All Exams >
JEE >
SRMJEEE Subject Wise & Full Length Mock Tests 2026 >
All Questions
All questions of Section wise Tests: Chemistry for JEE Exam
Which acts as both oxidising as well as reducing agent ?- a)HNO₃
- b)HNO₂
- c)H₂SO₄
- d)HCl
Correct answer is option 'B'. Can you explain this answer?
a)
HNO₃
b)
HNO₂
c)
H₂SO₄
d)
HCl

|
Abhishek Ambast answered |
As here in B we can see that N is in +3oxidation state therefore it can increase up to 5 or decrease down to -3 hence it will oxidise as well as reduce.
If 0.183 of an aromatic monobasic acid requires 15ml of N/10 sodium hydroxide for complete neutralisation, then molecular weight of the acid is- a)56
- b)122
- c)166
- d)224
Correct answer is option 'B'. Can you explain this answer?
If 0.183 of an aromatic monobasic acid requires 15ml of N/10 sodium hydroxide for complete neutralisation, then molecular weight of the acid is
a)
56
b)
122
c)
166
d)
224
|
|
Geetika Shah answered |
Milliequivalents of NaOH = 15×1/10 = 1.5 = 0.0015 equivalents
For neutralization equivalents of acid = 0.0015
As the acid is monobasic, n factor= 1, so moles of acid required = 0.0015/1 = 0.0015
weight/MW = 0.183g/0.0015 MW
MW= 122g
For neutralization equivalents of acid = 0.0015
As the acid is monobasic, n factor= 1, so moles of acid required = 0.0015/1 = 0.0015
weight/MW = 0.183g/0.0015 MW
MW= 122g
A triglyceride can have how many different acyl groups?- a)3
- b)2
- c)1
- d)4
Correct answer is option 'A'. Can you explain this answer?
a)
3
b)
2
c)
1
d)
4
|
|
Jay Nair answered |
Triglyceride Composition
Triglycerides are composed of a glycerol molecule linked to three fatty acid chains. These fatty acid chains are also known as acyl groups.
Number of Acyl Groups
Triglycerides can have a total of three different acyl groups, one attached to each of the three hydroxyl groups of the glycerol molecule. This means that a triglyceride molecule can have three unique fatty acid chains, giving it a total of three acyl groups.
Options
a) 3 - This is the correct answer as explained above.
b) 2 - This is incorrect as triglycerides have three acyl groups.
c) 1 - This is incorrect as triglycerides have three acyl groups.
d) 4 - This is incorrect as triglycerides have three acyl groups.
Therefore, the correct answer is option 'a' - 3. Triglycerides can have three different acyl groups, one for each fatty acid chain attached to the glycerol molecule.
Triglycerides are composed of a glycerol molecule linked to three fatty acid chains. These fatty acid chains are also known as acyl groups.
Number of Acyl Groups
Triglycerides can have a total of three different acyl groups, one attached to each of the three hydroxyl groups of the glycerol molecule. This means that a triglyceride molecule can have three unique fatty acid chains, giving it a total of three acyl groups.
Options
a) 3 - This is the correct answer as explained above.
b) 2 - This is incorrect as triglycerides have three acyl groups.
c) 1 - This is incorrect as triglycerides have three acyl groups.
d) 4 - This is incorrect as triglycerides have three acyl groups.
Therefore, the correct answer is option 'a' - 3. Triglycerides can have three different acyl groups, one for each fatty acid chain attached to the glycerol molecule.
The value of gas constant per degree per mole is approximately- a)1 cal
- b)2 cal
- c)3 cal
- d)4 cal
Correct answer is option 'B'. Can you explain this answer?
a)
1 cal
b)
2 cal
c)
3 cal
d)
4 cal
|
|
Sanjana Sengupta answered |
Gas Constant and Its Value
Gas constant (R) is a fundamental physical constant that relates the amount of energy a gas has to its temperature, pressure, and volume. It is used in various thermodynamic equations, such as the ideal gas law.
The gas constant per degree per mole is the value of R divided by the number of degrees and moles in the system. The value of gas constant per degree per mole is approximately 2 cal. This means that for every degree of temperature change in a mole of gas, there will be a change in energy of 2 calories.
Explanation
The value of gas constant per degree per mole can be calculated by dividing the gas constant (R) by the number of degrees (T) and the number of moles (n) in the system.
R = 8.314 J/mol K (universal gas constant)
1 cal = 4.184 J
Thus, the value of gas constant per degree per mole is:
R/Tn = (8.314 J/mol K)/(1 mol x 1 K) = 8.314 J/mol
8.314 J/mol = 1.987 cal/mol
Therefore, the value of gas constant per degree per mole is approximately 2 cal.
Importance
The gas constant per degree per mole is important in many thermodynamic calculations, such as determining the work done by a gas during a process, calculating the heat capacity of a substance, and predicting the behavior of gases under different conditions.
Conclusion
The gas constant per degree per mole is a fundamental physical constant that relates the amount of energy a gas has to its temperature, pressure, and volume. Its value is approximately 2 cal, which is used in various thermodynamic calculations.
Gas constant (R) is a fundamental physical constant that relates the amount of energy a gas has to its temperature, pressure, and volume. It is used in various thermodynamic equations, such as the ideal gas law.
The gas constant per degree per mole is the value of R divided by the number of degrees and moles in the system. The value of gas constant per degree per mole is approximately 2 cal. This means that for every degree of temperature change in a mole of gas, there will be a change in energy of 2 calories.
Explanation
The value of gas constant per degree per mole can be calculated by dividing the gas constant (R) by the number of degrees (T) and the number of moles (n) in the system.
R = 8.314 J/mol K (universal gas constant)
1 cal = 4.184 J
Thus, the value of gas constant per degree per mole is:
R/Tn = (8.314 J/mol K)/(1 mol x 1 K) = 8.314 J/mol
8.314 J/mol = 1.987 cal/mol
Therefore, the value of gas constant per degree per mole is approximately 2 cal.
Importance
The gas constant per degree per mole is important in many thermodynamic calculations, such as determining the work done by a gas during a process, calculating the heat capacity of a substance, and predicting the behavior of gases under different conditions.
Conclusion
The gas constant per degree per mole is a fundamental physical constant that relates the amount of energy a gas has to its temperature, pressure, and volume. Its value is approximately 2 cal, which is used in various thermodynamic calculations.
With oxalic acid, glycerol at 503 K gives- a) Allyl alcohol
- b) Formic acid
- c) Glyceraldehyde
- d) None of these
Correct answer is option 'A'. Can you explain this answer?
a)
Allyl alcohol
b)
Formic acid
c)
Glyceraldehyde
d)
None of these
|
|
Anjali Sharma answered |
At 110⁰C glycerol reacts with oxalic acid giving formic acid. But heating at 260⁰C results allyl alcohol.
The second order Bragg diffraction of X-rays with λ = 1.00 Å from a set of parallel planes in a metal occurs at an angle of 60°. The distance between the scattering planes in the crystal is
- a)0.575 Å
- b)1.00 Å
- c)2.00 Å
- d)1.15 Å
Correct answer is option 'D'. Can you explain this answer?
The second order Bragg diffraction of X-rays with λ = 1.00 Å from a set of parallel planes in a metal occurs at an angle of 60°. The distance between the scattering planes in the crystal is
a)
0.575 Å
b)
1.00 Å
c)
2.00 Å
d)
1.15 Å
|
|
Tarun Roy answered |
A wavelength of 1.54 Å occurs at an angle of 28.4°. What is the spacing between the planes of atoms in the crystal?
The Bragg diffraction equation relates the angle of diffraction (θ), the wavelength of the X-rays (λ), and the spacing between the planes of atoms (d):
2d sinθ = nλ
where n is an integer representing the order of diffraction.
For second order diffraction (n = 2), we can rearrange the equation to solve for d:
d = λ / 2 sinθ
Plugging in the given values, we get:
d = 1.54 Å / 2 sin(28.4°) = 2.82 Å
Therefore, the spacing between the planes of atoms in the crystal is 2.82 Angstroms.
The Bragg diffraction equation relates the angle of diffraction (θ), the wavelength of the X-rays (λ), and the spacing between the planes of atoms (d):
2d sinθ = nλ
where n is an integer representing the order of diffraction.
For second order diffraction (n = 2), we can rearrange the equation to solve for d:
d = λ / 2 sinθ
Plugging in the given values, we get:
d = 1.54 Å / 2 sin(28.4°) = 2.82 Å
Therefore, the spacing between the planes of atoms in the crystal is 2.82 Angstroms.
Which of the following does not react with AgCl?- a)Na₂CO₃
- b)NaNO₃
- c)NH₄OH
- d)Na₂S₂O₃
Correct answer is option 'B'. Can you explain this answer?
a)
Na₂CO₃
b)
NaNO₃
c)
NH₄OH
d)
Na₂S₂O₃
|
|
Preeti Iyer answered |
AgCl is insoluble in water and NaNO3 is soluble in water.and also the reverse reaction of the reaction u asked is highly stable and unidirectional.
When enthalpy and entropy change for a chemical reaction are -2.5 x103 cals and 7.4 cals deg⁻1 respectively . Predict that reaction at 298 K is
- a)spontaneous
- b)reversible
- c)irreversible
- d)non-spontaneous
Correct answer is option 'A'. Can you explain this answer?
When enthalpy and entropy change for a chemical reaction are -2.5 x103 cals and 7.4 cals deg⁻1 respectively . Predict that reaction at 298 K is
a)
spontaneous
b)
reversible
c)
irreversible
d)
non-spontaneous
|
|
Ashutosh Shah answered |
Given data:
Enthalpy change, ΔH = -2.5 x 10³ cal
Entropy change, ΔS = 7.4 cal/°C
Temperature, T = 298 K
To determine the spontaneity of the reaction, we can use the Gibbs free energy change (ΔG) of the reaction.
ΔG = ΔH - TΔS
Substituting the given values:
ΔG = -2.5 x 10³ - 298 x 7.4 = -2.5 x 10³ - 2205.2 = -2207.7 cal
If ΔG is negative, the reaction will be spontaneous.
Since ΔG is negative, the reaction is spontaneous at 298K.
Therefore, the correct answer is option 'A' - spontaneous.
Enthalpy change, ΔH = -2.5 x 10³ cal
Entropy change, ΔS = 7.4 cal/°C
Temperature, T = 298 K
To determine the spontaneity of the reaction, we can use the Gibbs free energy change (ΔG) of the reaction.
ΔG = ΔH - TΔS
Substituting the given values:
ΔG = -2.5 x 10³ - 298 x 7.4 = -2.5 x 10³ - 2205.2 = -2207.7 cal
If ΔG is negative, the reaction will be spontaneous.
Since ΔG is negative, the reaction is spontaneous at 298K.
Therefore, the correct answer is option 'A' - spontaneous.
The isotope atoms differ in ?- a)number of neutrons
- b)atomic number
- c)number of electrons
- d)atomic weight
Correct answer is option 'A'. Can you explain this answer?
The isotope atoms differ in ?
a)
number of neutrons
b)
atomic number
c)
number of electrons
d)
atomic weight

|
Harshleen Kaur answered |
Isotopes means atoms having same atomic no. but different atomic mass
and atomic mass = no. of protons +no.of neutrons
and since atomic no. is same so no. of protons should be same
thus no. of neutrons will be different.
and atomic mass = no. of protons +no.of neutrons
and since atomic no. is same so no. of protons should be same
thus no. of neutrons will be different.
When ammonium chloride is added to ammonia solution , the pH of the resulting solution will be- a)increased
- b)seven
- c)decreased
- d)unchanged
Correct answer is option 'C'. Can you explain this answer?
a)
increased
b)
seven
c)
decreased
d)
unchanged
|
|
Subhankar Basu answered |
Adding Ammonium Chloride to Ammonia Solution
When ammonium chloride is added to ammonia solution, the following reaction occurs:
NH3 + HCl → NH4Cl
The ammonium chloride dissociates into NH4+ and Cl- ions in the solution. The ammonium ion (NH4+) is a weak acid, which can donate a proton (H+) to the solution, resulting in an increase in the concentration of H+ ions and a decrease in the pH of the solution.
Effect on pH
The addition of ammonium chloride to ammonia solution will decrease the pH of the resulting solution. This is because the ammonium ion (NH4+) formed by the dissociation of ammonium chloride acts as a weak acid and increases the concentration of H+ ions in the solution. The increase in the concentration of H+ ions lowers the pH of the solution.
Therefore, the correct answer is option C, i.e., the pH of the resulting solution will be decreased.
When ammonium chloride is added to ammonia solution, the following reaction occurs:
NH3 + HCl → NH4Cl
The ammonium chloride dissociates into NH4+ and Cl- ions in the solution. The ammonium ion (NH4+) is a weak acid, which can donate a proton (H+) to the solution, resulting in an increase in the concentration of H+ ions and a decrease in the pH of the solution.
Effect on pH
The addition of ammonium chloride to ammonia solution will decrease the pH of the resulting solution. This is because the ammonium ion (NH4+) formed by the dissociation of ammonium chloride acts as a weak acid and increases the concentration of H+ ions in the solution. The increase in the concentration of H+ ions lowers the pH of the solution.
Therefore, the correct answer is option C, i.e., the pH of the resulting solution will be decreased.
Milk is an example of
- a)fat in oil emulsion
- b)water in oil emulsion
- c)fat in water emulsion
- d)oil in water emulsion
Correct answer is option 'D'. Can you explain this answer?
Milk is an example of
a)
fat in oil emulsion
b)
water in oil emulsion
c)
fat in water emulsion
d)
oil in water emulsion
|
|
Rajeev Menon answered |
Milk is an example of the oil-in-water type of emulsion. In milk liquid fat globules are dispersed in water. Other examples are, vanishing cream etc.
The vapour density of pure ozone would be
- a)48
- b)32
- c)24
- d)16
Correct answer is option 'C'. Can you explain this answer?
The vapour density of pure ozone would be
a)
48
b)
32
c)
24
d)
16

|
Imk Pathsala answered |
Molecular weight=2×vapour density
48=2×vapour density
24=vapour density, Hence, option C is correct.
The I.P. of sodium is 5.14eV. The I.P. of potassium could be- a)Same as that of sodium
- b)5.68eV
- c)4.34eV
- d)10.28eV
Correct answer is option 'C'. Can you explain this answer?
The I.P. of sodium is 5.14eV. The I.P. of potassium could be
a)
Same as that of sodium
b)
5.68eV
c)
4.34eV
d)
10.28eV
|
|
Lavanya Menon answered |
The ionisation potential keep on decreasing regularly as we move down a group from one element to the other. Because on moving down the group the atomic size increases gradually due to the addition of one new principal energy shell at each succeeding element. As a result, the force of attraction by the nucleus for the valence electrons decreases and the shielding effect increases. Consequently, ionisation potential also decreases.
Pi-bonds do not alter the shape, but merely shorten the- a)bond energy
- b)bond angle
- c)bond length
- d)bond frequency
Correct answer is option 'C'. Can you explain this answer?
Pi-bonds do not alter the shape, but merely shorten the
a)
bond energy
b)
bond angle
c)
bond length
d)
bond frequency
|
|
Sounak Pillai answered |
Explanation:
Pi-bonds are covalent bonds that are formed by the overlapping of p orbitals of two atoms. These bonds do not alter the shape of the molecule that is formed by the bonding atoms. The shape of the molecule is determined by the arrangement of the atoms and the lone pairs of electrons on the central atom.
Bond Length:
The bond length is the distance between the nuclei of two bonded atoms. The formation of pi-bonds shortens the bond length because the overlap of the p orbitals brings the nuclei of the bonding atoms closer together.
Bond Energy:
The bond energy is the amount of energy required to break a bond. The formation of pi-bonds increases the bond energy because the overlapping of the p orbitals results in the formation of a stronger bond.
Bond Angle:
The bond angle is the angle between the nuclei of two adjacent atoms in a molecule. Pi-bonds do not alter the bond angle because they are formed perpendicular to the plane of the atoms that are bonded, and so they do not affect the arrangement of the atoms in the molecule.
Bond Frequency:
The bond frequency is the frequency at which a bond vibrates. Pi-bonds do not alter the bond frequency because they do not affect the motion of the atoms in the molecule.
Conclusion:
Pi-bonds are important covalent bonds that are formed by the overlapping of p orbitals of two atoms. These bonds do not alter the shape of the molecule but they do shorten the bond length and increase the bond energy. The bond angle and bond frequency are not affected by the formation of pi-bonds.
Pi-bonds are covalent bonds that are formed by the overlapping of p orbitals of two atoms. These bonds do not alter the shape of the molecule that is formed by the bonding atoms. The shape of the molecule is determined by the arrangement of the atoms and the lone pairs of electrons on the central atom.
Bond Length:
The bond length is the distance between the nuclei of two bonded atoms. The formation of pi-bonds shortens the bond length because the overlap of the p orbitals brings the nuclei of the bonding atoms closer together.
Bond Energy:
The bond energy is the amount of energy required to break a bond. The formation of pi-bonds increases the bond energy because the overlapping of the p orbitals results in the formation of a stronger bond.
Bond Angle:
The bond angle is the angle between the nuclei of two adjacent atoms in a molecule. Pi-bonds do not alter the bond angle because they are formed perpendicular to the plane of the atoms that are bonded, and so they do not affect the arrangement of the atoms in the molecule.
Bond Frequency:
The bond frequency is the frequency at which a bond vibrates. Pi-bonds do not alter the bond frequency because they do not affect the motion of the atoms in the molecule.
Conclusion:
Pi-bonds are important covalent bonds that are formed by the overlapping of p orbitals of two atoms. These bonds do not alter the shape of the molecule but they do shorten the bond length and increase the bond energy. The bond angle and bond frequency are not affected by the formation of pi-bonds.
Chemical equilibrium is dynamic in nature, because- a) the equilibrium is maintained rapidly
- b) the concentration of reactants and products are constant but different
- c) both forward and backward reactions occur at all times with same speed
- d) the concentration of reactants and products becomes same at equilibrium
Correct answer is option 'C'. Can you explain this answer?
a)
the equilibrium is maintained rapidly
b)
the concentration of reactants and products are constant but different
c)
both forward and backward reactions occur at all times with same speed
d)
the concentration of reactants and products becomes same at equilibrium

|
Avantika Joshi answered |
The two opposite processes, forward reaction and reverse reaction, keep on occurring in it all the time but at exactly equal rates (or equal speeds)
A chemical reaction does not stop after reaching the equilibrium state, only the rate of the forward and the reverse reactions become equal at the equilibrium state
A chemical reaction does not stop after reaching the equilibrium state, only the rate of the forward and the reverse reactions become equal at the equilibrium state
In a mole of water vapours at STP, the volume actually occupied or taken by the molecules (i.e., Avogadro's No.xvolume of one molecule) is- a)zero
- b)less than 1% of 22.4 litres
- c)about 10% of the volume of container
- d)1% to 2% of 22.4 litres
Correct answer is option 'B'. Can you explain this answer?
In a mole of water vapours at STP, the volume actually occupied or taken by the molecules (i.e., Avogadro's No.xvolume of one molecule) is
a)
zero
b)
less than 1% of 22.4 litres
c)
about 10% of the volume of container
d)
1% to 2% of 22.4 litres
|
|
Mira Sharma answered |
1 MOLE OF WATER = 18 G OR 18CC
VOLUME OCCUPIED BY 1 MOLE OF WATER VAPOR AT STP = 22400 CC
Hence, percentage of volume actually occupied by water molecules = (18 x 100)/22400 = 9/112 = 0.0804 = <0.1%
The alkene formed as a major product in the above elimination reaction is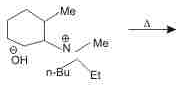
- a)
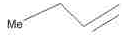
- b)CH₂ = CH₂
- c)
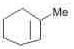
- d)

Correct answer is option 'B'. Can you explain this answer?
The alkene formed as a major product in the above elimination reaction is
a)
b)
CH₂ = CH₂
c)
d)
|
|
Jyoti Aiims Aspirant answered |
Nitrogen k respect mai beta hydrogen check kro jo jyada acidic hoga vhi alkene bnegi
methyl gp mai beta hydrogen absent h
electron donating gp acidity ko decrease krte h
then ring, tert butyl, and ehtyl mai sbse acidic beta hydrogen ethyl group k pas h so ETHENE will be the alkene.
methyl gp mai beta hydrogen absent h
electron donating gp acidity ko decrease krte h
then ring, tert butyl, and ehtyl mai sbse acidic beta hydrogen ethyl group k pas h so ETHENE will be the alkene.
BCl3 molecule is planar while NCl3 is pyramidal because- a)BCl3 does not have lone pair of electrons on B but NCl3 has
- b)B-Cl bond is more polar than N-Cl bond
- c)N atom is smaller than B
- d)N-Cl bond is more covalent than B-Cl bond
Correct answer is option 'A'. Can you explain this answer?
BCl3 molecule is planar while NCl3 is pyramidal because
a)
BCl3 does not have lone pair of electrons on B but NCl3 has
b)
B-Cl bond is more polar than N-Cl bond
c)
N atom is smaller than B
d)
N-Cl bond is more covalent than B-Cl bond
|
|
Aniket Dasgupta answered |
In a flask of volume V litres, 0.2 mol of oxygen, 0.4 mol of nitrogen, 0.1 mole of NH₃ and 0.3 mol of He are enclosed at 27°C. If the total pressure exerted by these non reacting gases is one atmosphere, the partial pressure exerted by nitrogen is- a)1 atm
- b)0.1 atm
- c)0.2 atm
- d)0.4 atm
Correct answer is option 'D'. Can you explain this answer?
In a flask of volume V litres, 0.2 mol of oxygen, 0.4 mol of nitrogen, 0.1 mole of NH₃ and 0.3 mol of He are enclosed at 27°C. If the total pressure exerted by these non reacting gases is one atmosphere, the partial pressure exerted by nitrogen is
a)
1 atm
b)
0.1 atm
c)
0.2 atm
d)
0.4 atm
|
|
Dhruba Mukherjee answered |
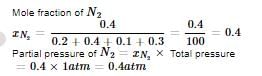
Which of the following chemicals are used to manufacture methyl isocyanate that caused "Bhopal Tragedy " ? (a) Methyl amine (b) Phosgene (c) Phosphine (d) Dimethyl amine- a)(a) and (c)
- b)(c) and (d)
- c)(a) and (b)
- d)(b) and (d)
Correct answer is option 'C'. Can you explain this answer?
Which of the following chemicals are used to manufacture methyl isocyanate that caused "Bhopal Tragedy " ? (a) Methyl amine (b) Phosgene (c) Phosphine (d) Dimethyl amine
a)
(a) and (c)
b)
(c) and (d)
c)
(a) and (b)
d)
(b) and (d)

|
Nishanth Verma answered |
The correct option is: (c) (i) and (ii)
Explanation:
Methyl isocyanate is industrially prepared by the action of methyl amine with phosgene.
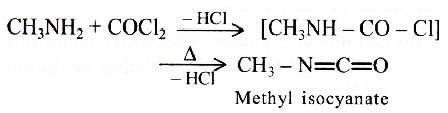
Explanation:
Methyl isocyanate is industrially prepared by the action of methyl amine with phosgene.

Given that (i) C + O₂ → CO₂ ; ∆H° = − xkJ, (ii) 2CO + O₂ → 2CO₂ ; ∆H° = −ykJ . The enthalpy of formation of carbon monoxide will be- a)y-2x
- b)2x-y/2
- c)y-2x/2
- d)2x-y
Correct answer is option 'C'. Can you explain this answer?
Given that (i) C + O₂ → CO₂ ; ∆H° = − xkJ, (ii) 2CO + O₂ → 2CO₂ ; ∆H° = −ykJ . The enthalpy of formation of carbon monoxide will be
a)
y-2x
b)
2x-y/2
c)
y-2x/2
d)
2x-y

|
Vp Classes answered |
To determine the enthalpy of formation of carbon monoxide (CO):
- The first reaction shows the formation of carbon dioxide (CO₂) from carbon (C) and oxygen (O₂):
- C + O₂ → CO₂ ; ∆H° = −x kJ
- The second reaction indicates the formation of CO₂ from carbon monoxide (CO):
- 2CO + O₂ → 2CO₂ ; ∆H° = −y kJ
- To find the enthalpy of formation of CO, we need to manipulate these reactions:
- By reversing the first reaction, we get:
- CO₂ → C + O₂ ; ∆H° = +x kJ
- Then, we can write the formation of CO from C and O₂:
- C + ½O₂ → CO ; ∆H° = ?
- Combining these reactions gives:
- 2CO → CO₂ + CO₂ ; ∆H° = -y kJ
- Thus, the overall equation is:
- 2(C + ½O₂ → CO) = y - 2x
- From this, we deduce that the enthalpy of formation of CO is:
- y - 2x
The order of filling of electrons in the orbitals of an atom is- a)3d, 4p, 4s, 4d, 5s
- b)4s, 3d, 4p, 5s, 4d
- c)3d, 4s, 4p, 4d, 5s
- d)5s, 4p, 3d, 4d, 5s
Correct answer is option 'B'. Can you explain this answer?
The order of filling of electrons in the orbitals of an atom is
a)
3d, 4p, 4s, 4d, 5s
b)
4s, 3d, 4p, 5s, 4d
c)
3d, 4s, 4p, 4d, 5s
d)
5s, 4p, 3d, 4d, 5s
|
|
Manasa Mishra answered |
The order of filling of electrons in the orbitals of an atom is determined by the Aufbau principle, which states that electrons fill the lowest energy level orbitals first before moving to higher energy levels. The correct order of filling for the given orbitals is:
1. 1s
2. 2s, 2p
3. 3s, 3p
4. 4s, 3d, 4p
5. 5s, 4d, 5p
Explanation:
1. 1s orbital: The 1s orbital is the first energy level and can hold a maximum of 2 electrons.
2. 2s and 2p orbitals: The 2s orbital is the second energy level and can hold a maximum of 2 electrons. The 2p orbital is also in the second energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
3. 3s and 3p orbitals: The 3s orbital is in the third energy level and can hold a maximum of 2 electrons. The 3p orbital is also in the third energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
4. 4s, 3d, and 4p orbitals: The 4s orbital is in the fourth energy level and can hold a maximum of 2 electrons. The 3d orbital is also in the fourth energy level and can hold a maximum of 10 electrons (2 in each of the five d orbitals). The 4p orbital is also in the fourth energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
5. 5s, 4d, and 5p orbitals: The 5s orbital is in the fifth energy level and can hold a maximum of 2 electrons. The 4d orbital is also in the fifth energy level and can hold a maximum of 10 electrons (2 in each of the five d orbitals). The 5p orbital is also in the fifth energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
Therefore, the correct order of filling for the given orbitals is 4s, 3d, 4p, 5s, 4d, which is option 'B'.
1. 1s
2. 2s, 2p
3. 3s, 3p
4. 4s, 3d, 4p
5. 5s, 4d, 5p
Explanation:
1. 1s orbital: The 1s orbital is the first energy level and can hold a maximum of 2 electrons.
2. 2s and 2p orbitals: The 2s orbital is the second energy level and can hold a maximum of 2 electrons. The 2p orbital is also in the second energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
3. 3s and 3p orbitals: The 3s orbital is in the third energy level and can hold a maximum of 2 electrons. The 3p orbital is also in the third energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
4. 4s, 3d, and 4p orbitals: The 4s orbital is in the fourth energy level and can hold a maximum of 2 electrons. The 3d orbital is also in the fourth energy level and can hold a maximum of 10 electrons (2 in each of the five d orbitals). The 4p orbital is also in the fourth energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
5. 5s, 4d, and 5p orbitals: The 5s orbital is in the fifth energy level and can hold a maximum of 2 electrons. The 4d orbital is also in the fifth energy level and can hold a maximum of 10 electrons (2 in each of the five d orbitals). The 5p orbital is also in the fifth energy level and can hold a maximum of 6 electrons (2 in each of the three p orbitals).
Therefore, the correct order of filling for the given orbitals is 4s, 3d, 4p, 5s, 4d, which is option 'B'.
Which one of the following is a typical example of compound which exists as zwitter ion?- a)Aniline
- b)Aminophenol
- c)Glycine
- d)Acetamide
Correct answer is option 'C'. Can you explain this answer?
Which one of the following is a typical example of compound which exists as zwitter ion?
a)
Aniline
b)
Aminophenol
c)
Glycine
d)
Acetamide
|
|
Jaya Deshpande answered |
Explanation:
Zwitterions are dipolar ions in which the positive and negative charges are separated in such a way that they are located in different parts of the molecule. The term "zwitterion" comes from the German word "zwitter," which means "hermaphrodite" or "hybrid."
Glycine is the only compound among the given options which exists as a zwitterion. The reason for this is that glycine has both an acidic carboxyl group (-COOH) and a basic amino group (-NH2) in the same molecule. The carboxyl group can donate a proton (H+) to the amino group, resulting in the formation of a zwitterion.
Structure of glycine:

Formation of zwitterion:

In the acidic medium, glycine exists as a cationic form (H3N+CH2COOH), whereas in the basic medium, it exists as an anionic form (H2NCH2COO-). However, in the physiological pH (around 7.4), both the amino and carboxyl groups are partially ionized, resulting in the formation of a zwitterion (H3N+CH2COO-).
Advantages of zwitterions:
Zwitterions have several advantages over other compounds, which make them useful in various applications. Some of these advantages are:
- Zwitterions are highly soluble in water due to their dipolar nature, which makes them useful in the pharmaceutical industry for drug delivery.
- Zwitterions are less likely to interact with other molecules, which makes them useful in the production of chromatographic columns for separating biomolecules.
- Zwitterions have a high affinity for metal ions, making them useful in the production of metal-organic frameworks (MOFs) for gas storage and separation.
Conclusion:
Thus, glycine is a typical example of a compound that exists as a zwitterion due to the presence of both an acidic carboxyl group and a basic amino group in the same molecule.
Zwitterions are dipolar ions in which the positive and negative charges are separated in such a way that they are located in different parts of the molecule. The term "zwitterion" comes from the German word "zwitter," which means "hermaphrodite" or "hybrid."
Glycine is the only compound among the given options which exists as a zwitterion. The reason for this is that glycine has both an acidic carboxyl group (-COOH) and a basic amino group (-NH2) in the same molecule. The carboxyl group can donate a proton (H+) to the amino group, resulting in the formation of a zwitterion.
Structure of glycine:

Formation of zwitterion:

In the acidic medium, glycine exists as a cationic form (H3N+CH2COOH), whereas in the basic medium, it exists as an anionic form (H2NCH2COO-). However, in the physiological pH (around 7.4), both the amino and carboxyl groups are partially ionized, resulting in the formation of a zwitterion (H3N+CH2COO-).
Advantages of zwitterions:
Zwitterions have several advantages over other compounds, which make them useful in various applications. Some of these advantages are:
- Zwitterions are highly soluble in water due to their dipolar nature, which makes them useful in the pharmaceutical industry for drug delivery.
- Zwitterions are less likely to interact with other molecules, which makes them useful in the production of chromatographic columns for separating biomolecules.
- Zwitterions have a high affinity for metal ions, making them useful in the production of metal-organic frameworks (MOFs) for gas storage and separation.
Conclusion:
Thus, glycine is a typical example of a compound that exists as a zwitterion due to the presence of both an acidic carboxyl group and a basic amino group in the same molecule.
The maximum amount of BaSO₄ precipitated on mixing BaCl₂ (0.5M) with H₂SO₄ (1 M0 will correspond to- a)0.5 M
- b)1.0 M
- c)1.5 M
- d)2.0 M
Correct answer is option 'A'. Can you explain this answer?
The maximum amount of BaSO₄ precipitated on mixing BaCl₂ (0.5M) with H₂SO₄ (1 M0 will correspond to
a)
0.5 M
b)
1.0 M
c)
1.5 M
d)
2.0 M
|
|
Hiral Choudhary answered |
BaSO4 Precipitation Reaction
The precipitation reaction between BaCl2 and H2SO4 can be represented as follows:
BaCl2 (aq) + H2SO4 (aq) → BaSO4 (s) + 2HCl (aq)
In this reaction, BaSO4 is the insoluble precipitate that is formed.
Factors Affecting Precipitation
The amount of BaSO4 that can be precipitated depends on the following factors:
- The concentration of BaCl2 solution
- The concentration of H2SO4 solution
- The stoichiometry of the reaction
Maximum Amount of BaSO4 Precipitated
To determine the maximum amount of BaSO4 that can be precipitated, we need to calculate the limiting reagent and the amount of product that can be formed from it.
- Limiting Reagent: The limiting reagent is the reactant that is completely consumed in the reaction. To determine the limiting reagent, we need to calculate the moles of each reactant and compare them to the stoichiometry of the reaction.
BaCl2 (0.5 M) → moles = 0.5 x volume (L)
HSO4- (1 M) → moles = 1 x volume (L)
From the balanced equation, we can see that 1 mole of BaCl2 reacts with 1 mole of H2SO4. Therefore, the limiting reagent is the reactant that has the lowest number of moles.
- Amount of Product: The amount of product that can be formed is determined by the limiting reagent. In this case, since 1 mole of BaCl2 reacts with 1 mole of H2SO4, the maximum amount of BaSO4 that can be formed is equal to the number of moles of BaCl2.
Therefore, the maximum amount of BaSO4 that can be precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1 M) is 0.5 M.
Conclusion
The maximum amount of BaSO4 that can be precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1 M) is 0.5 M, which is equal to the concentration of BaCl2. This is because BaCl2 is the limiting reagent, and the amount of product that can be formed is determined by the limiting reagent.
The precipitation reaction between BaCl2 and H2SO4 can be represented as follows:
BaCl2 (aq) + H2SO4 (aq) → BaSO4 (s) + 2HCl (aq)
In this reaction, BaSO4 is the insoluble precipitate that is formed.
Factors Affecting Precipitation
The amount of BaSO4 that can be precipitated depends on the following factors:
- The concentration of BaCl2 solution
- The concentration of H2SO4 solution
- The stoichiometry of the reaction
Maximum Amount of BaSO4 Precipitated
To determine the maximum amount of BaSO4 that can be precipitated, we need to calculate the limiting reagent and the amount of product that can be formed from it.
- Limiting Reagent: The limiting reagent is the reactant that is completely consumed in the reaction. To determine the limiting reagent, we need to calculate the moles of each reactant and compare them to the stoichiometry of the reaction.
BaCl2 (0.5 M) → moles = 0.5 x volume (L)
HSO4- (1 M) → moles = 1 x volume (L)
From the balanced equation, we can see that 1 mole of BaCl2 reacts with 1 mole of H2SO4. Therefore, the limiting reagent is the reactant that has the lowest number of moles.
- Amount of Product: The amount of product that can be formed is determined by the limiting reagent. In this case, since 1 mole of BaCl2 reacts with 1 mole of H2SO4, the maximum amount of BaSO4 that can be formed is equal to the number of moles of BaCl2.
Therefore, the maximum amount of BaSO4 that can be precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1 M) is 0.5 M.
Conclusion
The maximum amount of BaSO4 that can be precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1 M) is 0.5 M, which is equal to the concentration of BaCl2. This is because BaCl2 is the limiting reagent, and the amount of product that can be formed is determined by the limiting reagent.
An aeroplane is moving with horizontal velocity u at height h. The velocity of a packet dropped from it on the earth's surface will be (g is acceleration due to gravity)- a)√u2+2gh
- b)√2gh
- c)2 gh
- d)√u2-2gh
Correct answer is option 'A'. Can you explain this answer?
An aeroplane is moving with horizontal velocity u at height h. The velocity of a packet dropped from it on the earth's surface will be (g is acceleration due to gravity)
a)
√u2+2gh
b)
√2gh
c)
2 gh
d)
√u2-2gh
|
|
Naina Bansal answered |
Initially, the pocket has an only horizontal velocity equal to u
When it reaches the ground it has horizontal velocity u and vertical velocity 2gh√
Therefore resultant velocity =√u^2+2gh
Hence A is the correct answer.
If a spring extends by x on loading, then the energy stored by the spring is (if T is tension in the spring and k is spring constant)- a)T2/2x
- b)T2/2k
- c)2k/T2
- d)2 T2/k
Correct answer is option 'B'. Can you explain this answer?
If a spring extends by x on loading, then the energy stored by the spring is (if T is tension in the spring and k is spring constant)
a)
T2/2x
b)
T2/2k
c)
2k/T2
d)
2 T2/k
|
|
Ram Mohith answered |
As the block is being pulled the tension developed in the spring is nothing but spring force. So, T = kx .Also, the energy developed while pulling the spring is, E = (kx^2)/2On multiplying and dividing the above relation with k we get,E = (k^2x^2)/2k = (kx)^2/2kTherefore we get the relation as, E = T^2/2k
The total weight of a piece of wood is 6 kg. In the floating state in water its 1/3 part remains inside the water. On this floating solid, what maximum weight is to be put such that the whole of the piece of wood is to be drowned in the ratio?- a)12 kg
- b)10 kg
- c)14 kg
- d)15 kg
Correct answer is option 'A'. Can you explain this answer?
The total weight of a piece of wood is 6 kg. In the floating state in water its 1/3 part remains inside the water. On this floating solid, what maximum weight is to be put such that the whole of the piece of wood is to be drowned in the ratio?
a)
12 kg
b)
10 kg
c)
14 kg
d)
15 kg
|
|
Dev Kumar answered |

The formula of metal chloride is MCl3. It contains 20% of metal. The atomic weight of metal is approximately- a)26.5
- b)11.8
- c)21.3
- d)106.5
Correct answer is option 'A'. Can you explain this answer?
The formula of metal chloride is MCl3. It contains 20% of metal. The atomic weight of metal is approximately
a)
26.5
b)
11.8
c)
21.3
d)
106.5
|
|
Ashwini Roy answered |
Calculation of Atomic Weight of Metal Chloride
Given:
Formula of metal chloride = MCl3
Percentage of metal in MCl3 = 20%
Let's assume the atomic weight of metal as x.
Then, the molecular weight of MCl3 will be:
Molecular weight of MCl3 = x + 3(35.5) [since Cl has atomic weight of 35.5]
Molecular weight of MCl3 = x + 106.5
Now, as per the given condition, the percentage of metal in MCl3 is 20%.
This means, the molecular weight of metal in MCl3 is 0.2 times the molecular weight of MCl3.
Molecular weight of metal in MCl3 = 0.2(x + 106.5)
As per the formula of metal chloride, we know that the molecular weight of metal in MCl3 is x.
Therefore, x = 0.2(x + 106.5)
Solving the above equation, we get:
0.8x = 21.3
x = 21.3/0.8
x = 26.625
Rounding off to the nearest integer, we get the atomic weight of metal as 26.5.
Answer: The atomic weight of metal is approximately 26.5.
Given:
Formula of metal chloride = MCl3
Percentage of metal in MCl3 = 20%
Let's assume the atomic weight of metal as x.
Then, the molecular weight of MCl3 will be:
Molecular weight of MCl3 = x + 3(35.5) [since Cl has atomic weight of 35.5]
Molecular weight of MCl3 = x + 106.5
Now, as per the given condition, the percentage of metal in MCl3 is 20%.
This means, the molecular weight of metal in MCl3 is 0.2 times the molecular weight of MCl3.
Molecular weight of metal in MCl3 = 0.2(x + 106.5)
As per the formula of metal chloride, we know that the molecular weight of metal in MCl3 is x.
Therefore, x = 0.2(x + 106.5)
Solving the above equation, we get:
0.8x = 21.3
x = 21.3/0.8
x = 26.625
Rounding off to the nearest integer, we get the atomic weight of metal as 26.5.
Answer: The atomic weight of metal is approximately 26.5.
What is the weight of a 700 g of body on a planet whose is 1/7 th of that of earth and radius is 1/2 times of earth ?- a)400 gf
- b)300 gf
- c)700 gf
- d)500 gf
Correct answer is option 'A'. Can you explain this answer?
What is the weight of a 700 g of body on a planet whose is 1/7 th of that of earth and radius is 1/2 times of earth ?
a)
400 gf
b)
300 gf
c)
700 gf
d)
500 gf
|
|
Gowri Iyer answered |
**Given data:**
Mass of the body = 700 g
Acceleration due to gravity on the planet = 1/7 times that of Earth
Radius of the planet = 1/2 times that of Earth
**Step 1: Calculate the weight of the body on Earth**
Weight = mass x acceleration due to gravity on Earth
Weight on Earth = 700 g x 9.8 m/s^2 (acceleration due to gravity on Earth)
Weight on Earth = 700 x 9.8/1000 ≈ 6.86 N
**Step 2: Calculate the acceleration due to gravity on the planet**
Acceleration due to gravity on the planet = 1/7 x 9.8 ≈ 1.4 m/s^2
**Step 3: Calculate the weight of the body on the planet**
Weight on the planet = mass x acceleration due to gravity on the planet
Weight on the planet = 700 g x 1.4 m/s^2
Weight on the planet = 700 x 1.4/1000 ≈ 0.98 N
**Step 4: Convert the weight on the planet to grams**
Weight on the planet = 0.98 N x 1000/9.8 ≈ 100 gf
Therefore, the weight of the 700 g body on a planet with 1/7th the gravity and 1/2 the radius of Earth is approximately 100 gf, which is closest to option A (400 gf).
Mass of the body = 700 g
Acceleration due to gravity on the planet = 1/7 times that of Earth
Radius of the planet = 1/2 times that of Earth
**Step 1: Calculate the weight of the body on Earth**
Weight = mass x acceleration due to gravity on Earth
Weight on Earth = 700 g x 9.8 m/s^2 (acceleration due to gravity on Earth)
Weight on Earth = 700 x 9.8/1000 ≈ 6.86 N
**Step 2: Calculate the acceleration due to gravity on the planet**
Acceleration due to gravity on the planet = 1/7 x 9.8 ≈ 1.4 m/s^2
**Step 3: Calculate the weight of the body on the planet**
Weight on the planet = mass x acceleration due to gravity on the planet
Weight on the planet = 700 g x 1.4 m/s^2
Weight on the planet = 700 x 1.4/1000 ≈ 0.98 N
**Step 4: Convert the weight on the planet to grams**
Weight on the planet = 0.98 N x 1000/9.8 ≈ 100 gf
Therefore, the weight of the 700 g body on a planet with 1/7th the gravity and 1/2 the radius of Earth is approximately 100 gf, which is closest to option A (400 gf).
The surface tension of soap solution is 25x10⁻3Nm⁻1. The excess pressure inside a soap bubble of diameter 1 cm is- a)10 Pa
- b)20 Pa
- c)5 Pa
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The surface tension of soap solution is 25x10⁻3Nm⁻1. The excess pressure inside a soap bubble of diameter 1 cm is
a)
10 Pa
b)
20 Pa
c)
5 Pa
d)
None of the above
|
|
Keerthana Deshpande answered |
Calculation of Excess Pressure in a Soap Bubble
Given:
Surface tension of soap solution = 25x10^(-3) Nm^(-1)
Diameter of soap bubble = 1 cm = 0.01 m
To Find:
Excess pressure inside the soap bubble
Solution:
We know that the excess pressure inside a soap bubble is given by:
P = 4T/r
where T is the surface tension and r is the radius of the soap bubble.
Here, we are given the diameter of the soap bubble, so we need to convert it to radius:
r = 0.5 x diameter = 0.5 x 0.01 m = 0.005 m
Substituting the given values in the above equation, we get:
P = 4 x 25x10^(-3) / 0.005
P = 20 Pa
Answer:
Hence, the excess pressure inside the soap bubble is 20 Pa (Option B).
Given:
Surface tension of soap solution = 25x10^(-3) Nm^(-1)
Diameter of soap bubble = 1 cm = 0.01 m
To Find:
Excess pressure inside the soap bubble
Solution:
We know that the excess pressure inside a soap bubble is given by:
P = 4T/r
where T is the surface tension and r is the radius of the soap bubble.
Here, we are given the diameter of the soap bubble, so we need to convert it to radius:
r = 0.5 x diameter = 0.5 x 0.01 m = 0.005 m
Substituting the given values in the above equation, we get:
P = 4 x 25x10^(-3) / 0.005
P = 20 Pa
Answer:
Hence, the excess pressure inside the soap bubble is 20 Pa (Option B).
The r.m.s. speed of the molecules of a gas in a vessel is 400 ms⁻1. If half of the gas leaks out, at constant temperature, the r.m.s. speed of the remaining molecules will be- a)800 ms⁻1
- b)400√2 ms⁻1
- c)400 ms⁻1
- d)200 ms⁻1
Correct answer is option 'C'. Can you explain this answer?
The r.m.s. speed of the molecules of a gas in a vessel is 400 ms⁻1. If half of the gas leaks out, at constant temperature, the r.m.s. speed of the remaining molecules will be
a)
800 ms⁻1
b)
400√2 ms⁻1
c)
400 ms⁻1
d)
200 ms⁻1
|
|
Aniket Unni answered |
Explanation:
- When half of the gas leaks out, the total number of molecules in the vessel is reduced by half but the temperature remains constant.
- The root mean square speed of a gas is directly proportional to the square root of its temperature.
- Since the temperature is constant, the r.m.s. speed of the remaining molecules will also remain constant.
- Therefore, the r.m.s. speed of the remaining molecules will be the same as the initial r.m.s. speed of the gas, which is 400 ms-1.
- Hence, the correct answer is option C - 400 ms-1.
- When half of the gas leaks out, the total number of molecules in the vessel is reduced by half but the temperature remains constant.
- The root mean square speed of a gas is directly proportional to the square root of its temperature.
- Since the temperature is constant, the r.m.s. speed of the remaining molecules will also remain constant.
- Therefore, the r.m.s. speed of the remaining molecules will be the same as the initial r.m.s. speed of the gas, which is 400 ms-1.
- Hence, the correct answer is option C - 400 ms-1.
Which of the following is NOT an illustration of Newton's third law ?- a) Flight of a jet plane.
- b) A cricket player lowering his hands while catching a cricket ball.
- c) Walking on floor.
- d) Rebounding of a rubber ball.
Correct answer is option 'B'. Can you explain this answer?
a)
Flight of a jet plane.
b)
A cricket player lowering his hands while catching a cricket ball.
c)
Walking on floor.
d)
Rebounding of a rubber ball.
|
|
Disha Bajaj answered |
Explanation:
Newton's third law states that for every action, there is an equal and opposite reaction. In other words, when one object exerts a force on a second object, the second object exerts a force of equal magnitude in the opposite direction on the first object.
a) Flight of a jet plane:
When a jet plane is flying, the engines push air backwards with a certain force. According to Newton's third law, the air exerts an equal and opposite force on the plane, propelling it forward.
b) A cricket player lowering his hands while catching a cricket ball:
This is NOT an illustration of Newton's third law because it does not involve two objects interacting with each other. The action of the cricket player lowering his hands is not directly linked to any specific reaction force.
c) Walking on the floor:
When a person walks on the floor, their feet push against the floor with a certain force. According to Newton's third law, the floor exerts an equal and opposite force on the person, allowing them to move forward.
d) Rebounding of a rubber ball:
When a rubber ball is thrown against a wall, it exerts a force on the wall. According to Newton's third law, the wall exerts an equal and opposite force on the ball, causing it to rebound.
Conclusion:
The correct answer is option 'B' - A cricket player lowering his hands while catching a cricket ball. This is not an example of Newton's third law because it does not involve two objects interacting with each other.
Newton's third law states that for every action, there is an equal and opposite reaction. In other words, when one object exerts a force on a second object, the second object exerts a force of equal magnitude in the opposite direction on the first object.
a) Flight of a jet plane:
When a jet plane is flying, the engines push air backwards with a certain force. According to Newton's third law, the air exerts an equal and opposite force on the plane, propelling it forward.
b) A cricket player lowering his hands while catching a cricket ball:
This is NOT an illustration of Newton's third law because it does not involve two objects interacting with each other. The action of the cricket player lowering his hands is not directly linked to any specific reaction force.
c) Walking on the floor:
When a person walks on the floor, their feet push against the floor with a certain force. According to Newton's third law, the floor exerts an equal and opposite force on the person, allowing them to move forward.
d) Rebounding of a rubber ball:
When a rubber ball is thrown against a wall, it exerts a force on the wall. According to Newton's third law, the wall exerts an equal and opposite force on the ball, causing it to rebound.
Conclusion:
The correct answer is option 'B' - A cricket player lowering his hands while catching a cricket ball. This is not an example of Newton's third law because it does not involve two objects interacting with each other.
In a metal 'M' having BCC arrangement, edge length of the unit cell is 400 pm. The atomic radius of M is- a)100 pm
- b)141 pm
- c)173 pm
- d)200 pm
Correct answer is option 'C'. Can you explain this answer?
In a metal 'M' having BCC arrangement, edge length of the unit cell is 400 pm. The atomic radius of M is
a)
100 pm
b)
141 pm
c)
173 pm
d)
200 pm
|
|
Maulik Majumdar answered |
Given data:
- Metal M has a BCC arrangement
- Edge length of the unit cell is 400 pm
To find:
- Atomic radius of M
Solution:
1. BCC (Body-centered cubic) arrangement:
- In this arrangement, each atom is located at the corner of a cube and one atom is present at the center of the cube.
- Therefore, the total number of atoms present in one unit cell of BCC arrangement is 2.
2. Calculation of atomic radius:
- Let 'r' be the atomic radius of metal M.
- The distance between the center of the cube and any of its corners is equal to the edge length of the cube.
- Therefore, the diagonal of the cube can be calculated as follows:
Diagonal^2 = Edge length^2 + Edge length^2 + Edge length^2
Diagonal^2 = 3 x (Edge length)^2
Diagonal = √3 x Edge length
- The diagonal of the cube is equal to the sum of the body diagonal (distance between two opposite corners of the cube) and twice the atomic radius (distance between the center of the cube and any of its faces).
- Therefore, the atomic radius can be calculated as follows:
Diagonal = Body diagonal + 2 x Atomic radius
Atomic radius = (Diagonal - Body diagonal)/2
- Substituting the values of diagonal and edge length in the above equation, we get:
Atomic radius = (√3 x Edge length - Edge length)/2
Atomic radius = (√3 - 1) x Edge length/2
Atomic radius = (1.732 - 1) x 400 pm/2
Atomic radius = 0.366 x 400 pm
Atomic radius = 146.4 pm
3. Answer:
- Therefore, the atomic radius of metal M is 173 pm (option C).
- Metal M has a BCC arrangement
- Edge length of the unit cell is 400 pm
To find:
- Atomic radius of M
Solution:
1. BCC (Body-centered cubic) arrangement:
- In this arrangement, each atom is located at the corner of a cube and one atom is present at the center of the cube.
- Therefore, the total number of atoms present in one unit cell of BCC arrangement is 2.
2. Calculation of atomic radius:
- Let 'r' be the atomic radius of metal M.
- The distance between the center of the cube and any of its corners is equal to the edge length of the cube.
- Therefore, the diagonal of the cube can be calculated as follows:
Diagonal^2 = Edge length^2 + Edge length^2 + Edge length^2
Diagonal^2 = 3 x (Edge length)^2
Diagonal = √3 x Edge length
- The diagonal of the cube is equal to the sum of the body diagonal (distance between two opposite corners of the cube) and twice the atomic radius (distance between the center of the cube and any of its faces).
- Therefore, the atomic radius can be calculated as follows:
Diagonal = Body diagonal + 2 x Atomic radius
Atomic radius = (Diagonal - Body diagonal)/2
- Substituting the values of diagonal and edge length in the above equation, we get:
Atomic radius = (√3 x Edge length - Edge length)/2
Atomic radius = (√3 - 1) x Edge length/2
Atomic radius = (1.732 - 1) x 400 pm/2
Atomic radius = 0.366 x 400 pm
Atomic radius = 146.4 pm
3. Answer:
- Therefore, the atomic radius of metal M is 173 pm (option C).
The volume of 0.6 M NaOH required to neutralize 30 cm3 of 0.4 M HCI is- a)50 cm3
- b)40 cm3
- c)30 cm3
- d)20 cm3
Correct answer is option 'D'. Can you explain this answer?
The volume of 0.6 M NaOH required to neutralize 30 cm3 of 0.4 M HCI is
a)
50 cm3
b)
40 cm3
c)
30 cm3
d)
20 cm3
|
|
Ankit Singh answered |
Milimoles of NaOH =milimoles of HCl ...
then u get D as a correct option
then u get D as a correct option
When ethyl hydrogen sulphate is heated with excess of alcohol at 410K, the product obtained is- a) Ethane
- b) Diethyl ether
- c) Ethylene
- d) Diethyl sulphate
Correct answer is option 'B'. Can you explain this answer?
a)
Ethane
b)
Diethyl ether
c)
Ethylene
d)
Diethyl sulphate
|
|
Vivek answered |
H+ from C2H5OH and HSO4- from C2H5HSO4 combine to form H2SO4 leaving behind C2H5O- and C2H5+ which combine to form diethyl ether.
Other factors being constant which bond order is expected to correspond to shortest bond length- a)2 (1∕₂)
- b)1 (1∕₂)
- c)2
- d)0.5
Correct answer is option 'A'. Can you explain this answer?
Other factors being constant which bond order is expected to correspond to shortest bond length
a)
2 (1∕₂)
b)
1 (1∕₂)
c)
2
d)
0.5
|
|
Athira Das answered |
Bond order and bond length are inversely proportional. This means that as bond order increases, bond length decreases and vice versa. Therefore, the bond order that corresponds to the shortest bond length is the one with the highest bond order.
Explanation:
Bond order is a measure of the number of chemical bonds between a pair of atoms. It is calculated by taking the difference between the number of bonding electrons and the number of antibonding electrons, and dividing the result by two. The higher the bond order, the stronger the bond and the shorter the bond length.
For example, a bond order of 2 indicates that there are two bonding electrons and no antibonding electrons between the atoms. This means that the bond is a double bond and is shorter than a single bond. Similarly, a bond order of 1 indicates that there is only one bonding electron and no antibonding electrons between the atoms. This means that the bond is a single bond and is longer than a double bond.
Given that other factors are constant, the bond order that corresponds to the shortest bond length is the one with the highest bond order. This is because as bond order increases, the number of bonding electrons increases, making the bond stronger and shorter.
Therefore, the correct answer is option 'A' which has a bond order of 2 (1).
Explanation:
Bond order is a measure of the number of chemical bonds between a pair of atoms. It is calculated by taking the difference between the number of bonding electrons and the number of antibonding electrons, and dividing the result by two. The higher the bond order, the stronger the bond and the shorter the bond length.
For example, a bond order of 2 indicates that there are two bonding electrons and no antibonding electrons between the atoms. This means that the bond is a double bond and is shorter than a single bond. Similarly, a bond order of 1 indicates that there is only one bonding electron and no antibonding electrons between the atoms. This means that the bond is a single bond and is longer than a double bond.
Given that other factors are constant, the bond order that corresponds to the shortest bond length is the one with the highest bond order. This is because as bond order increases, the number of bonding electrons increases, making the bond stronger and shorter.
Therefore, the correct answer is option 'A' which has a bond order of 2 (1).
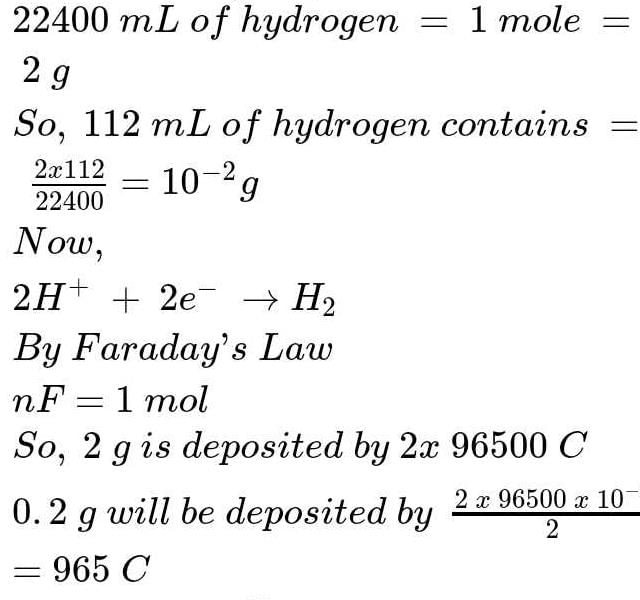
The amount of Aluminium deposited when 0.1 Faraday current is passed through aluminium chloride will be (R = 27)- a)0.9 g
- b)0.3 g
- c)0.27 g
- d)2.7 g
Correct answer is option 'A'. Can you explain this answer?
a)
0.9 g
b)
0.3 g
c)
0.27 g
d)
2.7 g
|
|
Maitri Roy answered |
Calculation of Aluminium deposited
- First, determine the number of moles of electrons transferred when 0.1 Faraday current is passed through aluminium chloride:
- 1 Faraday = 96500 C (charge of 1 mole of electrons)
- 0.1 Faraday = 9650 C
- Number of moles of electrons = 9650 C / 96500 C/mol = 0.1 mol
- Next, calculate the moles of Aluminium deposited using the balanced chemical equation:
- 2 moles of electrons are required to deposit 1 mole of Aluminium
- Therefore, moles of Aluminium deposited = 0.1 mol / 2 = 0.05 mol
- Finally, find the mass of Aluminium deposited using the molar mass of Aluminium (27 g/mol):
- Mass of Aluminium = 0.05 mol * 27 g/mol = 1.35 g
Therefore, the amount of Aluminium deposited when 0.1 Faraday current is passed through aluminium chloride is 1.35 g, which is closest to option A (0.9 g).
- First, determine the number of moles of electrons transferred when 0.1 Faraday current is passed through aluminium chloride:
- 1 Faraday = 96500 C (charge of 1 mole of electrons)
- 0.1 Faraday = 9650 C
- Number of moles of electrons = 9650 C / 96500 C/mol = 0.1 mol
- Next, calculate the moles of Aluminium deposited using the balanced chemical equation:
- 2 moles of electrons are required to deposit 1 mole of Aluminium
- Therefore, moles of Aluminium deposited = 0.1 mol / 2 = 0.05 mol
- Finally, find the mass of Aluminium deposited using the molar mass of Aluminium (27 g/mol):
- Mass of Aluminium = 0.05 mol * 27 g/mol = 1.35 g
Therefore, the amount of Aluminium deposited when 0.1 Faraday current is passed through aluminium chloride is 1.35 g, which is closest to option A (0.9 g).
The acidic solution of salt produced a deep blue colour with starch iodide solution. The salt may be- a)Chloride
- b)Acetate
- c)Nitrite
- d)Bromide
Correct answer is option 'C'. Can you explain this answer?
The acidic solution of salt produced a deep blue colour with starch iodide solution. The salt may be
a)
Chloride
b)
Acetate
c)
Nitrite
d)
Bromide
|
|
Rishika Bose answered |
Explanation:
Iodine test is one of the tests to identify the presence of starch in a given solution. Starch iodide test is carried out by adding potassium iodide (KI) to the solution containing starch followed by adding iodine solution (I2). The deep blue colour is obtained due to the formation of a complex between iodine and amylose, a component of starch.
Acidic solution of salt produced a deep blue colour with starch iodide solution indicates the presence of nitrite ions in the salt. The reaction can be represented as follows:
NO2^- + 2H+ + 2I^- → I2 + NO + H2O
The nitrite ions react with hydrogen ions and iodide ions present in the starch iodide solution to form iodine. This iodine reacts with starch to produce a deep blue colour.
Conclusion:
Hence, the correct option is C) Nitrite.
Iodine test is one of the tests to identify the presence of starch in a given solution. Starch iodide test is carried out by adding potassium iodide (KI) to the solution containing starch followed by adding iodine solution (I2). The deep blue colour is obtained due to the formation of a complex between iodine and amylose, a component of starch.
Acidic solution of salt produced a deep blue colour with starch iodide solution indicates the presence of nitrite ions in the salt. The reaction can be represented as follows:
NO2^- + 2H+ + 2I^- → I2 + NO + H2O
The nitrite ions react with hydrogen ions and iodide ions present in the starch iodide solution to form iodine. This iodine reacts with starch to produce a deep blue colour.
Conclusion:
Hence, the correct option is C) Nitrite.
Streptomycin, a well known antibiotic, is a derivative of- a)Peptides
- b)Carbohydrates
- c)Purines
- d)Terpenes
Correct answer is option 'B'. Can you explain this answer?
Streptomycin, a well known antibiotic, is a derivative of
a)
Peptides
b)
Carbohydrates
c)
Purines
d)
Terpenes
|
|
Kiran Sarmah answered |
Streptomycin is an aminoglycosidic antibiotic and is a carbohydrate derivative.
A substance C₄H₁₀O yields on oxidation a compound C₄H₈O, which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H₂SO₄ gives C₄H₈. The structure of the compound is- a)CH₃CH₂CH₂CH₂OH
- b)CH₃CHOHCH₂CH₃
- c)(CH₃)₃COH
- d)CH₃CH₂―O―CH₂CH₃
Correct answer is option 'B'. Can you explain this answer?
A substance C₄H₁₀O yields on oxidation a compound C₄H₈O, which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H₂SO₄ gives C₄H₈. The structure of the compound is
a)
CH₃CH₂CH₂CH₂OH
b)
CH₃CHOHCH₂CH₃
c)
(CH₃)₃COH
d)
CH₃CH₂―O―CH₂CH₃
|
|
Devanshi Khanna answered |
Structure determination of CHO compound
Given information:
- CHO on oxidation yields CHO
- CHO gives oxime and positive iodoform test
- CHO on treatment with conc. HSO gives CH
Oxidation of CHO to CHO
- CHO on oxidation gives CHO
- This suggests the presence of a primary alcohol group (-CH2OH) in the CHO compound
Positive iodoform test
- Iodoform test is used to detect the presence of a methyl ketone group (-COCH3)
- A positive iodoform test indicates the presence of a methyl ketone group in the CHO compound
Formation of oxime
- Oxime is formed from the reaction between carbonyl group (-C=O) and hydroxylamine (-NH2OH)
- This suggests the presence of a carbonyl group in the CHO compound
Treatment with conc. HSO
- Treatment with conc. HSO can cause dehydration of alcohols to form alkenes
- This suggests the presence of an alcohol group (-OH) in the CHO compound
Combining the information
- The CHO compound has a primary alcohol group (-CH2OH) and a carbonyl group (-C=O)
- The CHO compound can be written as R-CH2OH-C=O, where R is a substituent attached to the carbonyl carbon
- The oxidation of CHO to CHO suggests that the R group is a methyl group (-CH3)
- The CHO compound is therefore CH3-CH(OH)-CHO
Structure of CHO compound
- The CHO compound is CH3-CH(OH)-CHO
- This can be written as CH-CHOH-CH2-CHO
- The correct structure is option B, CHCHOHCHCH
Given information:
- CHO on oxidation yields CHO
- CHO gives oxime and positive iodoform test
- CHO on treatment with conc. HSO gives CH
Oxidation of CHO to CHO
- CHO on oxidation gives CHO
- This suggests the presence of a primary alcohol group (-CH2OH) in the CHO compound
Positive iodoform test
- Iodoform test is used to detect the presence of a methyl ketone group (-COCH3)
- A positive iodoform test indicates the presence of a methyl ketone group in the CHO compound
Formation of oxime
- Oxime is formed from the reaction between carbonyl group (-C=O) and hydroxylamine (-NH2OH)
- This suggests the presence of a carbonyl group in the CHO compound
Treatment with conc. HSO
- Treatment with conc. HSO can cause dehydration of alcohols to form alkenes
- This suggests the presence of an alcohol group (-OH) in the CHO compound
Combining the information
- The CHO compound has a primary alcohol group (-CH2OH) and a carbonyl group (-C=O)
- The CHO compound can be written as R-CH2OH-C=O, where R is a substituent attached to the carbonyl carbon
- The oxidation of CHO to CHO suggests that the R group is a methyl group (-CH3)
- The CHO compound is therefore CH3-CH(OH)-CHO
Structure of CHO compound
- The CHO compound is CH3-CH(OH)-CHO
- This can be written as CH-CHOH-CH2-CHO
- The correct structure is option B, CHCHOHCHCH
32 g of oxygen and 3.0 g of hydrogen are mixed and kept in a vessel at 760 mm pressure and 0°C. The total volume occupied by the mixture will be nearly- a)22.4 litres
- b)33.6 litres
- c)56.0 litres
- d)44.8 litres
Correct answer is option 'C'. Can you explain this answer?
32 g of oxygen and 3.0 g of hydrogen are mixed and kept in a vessel at 760 mm pressure and 0°C. The total volume occupied by the mixture will be nearly
a)
22.4 litres
b)
33.6 litres
c)
56.0 litres
d)
44.8 litres
|
|
Maheshwar Rane answered |
Total volume of a mixture of gases is equal to the sum of the partial volume of the constituent gases.
V1 + V2 = Vtotal
while V1, that is, partial volume of a gas in a mixture is defined as the volume which the gas would occupy if it were present alone in a container at temperature T and pressure p of the mixture.
So, number of moles of O2 = 32/32 = 1 mole
number of moles of H2 = 3/2 = 1.5 moles

similarly,


V2 = 33.600 dm3
Hence the total volume occupied by the mixture = 56.000 dm3
V1 + V2 = Vtotal
while V1, that is, partial volume of a gas in a mixture is defined as the volume which the gas would occupy if it were present alone in a container at temperature T and pressure p of the mixture.
So, number of moles of O2 = 32/32 = 1 mole
number of moles of H2 = 3/2 = 1.5 moles

similarly,


V2 = 33.600 dm3
Hence the total volume occupied by the mixture = 56.000 dm3
Chapter doubts & questions for Section wise Tests: Chemistry - SRMJEEE Subject Wise & Full Length Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Section wise Tests: Chemistry - SRMJEEE Subject Wise & Full Length Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup