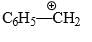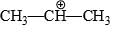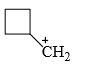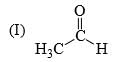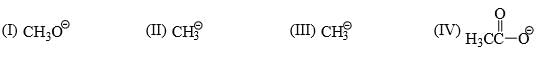All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Reaction Intermediates for Chemistry Exam
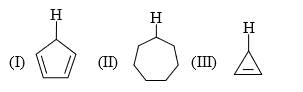
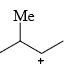
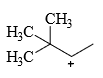
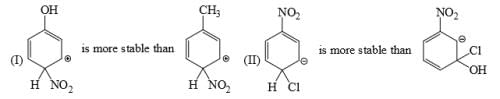
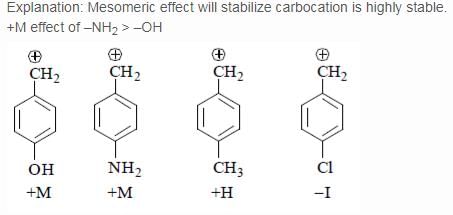
The relative stability of the following carbocations is:
- a) I > II > III
- b) II > III > I
- c) I > III > II
- d)III > II > I
Correct answer is option 'D'. Can you explain this answer?
The relative stability of the following carbocations is:


a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > II > I

|
Veda Institute answered |
Correct Answer :- d
Explanation : The dispersal of the charge stabilizes the carbocation. More the number of alkyl groups, the greater the dispersal of positive charge and therefore, more the stability of carbocation,is an electron donating group, thus it will increases the stability of carbocation, hence the expected order is, (iii)>(ii)>(i).
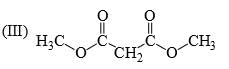
Identify correct C—O bond length order:
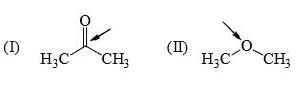
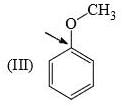
- a)I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'B'. Can you explain this answer?
Identify correct C—O bond length order:


a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Vedika Singh answered |
I is the shortest because of double bond.
III is next because of resonance.
II is last as there is no kind of bond strengthening.
Hence, B is correct.
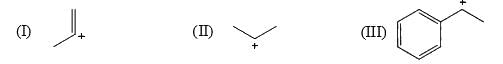
Arrange stability of the given carbocations in decreasing order:

- a)I < II < III < IV
- b) IV < III < II < I
- c)IV < II < III < I
- d)II < IV < III < I
Correct answer is option 'C'. Can you explain this answer?
Arrange stability of the given carbocations in decreasing order:


a)
I < II < III < IV
b)
IV < III < II < I
c)
IV < II < III < I
d)
II < IV < III < I

|
Veda Institute answered |
Correct Answer :- c
Explanation : The carbocation here is stabilized when there is an electron-donating group present.
More the activating effect of the group, more stable is the cation. Out of given options, I is most stable due to ability of oxygen to activate the ring with its lone pair of electrons.
Out of III and I,III is more stable as the lone pair of nitrogen in II is less available due to Ac group. IV is least stable due to electron- withdrawing effect of Cl atom.
The correct answer is : IV < II < III < I
Identify correct acidic strength order in the following compounds:
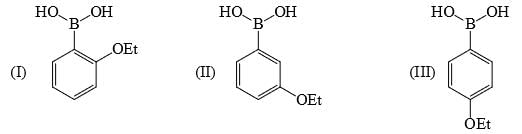
a)II > III > Ib) III > II > I c)I > II > IIId)III > I > IICorrect answer is option 'C'. Can you explain this answer?
b) III > II > I
c)I > II > III
d)III > I > II
Correct answer is option 'C'. Can you explain this answer?
|
|
Chirag Verma answered |
Correct Answer :- C
Explanation : The correct option is A. Since −OE+ which increases the acidity of the compound and is distance dependent. In I −OE+ group is nearer to the substituents. So, its more acidic than the other two. SO the correct order will be i>ii>iii.
Which is the most stable arenium carbocation:
- a)
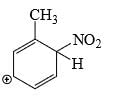
- b)
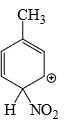
- c)
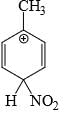
- d)
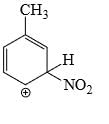
Correct answer is option 'C'. Can you explain this answer?
Which is the most stable arenium carbocation:
a)

b)

c)

d)


|
Beena Joshi answered |
No. of alpha hydrogens are more
The acidity for the following compounds increases in the order:CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH- a) I < II < III
- b) II < III < I
- c) III < II < I
- d) II < I < III
Correct answer is option 'C'. Can you explain this answer?
The acidity for the following compounds increases in the order:
CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH
a)
I < II < III
b)
II < III < I
c)
III < II < I
d)
II < I < III

|
Edurev.iitjam answered |
a. EWG ( e−− withdrawing groups) increases the acidic strength, whereas EDG ( e−− donating groups) decrease the acidic strength.
b. Nearer is the EWG to the source [(−COOH)group], stronger is the acid, i.e., α− substituted halo acid stronger than β−orγ− substituted halo acid.
Increasing order of acidic strength:
(III)<(II)<(I).
Increasing order of acidic strength:
(III)<(II)<(I).
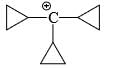
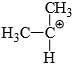
Which of the following carbonium ion is most stable?
- a)
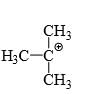
- b)
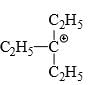
- c)
- d)
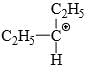
Correct answer is option 'A'. Can you explain this answer?
Which of the following carbonium ion is most stable?
a)

b)

c)

d)

|
|
Pooja Choudhury answered |
A is the correct option as 9- alpha Hydrogens are present. More alpha hydrogen, more hyperconjugation, more stability.
The relative nucleophilicity in polar, protic, solvents of the following is:
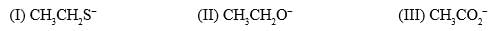
- a)I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'A'. Can you explain this answer?
The relative nucleophilicity in polar, protic, solvents of the following is:
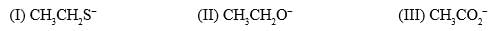
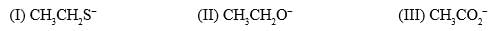
a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II

|
Veda Institute answered |
Correct Answer :- a
Explanation : In a polar protic solvent (CH3OH), nucleophilicity increases down a column of the periodic table. So S- is more nucleophilic than O-.
For two species with the same attacking atom, the more basic is the more nucleophilic, so CH3CH2O- is more nucleophilic than CH3CO2-
CO2- give identical str resonance hybrids more solvated, very less nucleophilic.
The relative acidity of the indicated H in each of the following is: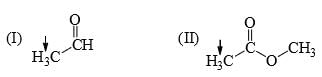
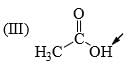
- a) I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'D'. Can you explain this answer?
The relative acidity of the indicated H in each of the following is:
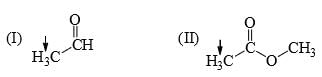

a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
In 3 the h is attached with acidic carboxyl gr
in 1 the -ve charge which generate after removing h will get stability by carbonyl gr of aldehyde
but in 2 the carbonyl gr is less available in ester gr
in 1 the -ve charge which generate after removing h will get stability by carbonyl gr of aldehyde
but in 2 the carbonyl gr is less available in ester gr
Reactivity of the following towards reaction with LiAlH4 is: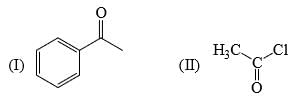
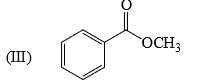
- a)II > I > III
- b) II > III > I
- c)I > III > II
- d)III > I > II
Correct answer is option 'A'. Can you explain this answer?
Reactivity of the following towards reaction with LiAlH4 is:
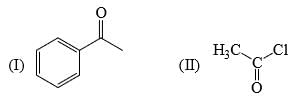

a)
II > I > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
Lah make complex with carbonyl gr
as the bulk increase the reactivity decrease
as the bulk increase the reactivity decrease
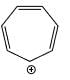
Which of the following systems are resonance contributions of the radical shown below:
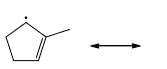
- a)
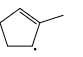
- b)
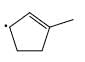
- c)
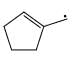
- d)
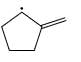
Correct answer is option 'A'. Can you explain this answer?
Which of the following systems are resonance contributions of the radical shown below:


a)

b)

c)

d)


|
Asf Institute answered |
Since, a free radical does the homolysis of pi bond.
The adjacent free radicals later form a bend.
The adjacent free radicals later form a bend.
Which intermediate is involved in the reaction given below?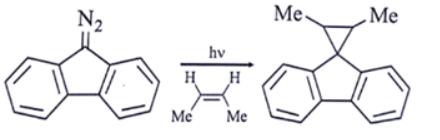
- a)free radical
- b)carbocation
- c) carbanion
- d)carbene
Correct answer is option 'D'. Can you explain this answer?
Which intermediate is involved in the reaction given below?
a)
free radical
b)
carbocation
c)
carbanion
d)
carbene

|
Edurev.iitjam answered |
Ans: d
Explanation: As we can see, the first step in the presence of light carbene is formed.
A compound 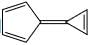 shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structures
can be used to adequately explain this observation:
- a)I only
- b) III and IV
- c) II and III
- d) IV only
Correct answer is option 'B'. Can you explain this answer?
A compound  shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structures
can be used to adequately explain this observation:
 shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structurescan be used to adequately explain this observation:

a)
I only
b)
III and IV
c)
II and III
d)
IV only
|
|
Pooja Choudhury answered |
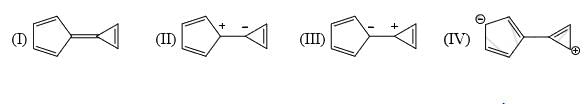
Both (IV) and (III) member ring are aromatic due to charge separation
in case of 5 member ring total 6 electron and 3 member ring 2 electron are present
according to 4n+2 rule they are aromatic
and all the carbon are sp2 hybridize
in case of 5 member ring total 6 electron and 3 member ring 2 electron are present
according to 4n+2 rule they are aromatic
and all the carbon are sp2 hybridize
Which of the following is not a resonance structure of the others:
- a)
- b)
- c)
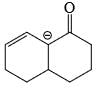
- d)
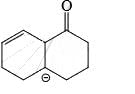
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a resonance structure of the others:
a)

b)

c)

d)

|
|
Vikram Kapoor answered |
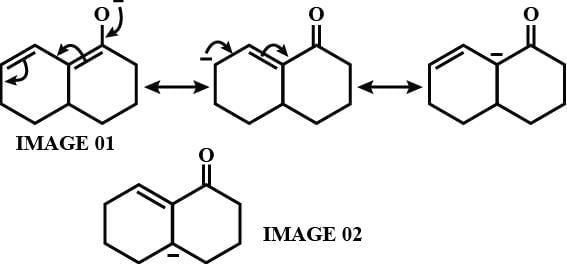
As from IMAGE 01 it is clear that here IMAGE 02 is not a resonance structure of others.
Rate of reaction of CH3COCl/AlCl3 with each of the following is: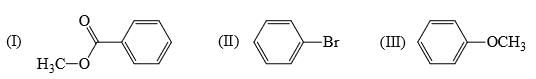
- a) III > II > I
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'A'. Can you explain this answer?
Rate of reaction of CH3COCl/AlCl3 with each of the following is:

a)
III > II > I
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
In iii the ome gr has strong +R effect
in ii the br gr has weak +R effect
in i the co gr has -R effect
hence we see the relative order iii>ii>i
in ii the br gr has weak +R effect
in i the co gr has -R effect
hence we see the relative order iii>ii>i
Which of the following is most reactive as a nucleophile?- a)PhO−
- b)PhCH2O−
- c)None of these
- d)PhS−
Correct answer is option 'D'. Can you explain this answer?
Which of the following is most reactive as a nucleophile?
a)
PhO−
b)
PhCH2O−
c)
None of these
d)
PhS−
|
|
Pooja Choudhury answered |
The most nucleophile is dependent on the compound which has a tendency to give an electron. In the compound there is oxygen and one has sulphur. The sulphur has big in size that why it has more tendency to donate an electron. The correct answer is PhS-
For the following compounds, which nitrogen is the least tendency to be protonated:
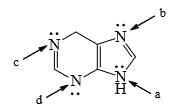
- a)Nitrogen indicated by arrow “b”
- b)Nitrogen indicated by arrow “a”
- c) Nitrogen indicated by arrow “c”
- d) Nitrogen indicated by arrow “d”
Correct answer is option 'B'. Can you explain this answer?
For the following compounds, which nitrogen is the least tendency to be protonated:


a)
Nitrogen indicated by arrow “b”
b)
Nitrogen indicated by arrow “a”
c)
Nitrogen indicated by arrow “c”
d)
Nitrogen indicated by arrow “d”

|
Himalika Pantola answered |
Because it's lone pairs are involved in conjugation and have no free bond to bound to incoming hydrogen
The acidity of the protons H in each of the following is:
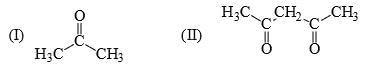
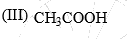
- a) III > II > I
- b) II > III > I
- c) I > III > II
- d)III > I > II
Correct answer is option 'A'. Can you explain this answer?
The acidity of the protons H in each of the following is:
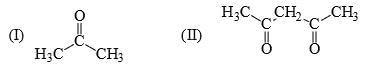
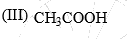
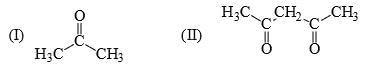
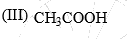
a)
III > II > I
b)
II > III > I
c)
I > III > II
d)
III > I > II

|
Akash Unplugged answered |
Ans is (a)
As we dont compare the acidity of acids with carbonyls so it is quite obvious that acetic acid will have more acidity than carbonyls.
and in ll option it is active methylene compound, which is more acidic than acetone.because in ll option there is two conjugation.
thanks,
As we dont compare the acidity of acids with carbonyls so it is quite obvious that acetic acid will have more acidity than carbonyls.
and in ll option it is active methylene compound, which is more acidic than acetone.because in ll option there is two conjugation.
thanks,
The relative nucleophilicity in polar, protic, solvents of the following is:
(I) CH3OH (II) CH3SH (III) CH3NH2
- a)I > II > III
- b) II > III > I
- c)I > III > II
- d) III > I > II
Correct answer is option 'B'. Can you explain this answer?
The relative nucleophilicity in polar, protic, solvents of the following is:
(I) CH3OH (II) CH3SH (III) CH3NH2
(I) CH3OH (II) CH3SH (III) CH3NH2
a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II

|
Aashna Shah answered |
< iii="" />< />
b)III < ii="" />< />
c)II < i="" />< />
d)I < ii="" />< />
Correct answer: d) I < ii="" />< />
Explanation:
In polar, protic solvents, nucleophilicity is generally directly proportional to basicity. The stronger the basicity of a species, the stronger its ability to donate a pair of electrons and act as a nucleophile.
Among the given species, CH3NH2 has the highest basicity due to the presence of a lone pair of electrons on the nitrogen atom. It can donate this lone pair easily and act as a strong nucleophile. Hence, it is the most nucleophilic species among the given options.
Next comes CH3SH, which is less basic than CH3NH2 but still more basic than CH3OH. Sulfur is less electronegative than oxygen, which makes the lone pair of electrons on the sulfur more available for donation. Hence, CH3SH is a stronger nucleophile than CH3OH.
Finally, CH3OH has the lowest basicity among the given options. Its lone pair of electrons is involved in hydrogen bonding with the solvent molecules, which reduces its availability for donation. Hence, it is the least nucleophilic species among the given options.
b)III < ii="" />< />
c)II < i="" />< />
d)I < ii="" />< />
Correct answer: d) I < ii="" />< />
Explanation:
In polar, protic solvents, nucleophilicity is generally directly proportional to basicity. The stronger the basicity of a species, the stronger its ability to donate a pair of electrons and act as a nucleophile.
Among the given species, CH3NH2 has the highest basicity due to the presence of a lone pair of electrons on the nitrogen atom. It can donate this lone pair easily and act as a strong nucleophile. Hence, it is the most nucleophilic species among the given options.
Next comes CH3SH, which is less basic than CH3NH2 but still more basic than CH3OH. Sulfur is less electronegative than oxygen, which makes the lone pair of electrons on the sulfur more available for donation. Hence, CH3SH is a stronger nucleophile than CH3OH.
Finally, CH3OH has the lowest basicity among the given options. Its lone pair of electrons is involved in hydrogen bonding with the solvent molecules, which reduces its availability for donation. Hence, it is the least nucleophilic species among the given options.
Tripitakas are sacred books of- a)Buddhists
- b)Hindus
- c)Jains
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Tripitakas are sacred books of
a)
Buddhists
b)
Hindus
c)
Jains
d)
None of the above
|
|
Priyanka answered |
The three pitaks are sutta pitak,vinya pitak and abhidam pitak.
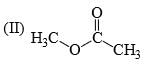
Which of the following statements is correct:
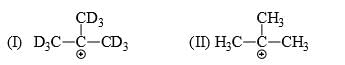
- a) I is more stable than II
- b)II is more stable than I
- c)Both are equally stable
- d) Stability criterion cannot be applied in this case
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements is correct:
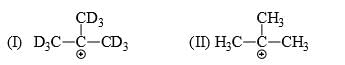
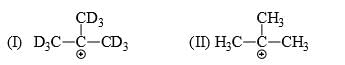
a)
I is more stable than II
b)
II is more stable than I
c)
Both are equally stable
d)
Stability criterion cannot be applied in this case

|
Adarsh Shukla answered |
Since C-D is a much stronger bond than C-H so there is more hyperconjugation in C-H bond and hence 2nd structure with 9 alpha hydrogen is more stable than 1st structure
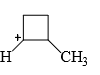
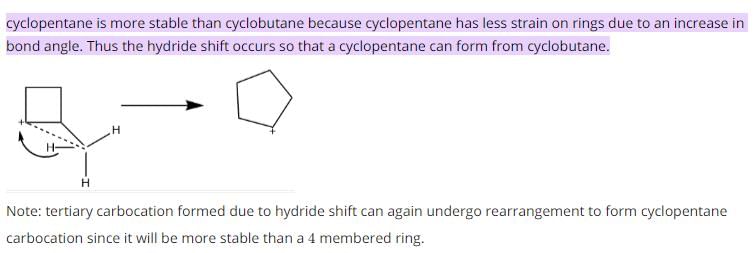
Which of the following carbocations would not likely rearrange to a more stable carbocation?
- a)
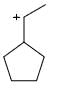
- b)
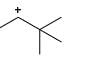
- c)
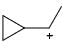
- d)
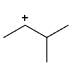
Correct answer is option 'C'. Can you explain this answer?
Which of the following carbocations would not likely rearrange to a more stable carbocation?
a)

b)

c)

d)


|
Anuradha Puri answered |
Correct option is c
because carbonation is stable because of dancing resonance
because carbonation is stable because of dancing resonance
Identify order of per ring resonance energies of each of the following:
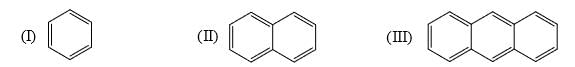
- a) I > II > III
- b) II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'A'. Can you explain this answer?
Identify order of per ring resonance energies of each of the following:


a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II

|
Subodh Sahoo answered |
Resonance energ per ring in benzene 36 kcal/mol
Naphthalene 30.5 kal/mol
Anthracene 27.7 kal /mol
Naphthalene 30.5 kal/mol
Anthracene 27.7 kal /mol
Reactivity of the following towards reaction with NaBH4 is: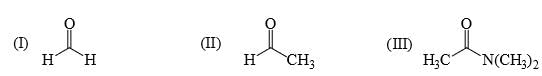
- a) I > II > III
- b)II > III > I
- c) I > III > II
- d)III > I > II
Correct answer is option 'A'. Can you explain this answer?
Reactivity of the following towards reaction with NaBH4 is:

a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
As crowding increase the rate of reduction reduce
The reaction of (+) 2-iodobutane and Nal* in acetone was studied by measuring the rate of incorporation of I* (ki) and the rate of racemisation(kr)(+) CH3CH(I)CH2CH3 + Nal* → CH3CH(I*)CH2CH3 + NalFor this reaction, the relationship between kr and ki is- a)ki = 2kr
- b)ki = (1/2)kr
- c)ki = kr
- d)ki = (1/3)kr
Correct answer is option 'B'. Can you explain this answer?
The reaction of (+) 2-iodobutane and Nal* in acetone was studied by measuring the rate of incorporation of I* (ki) and the rate of racemisation(kr)
(+) CH3CH(I)CH2CH3 + Nal* → CH3CH(I*)CH2CH3 + Nal
For this reaction, the relationship between kr and ki is
a)
ki = 2kr
b)
ki = (1/2)kr
c)
ki = kr
d)
ki = (1/3)kr
|
|
Pooja Choudhury answered |
The given reaction is : (+)CH3CH(I)CH2CH3 + Nal* → CH3CH(I*)CH2CH3 + Nal
This is an example of an SN2 type reaction.
This is an example of an SN2 type reaction.
- SN2 reaction involves the product formation with inversion of configuration. When one molecule of the original substances undergoes inversion, one molecule of product is formed with inversion of configuration.
- This trier that when one original optically active molecule undergoes inversion, the inverted product and another origins, optically active molecules lose their optical activity due to the occurrence of racemisation i.e. when one molecule is inverted, actually two molecules are racemised.
- Hence, it can be concluded that the rate of racemisation is double to the rate of inversion. Hence, mathematically, 2ki = kr
The increasing order of stability of the following free radicals is .- a)(CH3)2CH˙<(CH3)3C˙<(C6H5)2CH˙<(C6H5)3C˙
- b)(C6H5)3C˙<(C6H5)2CH˙<(CH3)3C˙<(CH3)2CH˙
- c)(C6H5)2CH˙<(C6H5)3C˙<(CH3)3C˙<(CH3)2CH˙
- d)(CH3)2CH˙<(CH3)3C˙<(C6H5)3C˙<(C6H5)2CH˙
Correct answer is option 'A'. Can you explain this answer?
The increasing order of stability of the following free radicals is .
a)
(CH3)2CH˙<(CH3)3C˙<(C6H5)2CH˙<(C6H5)3C˙
b)
(C6H5)3C˙<(C6H5)2CH˙<(CH3)3C˙<(CH3)2CH˙
c)
(C6H5)2CH˙<(C6H5)3C˙<(CH3)3C˙<(CH3)2CH˙
d)
(CH3)2CH˙<(CH3)3C˙<(C6H5)3C˙<(C6H5)2CH˙

|
Akshat Saini answered |
The increasing order of stability of the given free radicals is:
a) (CH3)2CH
In this radical, there are two methyl groups attached to the central carbon atom. The presence of these methyl groups increases the electron density around the central carbon atom, making it more stable. This is due to the inductive effect, where the methyl groups donate electron density through the sigma bonds to the central carbon atom. As a result, the central carbon atom is better able to stabilize the unpaired electron in the radical.
Therefore, (CH3)2CH is more stable than other radicals without any electron-donating groups.
a) (CH3)2CH
In this radical, there are two methyl groups attached to the central carbon atom. The presence of these methyl groups increases the electron density around the central carbon atom, making it more stable. This is due to the inductive effect, where the methyl groups donate electron density through the sigma bonds to the central carbon atom. As a result, the central carbon atom is better able to stabilize the unpaired electron in the radical.
Therefore, (CH3)2CH is more stable than other radicals without any electron-donating groups.
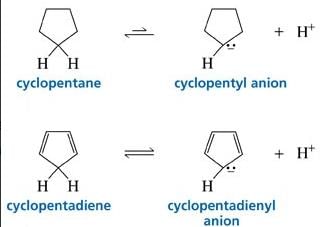
Cyclopentadiene has a pKa = 15, whereas cyclopentane has a pKa > 50. This is because:
- a) Cyclopentadiene is particularly unstable.
- b)Cyclopentadiene contains no lone pairs.
- c)Cyclopentadiene is a 4π anti-aromatic compound
- d)Cyclopentadiene is a 4π anti-aromatic compound and after deprotonation it is aromatic.
Correct answer is option 'D'. Can you explain this answer?
Cyclopentadiene has a pKa = 15, whereas cyclopentane has a pKa > 50. This is because:
a)
Cyclopentadiene is particularly unstable.
b)
Cyclopentadiene contains no lone pairs.
c)
Cyclopentadiene is a 4π anti-aromatic compound
d)
Cyclopentadiene is a 4π anti-aromatic compound and after deprotonation it is aromatic.

|
Raksha Pillai answered |
Since after deprotonation cyclopentadiene becomes stable by aromaticity, So, it will definitely have a higher acidic strength than that of cyclopentane and consequently lower pKa value.
Further, no such stability factor has a role in the case of cyclopentane so it has lower acidic strength comparatively.
Further, no such stability factor has a role in the case of cyclopentane so it has lower acidic strength comparatively.
Hyperconjugation is best described as:
- a)Delocalisation of p electrons into a nearby empty orbital
- b)Delocalisation of σ electrons into a nearby empty orbital
- c)The effect of the alkyl groups donating a small amount of electron density inductively into a carbocation.
- d) The migration of a carbon or hydrogen from one carbocation to another.
Correct answer is option 'B'. Can you explain this answer?
Hyperconjugation is best described as:
a)
Delocalisation of p electrons into a nearby empty orbital
b)
Delocalisation of σ electrons into a nearby empty orbital
c)
The effect of the alkyl groups donating a small amount of electron density inductively into a carbocation.
d)
The migration of a carbon or hydrogen from one carbocation to another.

|
Raghav Rane answered |
Sigma electrons into a nearby empty orbital
c)Transfer of electrons from one atom to another
d)Stabilization of a molecule through resonance
c)Transfer of electrons from one atom to another
d)Stabilization of a molecule through resonance
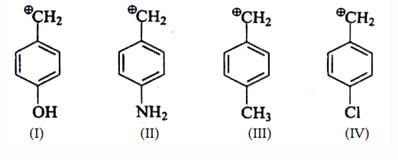
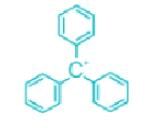
Which of the following carbocations is the most stable?- a)
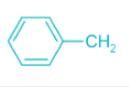
- b)

- c)
- d)
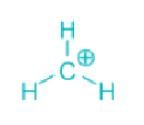
Correct answer is option 'C'. Can you explain this answer?
Which of the following carbocations is the most stable?
a)

b)

c)

d)


|
Asf Institute answered |
Concept:
- An organic compound having a positive charge on the carbon atom and six valence electrons is known as carbocation.
- Carbocations may be formed by the attack of a positively charged species on an unsaturated molecule.
- Carbocations may be classified as primary, secondary, and tertiary.
- Methyl carbocation having only one carbon is an exception.
- The positively charged carbon in the carbocation is sp2 hybridized.
- The three hybrid orbitals are used to form bonds with three other atoms.
- The carbocation has a planar structure.
Explanation:
- Carbocations are highly reactive and are used up as soon as they are generated in the reaction process.
- The relative order of their stabilities are:
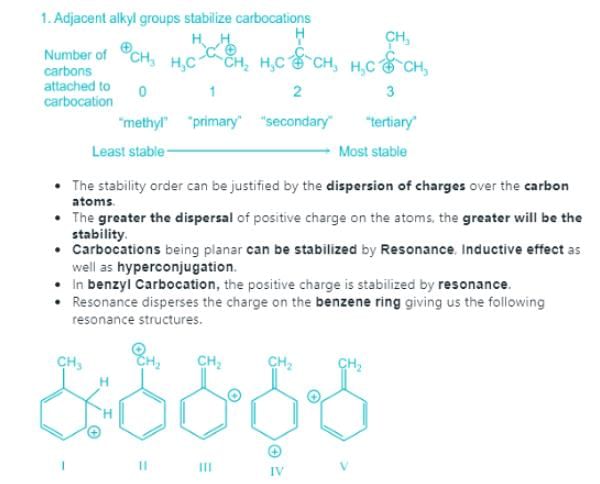
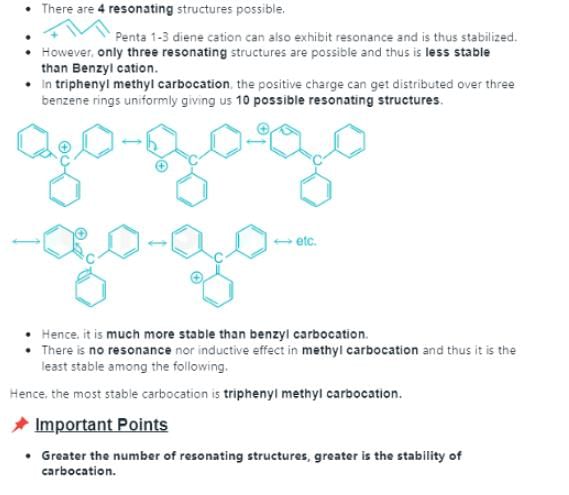
In each of the following pairs of ions, which ion is more stable:
- a)
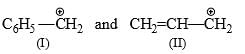
- b)
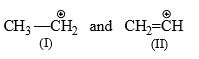
- c)
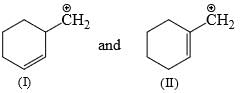
- d)
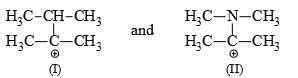
Correct answer is option 'A'. Can you explain this answer?
In each of the following pairs of ions, which ion is more stable:
a)
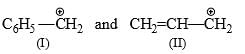
b)
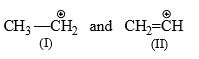
c)

d)
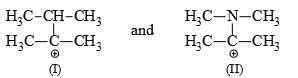

|
Kriti Alam answered |
In A there will be resonance stability in both the cases, 1st by benzyli conjugation and second by pi conjugation.
Which of the following forms most stable carbocation upon removal of OH–:
- a)
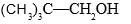
- b)
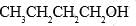
- c) C6H5CH2OH
- d) C6H5CH2CH2OH
Correct answer is option 'C'. Can you explain this answer?
Which of the following forms most stable carbocation upon removal of OH–:
a)
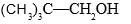
b)
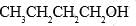
c)
C6H5CH2OH
d)
C6H5CH2CH2OH
|
|
Siddhant Kannoji answered |
In c positive charge on ch2 is in conjugation with benzene ring so is highly stable
Imidazole has a pKa = 7 with respect to its conjugate acid. Which N is protonated in this conjugate acid and why?
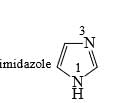
- a) N1 because imidazole is an aromatic heterocycle where n = 1 as per Huckel’s rule.
- b) N1 is protonated because it is sp3 hybridized.
- c) N3 is protonated because it is sp2 hybridized.
- d)N1 is protonated because the lone pair is part of the aromatic pi system
Correct answer is option 'C'. Can you explain this answer?
Imidazole has a pKa = 7 with respect to its conjugate acid. Which N is protonated in this conjugate acid and why?


a)
N1 because imidazole is an aromatic heterocycle where n = 1 as per Huckel’s rule.
b)
N1 is protonated because it is sp3 hybridized.
c)
N3 is protonated because it is sp2 hybridized.
d)
N1 is protonated because the lone pair is part of the aromatic pi system

|
Raksha answered |
The lone pair of N1 are participating in aromaticity so they are not available to donate
& lone pair of N5 are free to donate so N5 will be protonated
& lone pair of N5 are free to donate so N5 will be protonated
The resonance energy per ring of each of the following is:
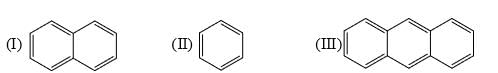
- a)I > II > III
- b) II > III > I
- c) I > III > II
- d)II > I > III
Correct answer is option 'D'. Can you explain this answer?
The resonance energy per ring of each of the following is:


a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
II > I > III

|
Edurev.iitjam answered |
Explanation : Resonance energy per ring decreases as ring size increases
So, II > I > III (Resonance energy/ring)
Anthracene < Naphthalene < Benzene.
Two of the great Mughals wrote their own memories. There were- a)Babar and Humayun
- b)Humayun and Jahangir
- c)Babar and Jahangir
- d)Jahangir and Shahjahan
Correct answer is option 'C'. Can you explain this answer?
Two of the great Mughals wrote their own memories. There were
a)
Babar and Humayun
b)
Humayun and Jahangir
c)
Babar and Jahangir
d)
Jahangir and Shahjahan
|
|
Arun Khatri answered |
The two great Mughals who wrote their own memories are Babar and Jahangir. Below is a detailed explanation of the answer:
1. Introduction:
- The question asks about two Mughals who wrote their own memories.
- We need to identify the correct pair of Mughals.
2. Possible Options:
The given options are:
A) Babar and Humayun
B) Humayun and Jahangir
C) Babar and Jahangir
D) Jahangir and Shahjahan
3. Analyzing the Options:
- We need to determine which pair of Mughals wrote their own memories.
- We will analyze each option to find the correct answer.
4. Option A) Babar and Humayun:
- It is known that Babar wrote his own memoir called "Baburnama."
- However, there is no record of Humayun writing his own memoirs.
- Therefore, this option is incorrect.
5. Option B) Humayun and Jahangir:
- While Humayun did not write his own memoirs, Jahangir did.
- The memoirs written by Jahangir are known as "Tuzk-e-Jahangiri."
- Therefore, this option is incorrect.
6. Option C) Babar and Jahangir:
- As mentioned earlier, Babar wrote his own memoir called "Baburnama."
- Jahangir also wrote his own memoirs known as "Tuzk-e-Jahangiri."
- Both Babar and Jahangir wrote their own memories, making this option correct.
7. Option D) Jahangir and Shahjahan:
- While Jahangir did write his own memoirs, there is no record of Shahjahan writing his own memoirs.
- Therefore, this option is incorrect.
8. Conclusion:
- After analyzing all the options, we can conclude that the correct answer is option C) Babar and Jahangir.
- Both Babar and Jahangir wrote their own memoirs, namely "Baburnama" and "Tuzk-e-Jahangiri," respectively.
Which of the following statements about resonance structures is false?
- a)Individual resonance structure are imaginary, not real.
- b)Resonance forms differ only in the placement of their -or non-bonding electron or unpairedelectron.
- c)In valid resonance structures, all atoms from the second row of the periodic table must have an octet of electrons.
- d)Different resonance structures of a substance do not have to be equivalent.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about resonance structures is false?
a)
Individual resonance structure are imaginary, not real.
b)
Resonance forms differ only in the placement of their -or non-bonding electron or unpairedelectron.
c)
In valid resonance structures, all atoms from the second row of the periodic table must have an octet of electrons.
d)
Different resonance structures of a substance do not have to be equivalent.

|
Vandana Gupta answered |
Explanation:
Resonance Structures:
- Resonance structures are a way of representing the delocalization of electrons in a molecule or ion.
- They are used when a single Lewis structure cannot accurately represent the bonding in a molecule.
- Resonance structures are not individual, real structures but rather theoretical representations of the possible electron distributions in a molecule.
Differences in Resonance Structures:
- Resonance forms differ only in the placement of their π or non-bonding electrons.
- The actual structure of the molecule is a combination or hybrid of all the resonance structures.
Equivalence of Resonance Structures:
- Different resonance structures of a substance do not have to be equivalent.
- Some resonance structures may contribute more to the overall structure of the molecule than others.
- The more stable resonance structures are typically those where formal charges are minimized and octet rules are obeyed.
Validity of Resonance Structures:
- In valid resonance structures, all atoms from the second row of the periodic table must have an octet of electrons.
- This means that elements like carbon, nitrogen, oxygen, and fluorine follow the octet rule in resonance structures.
Therefore, the false statement among the options provided is that different resonance structures of a substance do not have to be equivalent. In reality, some resonance structures may contribute more significantly to the overall structure of the molecule based on stability considerations.
Rank, from the most stabilized to the least stabilized, the following free radicals according to their stabilization energies:
(I) CH3CH2 (II) CH2CH3 (III) (CH3)2CH (IV) (CH2=CH—CH2) - a) IV > III > II > I
- b) I > IV > III > II
- c) III > IV > I > II
- d) III > IV > II > I
Correct answer is option 'A'. Can you explain this answer?
Rank, from the most stabilized to the least stabilized, the following free radicals according to their stabilization energies:
(I) CH3CH2 (II) CH2CH3 (III) (CH3)2CH (IV) (CH2=CH—CH2)
(I) CH3CH2 (II) CH2CH3 (III) (CH3)2CH (IV) (CH2=CH—CH2)
a)
IV > III > II > I
b)
I > IV > III > II
c)
III > IV > I > II
d)
III > IV > II > I

|
Jaya Sen answered |
To rank the free radicals according to their stabilization energies, we need to consider the factors that contribute to stabilization.
The factors that contribute to stabilization of free radicals include:
1. Hyperconjugation: The presence of adjacent sigma bonds can provide stabilization through the overlap of electron density from the sigma bond into the empty p orbital of the free radical.
2. Inductive effect: The presence of electron-donating alkyl groups can stabilize the free radical.
3. Resonance: If the free radical can form resonance structures, it will be more stabilized.
Now, let's analyze each free radical:
(I) CH3CH2: This free radical has one adjacent sigma bond and one alkyl group, providing some stabilization through hyperconjugation and the inductive effect.
(II) CH2CH3: This free radical also has one adjacent sigma bond and one alkyl group, providing the same level of stabilization as (I).
(III) (CH3)2CH: This free radical has two adjacent sigma bonds and one alkyl group, providing more stabilization through hyperconjugation and the inductive effect compared to (I) and (II).
(IV) (CH2=CH: This free radical has one adjacent sigma bond and one pi bond. The presence of the pi bond allows for resonance stabilization, which is a stronger stabilizing factor compared to hyperconjugation and the inductive effect.
Therefore, the ranking of the free radicals from most stabilized to least stabilized is as follows:
1. (CH2=CH
2. (CH3)2CH
3. CH3CH2
4. CH2CH3
The factors that contribute to stabilization of free radicals include:
1. Hyperconjugation: The presence of adjacent sigma bonds can provide stabilization through the overlap of electron density from the sigma bond into the empty p orbital of the free radical.
2. Inductive effect: The presence of electron-donating alkyl groups can stabilize the free radical.
3. Resonance: If the free radical can form resonance structures, it will be more stabilized.
Now, let's analyze each free radical:
(I) CH3CH2: This free radical has one adjacent sigma bond and one alkyl group, providing some stabilization through hyperconjugation and the inductive effect.
(II) CH2CH3: This free radical also has one adjacent sigma bond and one alkyl group, providing the same level of stabilization as (I).
(III) (CH3)2CH: This free radical has two adjacent sigma bonds and one alkyl group, providing more stabilization through hyperconjugation and the inductive effect compared to (I) and (II).
(IV) (CH2=CH: This free radical has one adjacent sigma bond and one pi bond. The presence of the pi bond allows for resonance stabilization, which is a stronger stabilizing factor compared to hyperconjugation and the inductive effect.
Therefore, the ranking of the free radicals from most stabilized to least stabilized is as follows:
1. (CH2=CH
2. (CH3)2CH
3. CH3CH2
4. CH2CH3
List the following carbocations in order of decreasing stability (starting with the most stable):
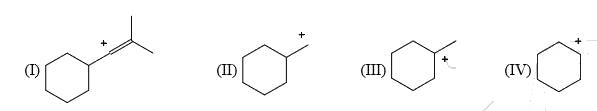
- a) II, III, I, IV
- b) III, IV, II, I
- c) III, IV, I, II
- d) I, II, IV, III
Correct answer is option 'B'. Can you explain this answer?
List the following carbocations in order of decreasing stability (starting with the most stable):


a)
II, III, I, IV
b)
III, IV, II, I
c)
III, IV, I, II
d)
I, II, IV, III

|
Isha Bose answered |
Correct Answer :- b
Explanation : I have an sp2 hybridized carbocation which is highly unstable. Hence, I will be the least stable of all. III will be the most stable as it is tertiary carbocation.
Among II and IV, IV has 4 alpha− hydrogens whereas II has only, α− hydrogens. Also IV is a secondary carbocation while II is a primary one. Hence IV will be more stable than II.
Hence, the decreasing order of stability or stabilization energy is III>IV>II>I
Which carbocation is the most stabilized:
- a)
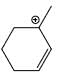
- b)
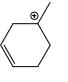
- c)
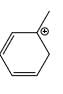
- d)
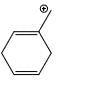
Correct answer is option 'C'. Can you explain this answer?
Which carbocation is the most stabilized:
a)

b)

c)

d)

|
|
Rahul Dps answered |
Two double bond in delocalisation with +ve charge and 6 alpha hydrogen present
Which of the following phenol would be the most acidic:
- a)
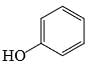
- b)
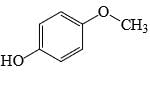
- c)
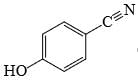
- d)
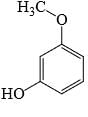
Correct answer is option 'C'. Can you explain this answer?
Which of the following phenol would be the most acidic:
a)

b)

c)

d)


|
Uma Bharti answered |
Ewg enhances acudity cyanide is ewg
Chapter doubts & questions for Reaction Intermediates - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Reaction Intermediates - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup