GATE Exam > GATE Questions > The following gas-phase reaction is carried o...
Start Learning for Free
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactor A + B → R + S
The reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.
Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants are CA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.
Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants are CA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.
The time at which R just starts condensing, rounded to 1 decimal place, is_____
Correct answer is '4'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The following gas-phase reaction is carried out in a constant-volume i...
A + B → R + S
Let m moles of A and B present initially in the reactor
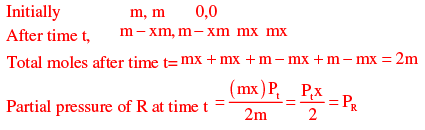
Let m moles of A and B present initially in the reactor
At Pr = 40 kPa. R will start condensing
Here, Pt = 100kPa, PR = 40 = 100x\2
x = 0.8
CA = (1 - X) = 1.25(1 - 0.8)
(1 - X) = 1.25(1 - 0.8)
CA = 2.5mol / m3
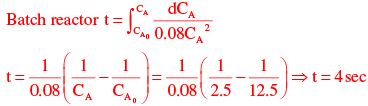
Here, Pt = 100kPa, PR = 40 = 100x\2
x = 0.8
CA =
CA = 2.5mol / m3
Most Upvoted Answer
The following gas-phase reaction is carried out in a constant-volume i...
The reaction can be represented by the equation:
A → B
In a constant-volume isothermal batch reactor, the volume remains constant throughout the reaction, and the temperature is kept constant. This means that the pressure inside the reactor may change as the reaction progresses.
The reaction involves the conversion of A into B. As the reaction proceeds, the concentration of A decreases, while the concentration of B increases. The rate of the reaction depends on the concentration of A and the reaction rate constant.
In a constant-volume isothermal batch reactor, the reaction will continue until the reactant A is completely converted into product B, or until the reaction reaches equilibrium if it is a reversible reaction. The reaction time required to reach completion or equilibrium will depend on the reaction rate constant and the initial concentrations of A and B.
The pressure inside the reactor may change during the reaction due to the change in the number of moles of gas. If the reaction involves a change in the number of moles of gas, the pressure may increase or decrease depending on the stoichiometry of the reaction. However, the overall volume of the reactor remains constant.
Overall, in a constant-volume isothermal batch reactor, the reaction proceeds until completion or equilibrium is reached, while the volume remains constant and the temperature is kept constant. The pressure may change due to the change in the number of moles of gas involved in the reaction.
A → B
In a constant-volume isothermal batch reactor, the volume remains constant throughout the reaction, and the temperature is kept constant. This means that the pressure inside the reactor may change as the reaction progresses.
The reaction involves the conversion of A into B. As the reaction proceeds, the concentration of A decreases, while the concentration of B increases. The rate of the reaction depends on the concentration of A and the reaction rate constant.
In a constant-volume isothermal batch reactor, the reaction will continue until the reactant A is completely converted into product B, or until the reaction reaches equilibrium if it is a reversible reaction. The reaction time required to reach completion or equilibrium will depend on the reaction rate constant and the initial concentrations of A and B.
The pressure inside the reactor may change during the reaction due to the change in the number of moles of gas. If the reaction involves a change in the number of moles of gas, the pressure may increase or decrease depending on the stoichiometry of the reaction. However, the overall volume of the reactor remains constant.
Overall, in a constant-volume isothermal batch reactor, the reaction proceeds until completion or equilibrium is reached, while the volume remains constant and the temperature is kept constant. The pressure may change due to the change in the number of moles of gas involved in the reaction.

|
Explore Courses for GATE exam
|

|
Similar GATE Doubts
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer?
Question Description
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer?.
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer?.
Solutions for The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer?, a detailed solution for The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? has been provided alongside types of The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The following gas-phase reaction is carried out in a constant-volume isothermal batch reactorA + B → R + SThe reactants A and B as well as the product S are non-condensable gases. At the operating temperature, the saturation pressure of the product R is 40 kPa.Initially, the batch reactor contains equimolar amounts of A and B (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants areCA,0 = CB,0 = 12.56 mol / m3. The rate of reaction is given by (−rA) = 0.08CA CB mol / m3.s.The time at which R just starts condensing, rounded to 1 decimal place, is_____Correct answer is '4'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















