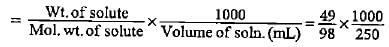All Exams >
NEET >
NCERTs at Fingertips: Textbooks, Tests & Solutions >
All Questions
All questions of Some Basic Concepts of Chemistry for NEET Exam
Which of the following options is not correct?
- a)2.300 + 0.02017 + 0.02015 = 2.340
- b)126,000 has 3 significant figures
- c)15.15 μs = 1.515 x 10-5 s
- d)0.0048 = 48 x 10-3
Correct answer is option 'D'. Can you explain this answer?
Which of the following options is not correct?
a)
2.300 + 0.02017 + 0.02015 = 2.340
b)
126,000 has 3 significant figures
c)
15.15 μs = 1.515 x 10-5 s
d)
0.0048 = 48 x 10-3
|
|
Dev Patel answered |
Rule: The result of an addition or subtraction is reported to the same number of decimal places as present in number with the least decimal places.
Since the least precise number is 2.300 and that has three digits after the decimal, therefore the result of the above addition is rounded off to three decimal figures as 2.340.
126,000 has 3 significant figures as zeros are not counted
15.15μs = 15.15×10−6 s = 1.515×10−5 s
0.0048 = 4.8×10−3
Hence, the correct option is D.
Match the prefixes present in column I with their multiples in column II and mark the appropriate choice.
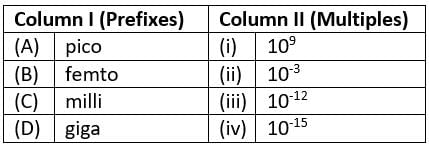
- a)(A)→(i), (B)→(ii), (C)→(iii), (D)→(iv)
- b)(A)→(ii), (B)→(i), (C)→(iv), (D)→(iii)
- c)(A)→(iv), (B)→(iii), (C)→(i), (D)→(ii)
- d)(A)→(iii), (B)→(iv), (C)→(ii), (D)→(i)
Correct answer is option 'D'. Can you explain this answer?
Match the prefixes present in column I with their multiples in column II and mark the appropriate choice.


a)
(A)→(i), (B)→(ii), (C)→(iii), (D)→(iv)
b)
(A)→(ii), (B)→(i), (C)→(iv), (D)→(iii)
c)
(A)→(iv), (B)→(iii), (C)→(i), (D)→(ii)
d)
(A)→(iii), (B)→(iv), (C)→(ii), (D)→(i)

|
Whatsapp Status answered |
D
What volume of 5 M Na2SO4 must be added to 25 mL of 1 M BaCl2 to produce 10 g of BaSO4?- a)8.58 mL
- b)7.2 mL
- c)10 mL
- d)12 mL
Correct answer is option 'A'. Can you explain this answer?
What volume of 5 M Na2SO4 must be added to 25 mL of 1 M BaCl2 to produce 10 g of BaSO4?
a)
8.58 mL
b)
7.2 mL
c)
10 mL
d)
12 mL
|
|
Anjali Sharma answered |
Na2SO4 + BaCI2 → BaSO4 + 2NaCl
No. of moles of BaSO4 = w/M = 10/233 = 0.0429
∴ No. of moles of Na2SO4 needed = M x V/1000
Or 0.0429 = 5 x V/1000
V = 8.58 mL
No. of moles of BaSO4 = w/M = 10/233 = 0.0429
∴ No. of moles of Na2SO4 needed = M x V/1000
Or 0.0429 = 5 x V/1000
V = 8.58 mL
What volume of water is to be added to 100 cm3 of 0.5 M NaOH solution to make it 0.1 M solution?- a)200 cm3
- b)400 cm3
- c)500 cm3
- d)100 cm3
Correct answer is option 'B'. Can you explain this answer?
What volume of water is to be added to 100 cm3 of 0.5 M NaOH solution to make it 0.1 M solution?
a)
200 cm3
b)
400 cm3
c)
500 cm3
d)
100 cm3

|
Siddhi Sonakshi answered |
N1=n2
M1V1=M2(V1+V H20)
.5×100=.1(100+VH20)
500=(100+VH20)
400=VH20
M1V1=M2(V1+V H20)
.5×100=.1(100+VH20)
500=(100+VH20)
400=VH20
How many seconds are there in 3 days?- a)259200 s
- b)172800 s
- c)24800 s
- d)72000 s
Correct answer is option 'A'. Can you explain this answer?
How many seconds are there in 3 days?
a)
259200 s
b)
172800 s
c)
24800 s
d)
72000 s
|
|
Trupti Thawkar answered |
Answer is A
1 hour = 3600 sec
24 hour = 86400
Therefore, 3 days have
3*86400 = 259200 sec
1 hour = 3600 sec
24 hour = 86400
Therefore, 3 days have
3*86400 = 259200 sec
How many grams of CaO are required to react with 852 g of P4O10?- a)852 g
- b)1008 g
- c)85 g
- d)7095 g
Correct answer is option 'B'. Can you explain this answer?
How many grams of CaO are required to react with 852 g of P4O10?
a)
852 g
b)
1008 g
c)
85 g
d)
7095 g
|
|
Dev Patel answered |
6CaO + P4O10 → 2Ca3(PO4)2
1 mole of P4O10 = molar mass, of P4O10 = 284g
852 g of P4O10 = 852/284 = 3mol
1 mole of P4O10 reacts with.6 moles of CaO
3 moles of P4O10 reacts with 18 moles of CaO Mass of 18 moles of CaO = 18 x 56 = 1008 g
1 mole of P4O10 = molar mass, of P4O10 = 284g
852 g of P4O10 = 852/284 = 3mol
1 mole of P4O10 reacts with.6 moles of CaO
3 moles of P4O10 reacts with 18 moles of CaO Mass of 18 moles of CaO = 18 x 56 = 1008 g
What mass of hydrochloric acid is needed to decompose 50 g of limestone?- a)36.5 g
- b)73 g
- c)50 g
- d)100 g
Correct answer is option 'A'. Can you explain this answer?
What mass of hydrochloric acid is needed to decompose 50 g of limestone?
a)
36.5 g
b)
73 g
c)
50 g
d)
100 g
|
|
Dev Patel answered |
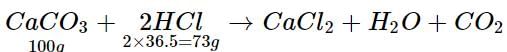
100 g of CaCO3 requires 73 g of HCl
∴ 50 g of CaCO3 requires 73/100 x 50 = 36.5 g of HCl
HCl is produced in the stomach which can be neutralised by Mg(OH)2 in the form of milk of magnesia. How much Mg(OH)2 is required to neutralise one mole of stomach acid?- a)29.16 g
- b)34.3 g
- c)58.33 g
- d)68.66 g
Correct answer is option 'A'. Can you explain this answer?
HCl is produced in the stomach which can be neutralised by Mg(OH)2 in the form of milk of magnesia. How much Mg(OH)2 is required to neutralise one mole of stomach acid?
a)
29.16 g
b)
34.3 g
c)
58.33 g
d)
68.66 g
|
|
Mira Joshi answered |
Mg(OH)2 + 2HCl → MgCl2 + 2H2O
No. of moles of Mg(OH)2 required for 2 moles of HCl = 1
No. of moles of Mg(OH)2 required for 1 mole of HCl = 0.5
Mass of 0.5 mol of Mg(OH)2 = 58.33 x 0.5 = 29.16 g
No. of moles of Mg(OH)2 required for 2 moles of HCl = 1
No. of moles of Mg(OH)2 required for 1 mole of HCl = 0.5
Mass of 0.5 mol of Mg(OH)2 = 58.33 x 0.5 = 29.16 g
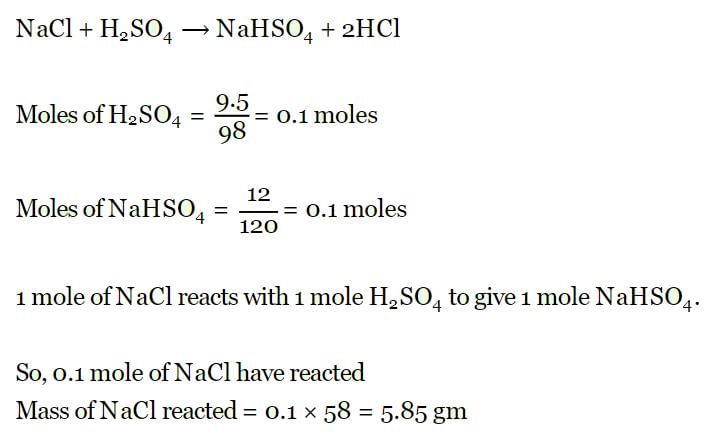
How much mass of silver nitrates will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride and 8.5 g of sodium nitrates if law of conservation of mass is followed?- a)22.85 g
- b)108 g
- c)17.0 g
- d)28.70 g
Correct answer is option 'C'. Can you explain this answer?
How much mass of silver nitrates will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride and 8.5 g of sodium nitrates if law of conservation of mass is followed?
a)
22.85 g
b)
108 g
c)
17.0 g
d)
28.70 g
|
|
Geetika Shah answered |
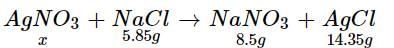
x + 5.85 = 8.5 + 14.35 ⇒ x = 17 g
The density of a gas is 1.78 g L-1 at STP. The weight of one mole of gas is- a)39.9 g
- b)22.4 g
- c)3.56 g
- d)29 g
Correct answer is option 'A'. Can you explain this answer?
The density of a gas is 1.78 g L-1 at STP. The weight of one mole of gas is
a)
39.9 g
b)
22.4 g
c)
3.56 g
d)
29 g
|
|
Mira Joshi answered |
1 mole occupies a volume of 22.4 L at STP
Mass of 1 mole of a gas = Density x Volume
= 1.78 x 22.4 = 39.9 g
Mass of 1 mole of a gas = Density x Volume
= 1.78 x 22.4 = 39.9 g
Carbon occurs in nature as a mixture of 12C and 13C. The average atomic mass of carbon is 12.011. What is the % abundance of 12C in nature?- a)88.9%
- b)98.9%
- c)89.9%
- d)79.9%
Correct answer is option 'B'. Can you explain this answer?
Carbon occurs in nature as a mixture of 12C and 13C. The average atomic mass of carbon is 12.011. What is the % abundance of 12C in nature?
a)
88.9%
b)
98.9%
c)
89.9%
d)
79.9%
|
|
Dev Patel answered |
Let % abundance of 12C = x
% abundance of 13C = 100 - x
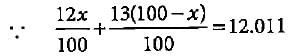
2x + 1300 - 13x = 1201.1 ⇒ x = 98.9%
Thus, abundance of 12C is 98.9%.
% abundance of 13C = 100 - x
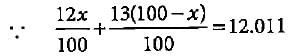
2x + 1300 - 13x = 1201.1 ⇒ x = 98.9%
Thus, abundance of 12C is 98.9%.
Choose the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively and its molecular mass is 160.- a)FeO
- b)Fe3O4
- c)Fe2O3
- d)FeO2
Correct answer is option 'C'. Can you explain this answer?
Choose the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively and its molecular mass is 160.
a)
FeO
b)
Fe3O4
c)
Fe2O3
d)
FeO2
|
|
Riya Banerjee answered |
For element Fe, mole of atoms = 69.9/56 = 1.25
For element O, Mole of atoms = 30.1/16 = 1.88
Mole ratio of Fe = 1.25/1.25 = 1
Mole ratio of O = 1.88/1.25 = 1.5
Simplest whole number ratio of Fe and O = 2, 3
Empirical formula of compoimd = Fe2O3
Molecular mass of Fe2O3 = 160
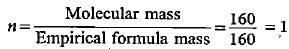
Molecular formula = Fe2O3
For element O, Mole of atoms = 30.1/16 = 1.88
Mole ratio of Fe = 1.25/1.25 = 1
Mole ratio of O = 1.88/1.25 = 1.5
Simplest whole number ratio of Fe and O = 2, 3
Empirical formula of compoimd = Fe2O3
Molecular mass of Fe2O3 = 160
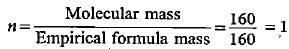
Molecular formula = Fe2O3
How many atoms in total are present in 1 kg of sugar?- a)7.92 x 1025 atoms
- b)6 x 1023 atoms
- c)6.022 x 1025 atoms
- d)1000 atoms
Correct answer is option 'A'. Can you explain this answer?
How many atoms in total are present in 1 kg of sugar?
a)
7.92 x 1025 atoms
b)
6 x 1023 atoms
c)
6.022 x 1025 atoms
d)
1000 atoms
|
|
Mira Joshi answered |
One molecule of sugar (C12H22O11) = 45 atoms
Number of moles of sugar = 1000/342 = 2.92
Number of molecules = 2.92 x 6.023 x 1023
= 17.60 x 1023
molecules Number of atoms = 45 x 17.60 x 1023
= 7.92 x 1025 atoms
Number of moles of sugar = 1000/342 = 2.92
Number of molecules = 2.92 x 6.023 x 1023
= 17.60 x 1023
molecules Number of atoms = 45 x 17.60 x 1023
= 7.92 x 1025 atoms
A mixture having 2 g of hydrogen and 32 g of oxygen occupies how much volume at NTP?- a)44.8 L
- b)22.4 L
- c)11.2 L
- d)67.2 L
Correct answer is option 'A'. Can you explain this answer?
A mixture having 2 g of hydrogen and 32 g of oxygen occupies how much volume at NTP?
a)
44.8 L
b)
22.4 L
c)
11.2 L
d)
67.2 L
|
|
Anjali Sharma answered |
2 g of H2 = 1 mole, 32 g of O2 = 1 mole
Total volume of 2 moles of gases at NTP = 2 x 22.4 L
= 44.8L
Total volume of 2 moles of gases at NTP = 2 x 22.4 L
= 44.8L
Mark the rule which is not correctly stated about the determination of significant figures.- a)Zeros preceding to first non-zero digit are not significant
- b)Zeros between two non-zero digits are not significant
- c)Zeros at the end or right of the number are significant if they are on the right side of the decimal point
- d)All non-zero digits are significant
Correct answer is option 'B'. Can you explain this answer?
Mark the rule which is not correctly stated about the determination of significant figures.
a)
Zeros preceding to first non-zero digit are not significant
b)
Zeros between two non-zero digits are not significant
c)
Zeros at the end or right of the number are significant if they are on the right side of the decimal point
d)
All non-zero digits are significant
|
|
Jyoti Sengupta answered |
Zero between two non - zero digits are significant.
Given below are few statements. Mark the statement which is not correct.- a)Gram atomic mass of an element may be defined as the mass of Avogadro's number of atoms
- b)The molecular mass of a diatomic elementary gas is twice its atomic mass
- c)Gay Lussac's law of chemical combination is valid for all substances
- d)A pure compound has always a fixed proportion of masses of its constituents
Correct answer is option 'C'. Can you explain this answer?
Given below are few statements. Mark the statement which is not correct.
a)
Gram atomic mass of an element may be defined as the mass of Avogadro's number of atoms
b)
The molecular mass of a diatomic elementary gas is twice its atomic mass
c)
Gay Lussac's law of chemical combination is valid for all substances
d)
A pure compound has always a fixed proportion of masses of its constituents
|
|
Harshitha Chakraborty answered |
Explanation:
The correct answer is option 'C' - Gay Lussac's law of chemical combination is not valid for all substances.
Explanation of the other options:
a) Gram atomic mass of an element may be defined as the mass of Avogadro's number of atoms:
This statement is correct. The gram atomic mass of an element is defined as the mass of one mole of atoms of that element. One mole of any substance contains Avogadro's number of particles, which is approximately 6.022 × 10^23. Therefore, the gram atomic mass is the molar mass of the element expressed in grams.
b) The molecular mass of a diatomic elementary gas is twice its atomic mass:
This statement is also correct. Diatomic elementary gases exist as molecules with two atoms bonded together. Since each atom has the same atomic mass, the molecular mass of the diatomic gas is twice the atomic mass.
d) A pure compound always has a fixed proportion of masses of its constituents:
This statement is correct. A pure compound is a substance made up of two or more elements chemically combined in a fixed proportion by mass. The ratio of the masses of the constituent elements in a pure compound is always constant.
Explanation of option 'C':
Gay Lussac's law of chemical combination states that when gases react, the volumes of the reacting gases and the volumes of the gaseous products, if they are also gases, are in a simple whole-number ratio, provided all gases are at the same temperature and pressure. However, this law is not valid for all substances.
The law is only applicable to gases because it is based on the assumption that gases behave ideally, meaning that their particles do not interact with each other and occupy negligible volumes. In reality, not all substances exist as gases, and even when they do, they may not behave ideally.
For example, solids and liquids do not follow Gay Lussac's law because their particles are closely packed and have strong intermolecular forces that prevent them from behaving like ideal gases. Additionally, some gases may deviate from ideal behavior under certain conditions, such as high pressures or low temperatures.
Therefore, Gay Lussac's law of chemical combination is only applicable to gases and cannot be generalized to all substances.
The correct answer is option 'C' - Gay Lussac's law of chemical combination is not valid for all substances.
Explanation of the other options:
a) Gram atomic mass of an element may be defined as the mass of Avogadro's number of atoms:
This statement is correct. The gram atomic mass of an element is defined as the mass of one mole of atoms of that element. One mole of any substance contains Avogadro's number of particles, which is approximately 6.022 × 10^23. Therefore, the gram atomic mass is the molar mass of the element expressed in grams.
b) The molecular mass of a diatomic elementary gas is twice its atomic mass:
This statement is also correct. Diatomic elementary gases exist as molecules with two atoms bonded together. Since each atom has the same atomic mass, the molecular mass of the diatomic gas is twice the atomic mass.
d) A pure compound always has a fixed proportion of masses of its constituents:
This statement is correct. A pure compound is a substance made up of two or more elements chemically combined in a fixed proportion by mass. The ratio of the masses of the constituent elements in a pure compound is always constant.
Explanation of option 'C':
Gay Lussac's law of chemical combination states that when gases react, the volumes of the reacting gases and the volumes of the gaseous products, if they are also gases, are in a simple whole-number ratio, provided all gases are at the same temperature and pressure. However, this law is not valid for all substances.
The law is only applicable to gases because it is based on the assumption that gases behave ideally, meaning that their particles do not interact with each other and occupy negligible volumes. In reality, not all substances exist as gases, and even when they do, they may not behave ideally.
For example, solids and liquids do not follow Gay Lussac's law because their particles are closely packed and have strong intermolecular forces that prevent them from behaving like ideal gases. Additionally, some gases may deviate from ideal behavior under certain conditions, such as high pressures or low temperatures.
Therefore, Gay Lussac's law of chemical combination is only applicable to gases and cannot be generalized to all substances.
A compound contains two elements 'x' and 'Y' in the ratio of 50% each. Atomic mass of 'x' is 20 and 'Y' is 40. What can be its simplest formula?- a)XY
- b)X2Y
- c)XY2
- d)X2Y3
Correct answer is option 'B'. Can you explain this answer?
A compound contains two elements 'x' and 'Y' in the ratio of 50% each. Atomic mass of 'x' is 20 and 'Y' is 40. What can be its simplest formula?
a)
XY
b)
X2Y
c)
XY2
d)
X2Y3
|
|
Gayatri Singh answered |
Explanation:
To determine the simplest formula of a compound, we need to find the ratio of the elements present in the compound. In this case, the compound contains two elements, X and Y, in a ratio of 50% each.
Step 1: Determine the atomic masses of X and Y
The atomic mass of X is given as 20, and the atomic mass of Y is given as 40.
Step 2: Convert the atomic masses to moles
To find the ratio of the elements, we need to convert the atomic masses to moles by dividing them by their respective atomic masses.
Moles of X = 20/20 = 1
Moles of Y = 40/40 = 1
Step 3: Determine the simplest ratio of X and Y
Since the ratio of X and Y is 1:1, the simplest ratio can be expressed as X1Y1, which can be simplified to XY.
Step 4: Write the simplest formula
The simplest formula of the compound is XY.
Therefore, the correct answer is option B, XY.
Note: It is important to note that the ratio of elements in a compound is determined by the number of moles, not the percentage. The 50% each mentioned in the question is used to determine the ratio of the elements, but the ratio is expressed in moles, not percentages.
To determine the simplest formula of a compound, we need to find the ratio of the elements present in the compound. In this case, the compound contains two elements, X and Y, in a ratio of 50% each.
Step 1: Determine the atomic masses of X and Y
The atomic mass of X is given as 20, and the atomic mass of Y is given as 40.
Step 2: Convert the atomic masses to moles
To find the ratio of the elements, we need to convert the atomic masses to moles by dividing them by their respective atomic masses.
Moles of X = 20/20 = 1
Moles of Y = 40/40 = 1
Step 3: Determine the simplest ratio of X and Y
Since the ratio of X and Y is 1:1, the simplest ratio can be expressed as X1Y1, which can be simplified to XY.
Step 4: Write the simplest formula
The simplest formula of the compound is XY.
Therefore, the correct answer is option B, XY.
Note: It is important to note that the ratio of elements in a compound is determined by the number of moles, not the percentage. The 50% each mentioned in the question is used to determine the ratio of the elements, but the ratio is expressed in moles, not percentages.
How many number of molecules and atoms respectively are present in 2.8 litres of a diatomic gas at STP?- a)6.023 x 1023, 7.5 x 1023
- b)6.023 x 1023, 15 x 1022
- c)7.5 x 1022, 15 x 1022
- d)15 x 1022, 7.5 x 1023
Correct answer is option 'C'. Can you explain this answer?
How many number of molecules and atoms respectively are present in 2.8 litres of a diatomic gas at STP?
a)
6.023 x 1023, 7.5 x 1023
b)
6.023 x 1023, 15 x 1022
c)
7.5 x 1022, 15 x 1022
d)
15 x 1022, 7.5 x 1023
|
|
Priya Menon answered |
Number of molecules of gas at STP
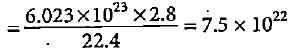 molecules
molecules
Number of atoms in diatomic molecule
= 2 x 7.5 x 1022 = 15 x 1022 atoms
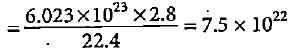 molecules
moleculesNumber of atoms in diatomic molecule
= 2 x 7.5 x 1022 = 15 x 1022 atoms
What should be the volume of the milk (in m3) which measures 5 L?- a)5 x 10-3 m3
- b)5 x 103 m3
- c)5 x 1000 m3
- d)5 x 106 m3
Correct answer is option 'A'. Can you explain this answer?
What should be the volume of the milk (in m3) which measures 5 L?
a)
5 x 10-3 m3
b)
5 x 103 m3
c)
5 x 1000 m3
d)
5 x 106 m3
|
|
Nishanth Chawla answered |
Conversion of units
The given question requires us to convert the volume of milk from liters (L) to cubic meters (m^3). In order to do this conversion, we need to know the relationship between these two units.
Conversion factor
1 liter (L) is equal to 0.001 cubic meters (m^3). This means that to convert liters to cubic meters, we need to multiply the volume in liters by the conversion factor of 0.001.
Calculating the volume in cubic meters
Given that the volume of milk is 5 L, we can calculate the volume in cubic meters using the conversion factor:
Volume in m^3 = 5 L * 0.001 m^3/L
Simplifying the calculation
To simplify the calculation, we can rewrite the conversion factor as a power of 10:
0.001 m^3/L = 10^-3 m^3/L
Now we can substitute this value back into the formula:
Volume in m^3 = 5 L * 10^-3 m^3/L
Final answer
By multiplying the volume in liters (5 L) by the conversion factor (10^-3 m^3/L), we find that the volume of milk is 5 x 10^-3 m^3. Therefore, the correct answer is option A: 5 x 10^-3 m^3.
The result of the operation 2.5 x 1.25 should be which of the following on the basis of significant figures?- a)3.125
- b)3.13
- c)3.1
- d)31.25
Correct answer is option 'C'. Can you explain this answer?
The result of the operation 2.5 x 1.25 should be which of the following on the basis of significant figures?
a)
3.125
b)
3.13
c)
3.1
d)
31.25
|
|
Jyoti Sengupta answered |
2.5 x 1.25 = 3.125
Since 2.5 has two significant figures, the result should not have more than two significant figures. Hence, the answer will be 3.1.
Since 2.5 has two significant figures, the result should not have more than two significant figures. Hence, the answer will be 3.1.
What will be the answer in appropriate significant figures as a result of addition of 3.0223 and 5.041?- a)80.633
- b)8.0633
- c)8.063
- d)806.33
Correct answer is option 'C'. Can you explain this answer?
What will be the answer in appropriate significant figures as a result of addition of 3.0223 and 5.041?
a)
80.633
b)
8.0633
c)
8.063
d)
806.33
|
|
Shraddha Bajaj answered |
When performing addition or subtraction with numbers, the result should be rounded to the same number of decimal places as the measurement with the fewest decimal places.
Given that the numbers being added are 3.0223 and 5.041, we can determine the answer with appropriate significant figures as follows:
1. Identify the number with the fewest decimal places: In this case, it is 5.041, which has three decimal places.
2. Round the other number to the same number of decimal places: The number 3.0223 has four decimal places, so it should be rounded to three decimal places.
3. Add the rounded numbers: 5.041 + 3.022 = 8.063
4. Round the result to the same number of decimal places as the number with the fewest decimal places: Since 5.041 has three decimal places, the final answer should be rounded to three decimal places.
5. The correct answer, therefore, is 8.063, which corresponds to option 'C'.
In this case, the significant figures rule is used to determine the appropriate number of decimal places to round to. The number 5.041 has three significant figures (because the zero between the decimal point and the first non-zero digit is not significant), and the number 3.0223 has four significant figures. Therefore, the final answer should also have three significant figures.
By following the rules for significant figures, we can ensure that the result of the addition is reported with the appropriate level of precision and accuracy.
Given that the numbers being added are 3.0223 and 5.041, we can determine the answer with appropriate significant figures as follows:
1. Identify the number with the fewest decimal places: In this case, it is 5.041, which has three decimal places.
2. Round the other number to the same number of decimal places: The number 3.0223 has four decimal places, so it should be rounded to three decimal places.
3. Add the rounded numbers: 5.041 + 3.022 = 8.063
4. Round the result to the same number of decimal places as the number with the fewest decimal places: Since 5.041 has three decimal places, the final answer should be rounded to three decimal places.
5. The correct answer, therefore, is 8.063, which corresponds to option 'C'.
In this case, the significant figures rule is used to determine the appropriate number of decimal places to round to. The number 5.041 has three significant figures (because the zero between the decimal point and the first non-zero digit is not significant), and the number 3.0223 has four significant figures. Therefore, the final answer should also have three significant figures.
By following the rules for significant figures, we can ensure that the result of the addition is reported with the appropriate level of precision and accuracy.
How many number of aluminium ions are present in 0.051 g of aluminium oxide?- a)6.023 x 1020 ions
- b)3 ions
- c)6.023 x 1023 ions
- d)9 ions
Correct answer is option 'A'. Can you explain this answer?
How many number of aluminium ions are present in 0.051 g of aluminium oxide?
a)
6.023 x 1020 ions
b)
3 ions
c)
6.023 x 1023 ions
d)
9 ions
|
|
Geetika Shah answered |
Mass of AI2O3 = 2 x 27 + 3 x 16 = 102
0.051 g of AI2O3 = 0.051/102 = 0.0005 mol
1 mol of AI2O3 contains 2 x 6.023 x 1023 Al3+ ions
0.0005 mol of AI2O3 contains 2 x 0.0005 x 6.023 x 1023
Al3+ ions = 6.023 x 1020 Al3+ ions
0.051 g of AI2O3 = 0.051/102 = 0.0005 mol
1 mol of AI2O3 contains 2 x 6.023 x 1023 Al3+ ions
0.0005 mol of AI2O3 contains 2 x 0.0005 x 6.023 x 1023
Al3+ ions = 6.023 x 1020 Al3+ ions
Which of the following rules regarding the significant figures and calculations involving them is not correct?
- a)The result of an addition or subtraction is reported to the same number of decimal places as present in number with least decimal places
- b)Result of multiplication or division should have same number of significant figures as present in most precise figure
- c)The result of multiplication or division should be rounded off to same number of significant figures as present in least precise figure
- d)The non-significant figures in the measurements are rounded off
Correct answer is option 'B'. Can you explain this answer?
Which of the following rules regarding the significant figures and calculations involving them is not correct?
a)
The result of an addition or subtraction is reported to the same number of decimal places as present in number with least decimal places
b)
Result of multiplication or division should have same number of significant figures as present in most precise figure
c)
The result of multiplication or division should be rounded off to same number of significant figures as present in least precise figure
d)
The non-significant figures in the measurements are rounded off
|
|
Dev Patel answered |
1. The result of an addition or subtraction is reported to the same number of decimal places as present in number with the least decimal places.
2. The result of multiplication or division should be rounded off to the same number of significant figures as present in number with the least significant number.
3. The result of multiplication or division should be rounded off to the same number of significant figures as present in the least precise figure.
4. The non-significant figures in the measurements are rounded off.
option B is correct
What will be the weight of CO having the same number of oxygen atoms as present in 22 g of CO2?- a)28 g
- b)22 g
- c)44 g
- d)72 g
Correct answer is option 'A'. Can you explain this answer?
What will be the weight of CO having the same number of oxygen atoms as present in 22 g of CO2?
a)
28 g
b)
22 g
c)
44 g
d)
72 g
|
|
Geetika Shah answered |
No. of O atoms in CO2 = 2
Molar mass of CO2 = 44 g
44 g = 1 mol ⇒ 22 g = 0.5 mol
1 mole of CO2 contains = 2 x 6.023 x 1023 O atoms
0.5 mole of CO2 = 6.023 x 1023 O atoms
1 mole of CO = 6.023 x 1023 O atoms
Mass of 1 mole of CO = 12 + 16 = 28 g
Molar mass of CO2 = 44 g
44 g = 1 mol ⇒ 22 g = 0.5 mol
1 mole of CO2 contains = 2 x 6.023 x 1023 O atoms
0.5 mole of CO2 = 6.023 x 1023 O atoms
1 mole of CO = 6.023 x 1023 O atoms
Mass of 1 mole of CO = 12 + 16 = 28 g
1 g of Mg is burnt in a closed vessel containing 0.5 g of O2. Which reactant is limiting reagent and how much of the excess reactant will be left?- a)O2 is a limiting reagent and Mg is in excess by 0.25 g
- b)Mg is a limiting reagent and is in excess by 0.5 g
- c)O2 is a limiting reagent and is in excess by 0.25 g
- d)O2 is a limiting reagent and Mg is in excess by 0.75 g
Correct answer is option 'A'. Can you explain this answer?
1 g of Mg is burnt in a closed vessel containing 0.5 g of O2. Which reactant is limiting reagent and how much of the excess reactant will be left?
a)
O2 is a limiting reagent and Mg is in excess by 0.25 g
b)
Mg is a limiting reagent and is in excess by 0.5 g
c)
O2 is a limiting reagent and is in excess by 0.25 g
d)
O2 is a limiting reagent and Mg is in excess by 0.75 g
|
|
Mira Joshi answered |
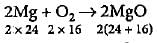
48 g of Mg requires 32 g of O2
1 g of Mg requires 32/48 = 0.66 g of O2
Oxygen available = 0.5 g
Hence, O2 is limiting reagent.
32 g of O2 reacts with 48 g of Mg
0.5 g of O2 will react with 48/32 x 0.5 = 0.75 g of Mg
Excess of Mg = (1.0 - 0.75) = 0.25 g
Total number of atoms present in 34g of NH3 is- a)4 x 1023
- b)48 x 1021
- c)2 x 1023
- d)48 x 1023
Correct answer is option 'D'. Can you explain this answer?
Total number of atoms present in 34g of NH3 is
a)
4 x 1023
b)
48 x 1021
c)
2 x 1023
d)
48 x 1023
|
|
Raghav Bansal answered |
No. of moles of 34 g of NH3 = 34/17 = 2
No. of molecules = 2 x 6.023 x 1023
No. of atoms in one molecule of NH3 = 4
No. of atoms in 2 molecules of NH3
= 4 x 2 x 6.023 x 1023 = 48.18 x 1023
No. of molecules = 2 x 6.023 x 1023
No. of atoms in one molecule of NH3 = 4
No. of atoms in 2 molecules of NH3
= 4 x 2 x 6.023 x 1023 = 48.18 x 1023
What quantity of copper oxide will react with 2.80 L of hydrogen at NTP?- a)79.5 g
- b)2 g
- c)9.9 g
- d)22.4 g
Correct answer is option 'C'. Can you explain this answer?
What quantity of copper oxide will react with 2.80 L of hydrogen at NTP?
a)
79.5 g
b)
2 g
c)
9.9 g
d)
22.4 g
|
|
Geetika Shah answered |
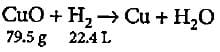
22.4 L of H2 = 79.5g of CuO
2.80 L of H2 = 79.5/22.4 x 2.80 = 9.9 g of CuO
The number of oxygen atoms present in 1 mole of oxalic acid dihydrate is- a)6 x 1023
- b)6.022 x 1034
- c)7.22 x 1023
- d)36.13 x 1023
Correct answer is option 'D'. Can you explain this answer?
The number of oxygen atoms present in 1 mole of oxalic acid dihydrate is
a)
6 x 1023
b)
6.022 x 1034
c)
7.22 x 1023
d)
36.13 x 1023
|
|
Anshu Rane answered |
The correct answer is option 'D' which is 36.13 x 10^23. Let's break down the calculation to understand why.
1. Oxalic acid dihydrate has the chemical formula C2H2O4 · 2H2O. This means that it contains two carbon atoms, two hydrogen atoms, four oxygen atoms from the oxalic acid, and two additional oxygen atoms from the water molecule.
2. To calculate the number of oxygen atoms in one mole of oxalic acid dihydrate, we need to find the molar mass of the compound and then determine the number of moles of oxygen atoms.
3. The molar mass of oxalic acid dihydrate can be calculated by adding up the molar masses of each element present in the compound. The molar mass of carbon (C) is 12.01 g/mol, the molar mass of hydrogen (H) is 1.01 g/mol, and the molar mass of oxygen (O) is 16.00 g/mol.
4. The molar mass of oxalic acid dihydrate can be calculated as follows:
(2 * molar mass of carbon) + (2 * molar mass of hydrogen) + (4 * molar mass of oxygen) + (2 * molar mass of oxygen from water)
= (2 * 12.01 g/mol) + (2 * 1.01 g/mol) + (4 * 16.00 g/mol) + (2 * 16.00 g/mol)
= 24.02 g/mol + 2.02 g/mol + 64.00 g/mol + 32.00 g/mol
= 122.04 g/mol
5. Now, we can calculate the number of moles of oxygen atoms in 122.04 g of oxalic acid dihydrate. Since the molar mass of oxygen is 16.00 g/mol, the number of moles of oxygen atoms can be calculated as follows:
Number of moles = Mass / Molar mass
= 122.04 g / 16.00 g/mol
= 7.6275 mol
6. Finally, we can determine the number of oxygen atoms in one mole of oxalic acid dihydrate by multiplying the number of moles of oxygen atoms by Avogadro's number, which is 6.022 x 10^23 atoms/mol.
Number of oxygen atoms = Number of moles * Avogadro's number
= 7.6275 mol * 6.022 x 10^23 atoms/mol
= 4.591 x 10^24 atoms
Thus, the correct answer is option 'D', which is 36.13 x 10^23 (rounded to two significant figures).
1. Oxalic acid dihydrate has the chemical formula C2H2O4 · 2H2O. This means that it contains two carbon atoms, two hydrogen atoms, four oxygen atoms from the oxalic acid, and two additional oxygen atoms from the water molecule.
2. To calculate the number of oxygen atoms in one mole of oxalic acid dihydrate, we need to find the molar mass of the compound and then determine the number of moles of oxygen atoms.
3. The molar mass of oxalic acid dihydrate can be calculated by adding up the molar masses of each element present in the compound. The molar mass of carbon (C) is 12.01 g/mol, the molar mass of hydrogen (H) is 1.01 g/mol, and the molar mass of oxygen (O) is 16.00 g/mol.
4. The molar mass of oxalic acid dihydrate can be calculated as follows:
(2 * molar mass of carbon) + (2 * molar mass of hydrogen) + (4 * molar mass of oxygen) + (2 * molar mass of oxygen from water)
= (2 * 12.01 g/mol) + (2 * 1.01 g/mol) + (4 * 16.00 g/mol) + (2 * 16.00 g/mol)
= 24.02 g/mol + 2.02 g/mol + 64.00 g/mol + 32.00 g/mol
= 122.04 g/mol
5. Now, we can calculate the number of moles of oxygen atoms in 122.04 g of oxalic acid dihydrate. Since the molar mass of oxygen is 16.00 g/mol, the number of moles of oxygen atoms can be calculated as follows:
Number of moles = Mass / Molar mass
= 122.04 g / 16.00 g/mol
= 7.6275 mol
6. Finally, we can determine the number of oxygen atoms in one mole of oxalic acid dihydrate by multiplying the number of moles of oxygen atoms by Avogadro's number, which is 6.022 x 10^23 atoms/mol.
Number of oxygen atoms = Number of moles * Avogadro's number
= 7.6275 mol * 6.022 x 10^23 atoms/mol
= 4.591 x 10^24 atoms
Thus, the correct answer is option 'D', which is 36.13 x 10^23 (rounded to two significant figures).
If 40 g of CaCO3 is treated with 40 g of HCl, which of the reactants will act as limiting reagent?- a)CaCO3
- b)HCl
- c)Both are equal
- d)Cannot be calculated
Correct answer is option 'A'. Can you explain this answer?
If 40 g of CaCO3 is treated with 40 g of HCl, which of the reactants will act as limiting reagent?
a)
CaCO3
b)
HCl
c)
Both are equal
d)
Cannot be calculated
|
|
Suresh Iyer answered |
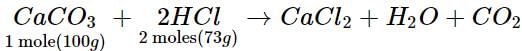
100 g of CaCO3 reacts with 73 g of HCl
40 g of CaCO3 will react with 73/100 x 40 = 29.2 g of HCl
Since CaCO3 is completely consumed and some amount (40 - 29.2 = 10.8g) of HCl remains unreacted and hence, CaCO3 is limiting reagent.
How much copper is present in 50 g of CuSO4?- a)19.90 g
- b)39.81 g
- c)63.5 g
- d)31.71 g
Correct answer is option 'A'. Can you explain this answer?
How much copper is present in 50 g of CuSO4?
a)
19.90 g
b)
39.81 g
c)
63.5 g
d)
31.71 g
|
|
Preeti Iyer answered |
Molar mass of CuSO4 = 63.5 + 32 + 4 x 16 = 159.5 g
Mass of copper present in 159.5 g of CuSO4 = 63.5 g
∴ Mass of copper present in 50 g of CuSO4
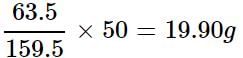
Mass of copper present in 159.5 g of CuSO4 = 63.5 g
∴ Mass of copper present in 50 g of CuSO4
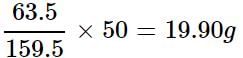
The empirical formula of a compound is CH2O2.What could be its molecular formula?- a)C2H2O2
- b)C2H2O4
- c)C2H4O4
- d)CH4O4
Correct answer is option 'C'. Can you explain this answer?
The empirical formula of a compound is CH2O2.What could be its molecular formula?
a)
C2H2O2
b)
C2H2O4
c)
C2H4O4
d)
CH4O4
|
|
Raghav Bansal answered |
Since empirical formula is multiplied by n to get molecular formula.
CH2O2 will give only C2H4O4 as its molecular formula. (CH2O2)n where n = 1, 2, 3,... etc.
CH2O2 will give only C2H4O4 as its molecular formula. (CH2O2)n where n = 1, 2, 3,... etc.
How many moles of oxygen gas can be produced during electrolytic decomposition of 180 g of water?- a)2.5 moles
- b)5 moles
- c)10 moles
- d)7 moles
Correct answer is option 'B'. Can you explain this answer?
How many moles of oxygen gas can be produced during electrolytic decomposition of 180 g of water?
a)
2.5 moles
b)
5 moles
c)
10 moles
d)
7 moles
|
|
Gaurav Kumar answered |
2H2O → 2H2 + O2
2 x 18 = 36g
36 g of water produces 1 mole of O2 gas.
180 g of water will produce 180/36 = 5 moles of O2 gas.
2 x 18 = 36g
36 g of water produces 1 mole of O2 gas.
180 g of water will produce 180/36 = 5 moles of O2 gas.
Atomic masses of elements are usually fractional because- a)These are mixtures of isotopes
- b)They contain impurities of other atoms
- c)They are mixtures of isobars
- d)Atomic masses cannot be weighed accurately
Correct answer is option 'A'. Can you explain this answer?
Atomic masses of elements are usually fractional because
a)
These are mixtures of isotopes
b)
They contain impurities of other atoms
c)
They are mixtures of isobars
d)
Atomic masses cannot be weighed accurately
|
|
Ajay Yadav answered |
Atomic masses are the average atomic masses of the mixture of isotopes and calculated on the basis of relative abundance, hence they are usually fractional.
The final molarity of a solution made by mixing 50 mL of 0.5 M HCl, 150 mL of 0.25 M HCl and water to make the volume 250 mL is- a)0.5 M
- b)1 M
- c)0.75 M
- d)0.25 M
Correct answer is option 'D'. Can you explain this answer?
The final molarity of a solution made by mixing 50 mL of 0.5 M HCl, 150 mL of 0.25 M HCl and water to make the volume 250 mL is
a)
0.5 M
b)
1 M
c)
0.75 M
d)
0.25 M
|
|
Swara Dey answered |
To find the final molarity of the solution, we need to consider the principle of conservation of moles. This principle states that the total number of moles of solute before and after mixing should remain the same.
- Initial moles of HCl in 50 mL of 0.5 M HCl:
Moles = Molarity x Volume
Moles = 0.5 M x 0.050 L = 0.025 moles
- Initial moles of HCl in 150 mL of 0.25 M HCl:
Moles = Molarity x Volume
Moles = 0.25 M x 0.150 L = 0.0375 moles
- Total moles of HCl before mixing:
Total moles = 0.025 moles + 0.0375 moles = 0.0625 moles
- Final volume of the solution = 250 mL = 0.250 L
- Final molarity of the solution:
Molarity = Total moles / Final volume
Molarity = 0.0625 moles / 0.250 L = 0.25 M
Therefore, the final molarity of the solution is 0.25 M.
- Initial moles of HCl in 50 mL of 0.5 M HCl:
Moles = Molarity x Volume
Moles = 0.5 M x 0.050 L = 0.025 moles
- Initial moles of HCl in 150 mL of 0.25 M HCl:
Moles = Molarity x Volume
Moles = 0.25 M x 0.150 L = 0.0375 moles
- Total moles of HCl before mixing:
Total moles = 0.025 moles + 0.0375 moles = 0.0625 moles
- Final volume of the solution = 250 mL = 0.250 L
- Final molarity of the solution:
Molarity = Total moles / Final volume
Molarity = 0.0625 moles / 0.250 L = 0.25 M
Therefore, the final molarity of the solution is 0.25 M.
Iron can be obtained by reduction, of iron oxide (FC3O4) with CO according to the reaction:
Fe3O4 + 4CO → 3Fe + 4CO2
How many kg of Fe3O4 should be heated with CO to get 3 kg of iron?- a)8.12 kg
- b)4.14 kg
- c)6.94 kg
- d)16.8 kg
Correct answer is option 'B'. Can you explain this answer?
Iron can be obtained by reduction, of iron oxide (FC3O4) with CO according to the reaction:
Fe3O4 + 4CO → 3Fe + 4CO2
How many kg of Fe3O4 should be heated with CO to get 3 kg of iron?
Fe3O4 + 4CO → 3Fe + 4CO2
How many kg of Fe3O4 should be heated with CO to get 3 kg of iron?
a)
8.12 kg
b)
4.14 kg
c)
6.94 kg
d)
16.8 kg
|
|
Riya Banerjee answered |
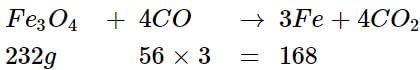
3 moles of Fe is produced from 1 mole of Fe3O4
168 g of Fe is producedfrom 232 g of Fe3O4.
3 kg of Fe will be produced from 232/168 x 3000 g
= 4142.8 g.or 4.14 kg of Fe3O4
What is the concentration of copper sulphate (in mol L-1) if 80 g of it is dissolved in enough water to make a final volume of 3 L?- a)0.0167
- b)0.167
- c)1.067
- d)10.67
Correct answer is option 'B'. Can you explain this answer?
What is the concentration of copper sulphate (in mol L-1) if 80 g of it is dissolved in enough water to make a final volume of 3 L?
a)
0.0167
b)
0.167
c)
1.067
d)
10.67
|
|
Dev Patel answered |
Molar mass of CUSO4 = 63.5 + 32 + 64 = 159.5
Moles of CUSO4 = 80/159.5 = O.50
Volume of solution = 3 L
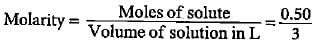
= 0.167 mol L-1
Moles of CUSO4 = 80/159.5 = O.50
Volume of solution = 3 L
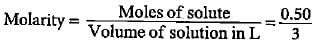
= 0.167 mol L-1
What is the mass percent of oxygen in ethanol?- a)52.14%
- b)13.13%
- c)16%
- d)34.73%
Correct answer is option 'D'. Can you explain this answer?
What is the mass percent of oxygen in ethanol?
a)
52.14%
b)
13.13%
c)
16%
d)
34.73%
|
|
Dev Patel answered |
Molecular formula of ethanol = C2H5OH
Molar mass of ethanol of 2 x 12.01 + 6 x 1.008 + 16 = 46.068g
Mass percent of oxygen = 16/46.068 x 100 = 34.73%
Molar mass of ethanol of 2 x 12.01 + 6 x 1.008 + 16 = 46.068g
Mass percent of oxygen = 16/46.068 x 100 = 34.73%
An impure sample of silver (1.5 g) is heated with S to form 0.124 g of Ag2S. What was the per cent yield of Ag2S?- a)21.6%
- b)7.2%
- c)1.7%
- d)24.8%
Correct answer is option 'B'. Can you explain this answer?
An impure sample of silver (1.5 g) is heated with S to form 0.124 g of Ag2S. What was the per cent yield of Ag2S?
a)
21.6%
b)
7.2%
c)
1.7%
d)
24.8%
|
|
Ananya Das answered |
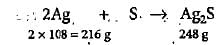
216 g of Ag forms 248 g of Ag2S
1.5 g of Ag forms 248/216 x 1.5 = 1.722 g of Ag2S
% yield of Ag2S = 0.124/1.722 x 100 = 7.2%
1.5 g of Ag forms 248/216 x 1.5 = 1.722 g of Ag2S
% yield of Ag2S = 0.124/1.722 x 100 = 7.2%
1.4 moles of phosphorus trichloride are present in a sample. How many atoms are there in the sample?- a)5.6
- b)34
- c)2.4 x 1023
- d)3.372 x 1024
Correct answer is option 'D'. Can you explain this answer?
1.4 moles of phosphorus trichloride are present in a sample. How many atoms are there in the sample?
a)
5.6
b)
34
c)
2.4 x 1023
d)
3.372 x 1024
|
|
Baishali Khanna answered |
To determine the number of atoms present in a sample of phosphorus trichloride, we need to use Avogadro's number, which states that 1 mole of any substance contains 6.022 × 10^23 entities (atoms, molecules, or ions).
Given:
Moles of phosphorus trichloride = 1.4 moles
To find the number of atoms, we need to multiply the number of moles by Avogadro's number:
Number of atoms = Moles of substance × Avogadro's number
Let's calculate the number of atoms in the sample:
Number of atoms = 1.4 moles × 6.022 × 10^23 atoms/mole
Solving this equation, we get:
Number of atoms = 8.4308 × 10^23 atoms
Therefore, the correct answer is option D: 3.372 × 10^24 atoms.
Given:
Moles of phosphorus trichloride = 1.4 moles
To find the number of atoms, we need to multiply the number of moles by Avogadro's number:
Number of atoms = Moles of substance × Avogadro's number
Let's calculate the number of atoms in the sample:
Number of atoms = 1.4 moles × 6.022 × 10^23 atoms/mole
Solving this equation, we get:
Number of atoms = 8.4308 × 10^23 atoms
Therefore, the correct answer is option D: 3.372 × 10^24 atoms.
At NTP, 1L of O2 reacts with 3 L of carbon monoxide. What will be the volume of CO and CO2 after the reaction?- a)1L CO2, 1L CO
- b)2L CO2, 2L CO
- c)1L CO2, 2L CO
- d)2L CO2, 1L CO
Correct answer is option 'D'. Can you explain this answer?
At NTP, 1L of O2 reacts with 3 L of carbon monoxide. What will be the volume of CO and CO2 after the reaction?
a)
1L CO2, 1L CO
b)
2L CO2, 2L CO
c)
1L CO2, 2L CO
d)
2L CO2, 1L CO
|
|
Ananya Das answered |
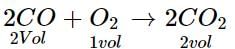
1 vol of CO2 reacts with 2 vol of CO
1 L of CO2 reacts with 2 L of CO
CO left after reaction = 3 - 2 = 11
1 L of O2 produces 2 L of CO2.
Hence, after the reaction, CO = 1 L, CO2 = 2 L
Which of the following gases will have least volume if 10 g of each gas is taken at same temperature and pressure?- a)CO2
- b)N2
- c)CH4
- d)HCl
Correct answer is option 'A'. Can you explain this answer?
Which of the following gases will have least volume if 10 g of each gas is taken at same temperature and pressure?
a)
CO2
b)
N2
c)
CH4
d)
HCl
|
|
Priya Menon answered |
Number of moles ∝ 1/Molecular mass Molecular mass of CO2 = 44, N2 = 28, CH4 = 16, HCl = 36.5
CO2 will have least volume, as no. of moles is directly proportional to volume at constant P and T.
CO2 will have least volume, as no. of moles is directly proportional to volume at constant P and T.
Few quantities with their units are listed below. Mark the units which are not correctly matched.
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m- a)(ii) and (iv)
- b)(i) and (iii)
- c)(iii) and (v)
- d)(iv) and (v)
Correct answer is option 'C'. Can you explain this answer?
Few quantities with their units are listed below. Mark the units which are not correctly matched.
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
a)
(ii) and (iv)
b)
(i) and (iii)
c)
(iii) and (v)
d)
(iv) and (v)
|
|
Preeti Iyer answered |
Planck's constant = J s
Force = kg m s-2
Force = kg m s-2
The weight of AgCl precipitated when a solution containing 5.85 g of NaCl is added to a solution containing 3.4 g of AgNO3 is- a)28 g
- b)9.25 g
- c)2.870 g
- d)58 g
Correct answer is option 'C'. Can you explain this answer?
The weight of AgCl precipitated when a solution containing 5.85 g of NaCl is added to a solution containing 3.4 g of AgNO3 is
a)
28 g
b)
9.25 g
c)
2.870 g
d)
58 g
|
|
Dev Patel answered |
AgNO3 + NaCl → AgCl + NaNO3
No. of moles of AgNO3 = 3.4/170 = 0.02
No, of moles of NaCl = 5.85/58.5 = 0.1
Limiting reagent = AgNO3
1 mole of AgNO3 produces 1 mole of AgCl
0.02 mole of AgNO3 will produce 0.02 mole of AgCl
Weight of AgCl produced = 0.02 x 143.5 = 2.870 g.
No. of moles of AgNO3 = 3.4/170 = 0.02
No, of moles of NaCl = 5.85/58.5 = 0.1
Limiting reagent = AgNO3
1 mole of AgNO3 produces 1 mole of AgCl
0.02 mole of AgNO3 will produce 0.02 mole of AgCl
Weight of AgCl produced = 0.02 x 143.5 = 2.870 g.
What is the mass of carbon dioxide which contains the same number of molecules as are contained in 40 g of oxygen?- a)40g
- b)55g
- c)32g
- d)44g
Correct answer is option 'B'. Can you explain this answer?
What is the mass of carbon dioxide which contains the same number of molecules as are contained in 40 g of oxygen?
a)
40g
b)
55g
c)
32g
d)
44g
|
|
Lavanya Menon answered |
Molar mass of O2 = 32 g mol-1
32 g of O2 = 6.023 x 1023 molecules
40 g of O2 =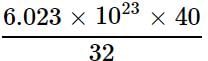 = 7.529 x 1023 molecules
= 7.529 x 1023 molecules
Mass of 6.023 x 1023 molecules of CO2 = 44 g
Mass of 7.529 x 1023 molecules of CO2
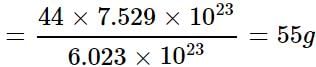
32 g of O2 = 6.023 x 1023 molecules
40 g of O2 =
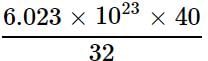 = 7.529 x 1023 molecules
= 7.529 x 1023 moleculesMass of 6.023 x 1023 molecules of CO2 = 44 g
Mass of 7.529 x 1023 molecules of CO2
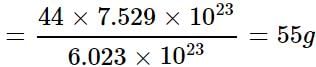
How much oxygen is required for complete combustion of 560 g of ethene?- a)6.4 kg
- b)1.92 kg
- c)2.8 kg
- d)9.6 kg
Correct answer is option 'B'. Can you explain this answer?
How much oxygen is required for complete combustion of 560 g of ethene?
a)
6.4 kg
b)
1.92 kg
c)
2.8 kg
d)
9.6 kg
|
|
Hansa Sharma answered |
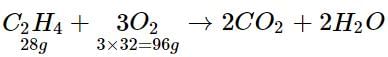
28 g of C2H4 requires 96 g of O2
560 g of C2H4 requires 96/28 x 560
= 1920 g or 1.92 kg of O2
A gas has molecular formula (CH)n. If vapour density of the gas is 39, what should be the formula of the compound?- a)C3H3
- b)C4H4
- c)C2H2
- d)C6H6
Correct answer is option 'D'. Can you explain this answer?
A gas has molecular formula (CH)n. If vapour density of the gas is 39, what should be the formula of the compound?
a)
C3H3
b)
C4H4
c)
C2H2
d)
C6H6
|
|
Riya Banerjee answered |
Mol. wt. = 2 x V.D. = 2 x 39 = 78
(CH)n = (13)n or 13 x n = 78
n = 78/13 = 6
∴ Molecular formula = C6H6
(CH)n = (13)n or 13 x n = 78
n = 78/13 = 6
∴ Molecular formula = C6H6
Chapter doubts & questions for Some Basic Concepts of Chemistry - NCERTs at Fingertips: Textbooks, Tests & Solutions 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Some Basic Concepts of Chemistry - NCERTs at Fingertips: Textbooks, Tests & Solutions in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily