to lower the freezing point.
Therefore, the depression in freezing point method measures the degree of dimerization of benzoic acid in benzene, which in turn provides information about the molecular weight of benzoic acid in benzene.
In conclusion, the depression in freezing point method is used to determine the molecular weight of benzoic acid in benzene, and the observed depression corresponds to the dimerization of benzoic acid in the solution.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer?
Question Description
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer?.
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds toa)ionization of benzoic acidb)dimerisation of benzoic acidc)trimerisation of benzoic acidd)solution of benzoic acidCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























